Correlation Analysis of Microbial Contamination and Alkaline Phosphatase Activity in Raw Milk and Dairy Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Microbiological Analyses and Enzymatic Activity Assays
2.3. Statistical Analysis
3. Results
3.1. Microbiological and Enzymatic Activity Analyses
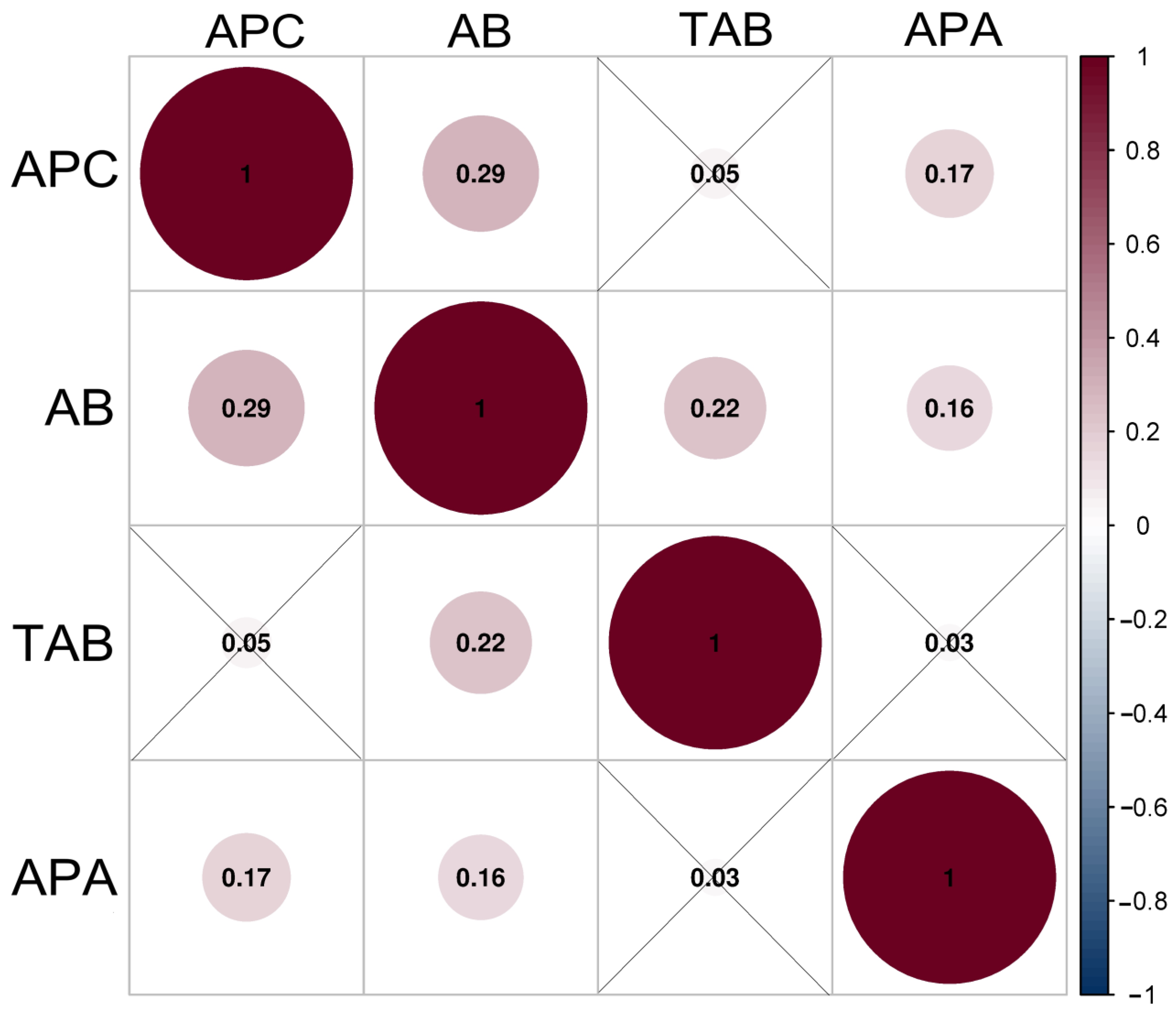
3.2. Correlation Analysis of Microbial Contamination and Alkaline Phosphatase Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belli, P.; Cantafora, A.; Stella, S.; Barbieri, S.; Crimella, C. Microbiological survey of milk and dairy products from a small scale dairy processing unit in Maroua (Cameroon). Food Control 2013, 32, 366–370. [Google Scholar] [CrossRef]
- Keba, A.; Rolon, M.L.; Tamene, A.; Dessie, K.; Vipham, J.; Kovac, J.; Zewdu, A. Review of the prevalence of foodborne pathogens in milk and dairy products in Ethiopia. Int. Dairy J. 2020, 109, 104762. [Google Scholar] [CrossRef] [PubMed]
- Berhanu, L.; Gume, B.; Kassa, T.; Dadi, L.S.; Tegegne, D.; Getnet, M.; Bediru, H.; Getaneh, A.; Suleman, S.; Mereta, S.T. Microbial quality of raw cow milk and its predictors along the dairy value chain in Southwest Ethiopia. Int. J. Food Microbiol. 2021, 350, 109228. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lu, Y.; Xu, H.; Lv, M.; Hu, D.; He, Z.; Liu, L.; Wang, Z.; Feng, Y. Challenges to improve the safety of dairy products in China. Trends Food Sci. Technol. 2018, 76, 6–14. [Google Scholar] [CrossRef]
- Boor, K.J.; Wiedmann, M.; Murphy, S.; Alcaine, S. A 100-Year Review: Microbiology and safety of milk handling. J. Dairy Sci. 2017, 100, 9933–9951. [Google Scholar] [CrossRef]
- Benahmed, M.; Leguerinel, I.; Moussa-Boudjemaa, B. Biodiversity, spoilage capacity and heat resistance of mesophilic aerobic spores isolated from milk powders marketed in Algeria. Int. J. Dairy Technol. 2020, 73, 771–780. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Flint, S.; Yuan, L.; Li, Y.; Liu, T.; He, G. Propensity for biofilm formation by aerobic mesophilic and thermophilic spore forming bacteria isolated from Chinese milk powders. Int. J. Food Microbiol. 2017, 262, 89–98. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Li, Y.; Liu, T.; Flint, S.; Zhang, G.; Yuan, L.; Pei, Z.; He, G. The heat resistance and spoilage potential of aerobic mesophilic and thermophilic spore forming bacteria isolated from Chinese milk powders. Int. J. Food Microbiol. 2016, 238, 193–201. [Google Scholar] [CrossRef]
- Ziobro, G.C.; McElroy, K.M. Fluorometric detection of active alkaline phosphatase and gamma-glutamyl transferase in fluid dairy products from multiple species. J. Food Prot. 2013, 76, 892–898. [Google Scholar] [CrossRef]
- Ying, L.; Pei, X.; Zhang, X.; Wu, L.; Liu, Y.; Zhou, H.; Ma, G.; Chen, Q.; Liang, H.; Yang, D. A surveillance of microbiological contamination on raw poultry meat at retail markets in China. Food Control 2019, 104, 99–104. [Google Scholar]
- Wang, H.; Tao, Y.; Gao, D.; Liu, G.; Chen, C.; Ren, N.; van Lier, J.B.; de Kreuk, M. Microbial population dynamics in response to increasing loadings of pre-hydrolyzed pig manure in an expanded granular sludge bed. Water Res. 2015, 87, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Eijlander, R.T.; Hekezen, R.V.; Bienvenue, A.; Girard, V.; Hoornstra, E.; Johnson, N.B.; Meyer, R.; Wagendorp, A.; Walker, D.C.; Wells-Bennik, M.H.J. Spores in dairy—New insights in detection, enumeration and risk assessment. Int. J. Dairy Technol. 2019, 72, 303–315. [Google Scholar] [CrossRef]
- Albillos, S.M.; Reddy, R.; Salter, R. Evaluation of alkaline phosphatase detection in dairy products using a modified rapid chemiluminescent method and official methods. J. Food Prot. 2011, 74, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- SPSS 16.0, Command Syntax Reference; SPSS Inc.: Chicago, IL, USA, 2007.
- Che, L.; Yu, C.; Chen, G.; Lin, J.; Xie, Z.; Xia, T.; Luo, W.; Cai, X.; Liu, S. The Inflammatory Response Induced by RELMβ Upregulates IL-8 and IL-1β Expression in Bronchial Epithelial Cells in COPD. Int. J. Chronic Obstr. 2021, 16, 2503–2513. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhu, X.; Kong, F.; Guo, W. A rapid method on identifying disqualified raw goat’s milk based on total bacterial count by using dielectric spectra. J. Food Eng. 2018, 239, 40–51. [Google Scholar] [CrossRef]
- Ding, R.; Yang, S.; Geng, L.; Liu, Y.; He, B.; Liu, L.; Yue, X.; Wu, R.; Wu, J. Characterization of the core microflora and nutrient composition in packaged pasteurized milk products during storage. Food Sci. Hum. Wellness 2023, 12, 1279–1286. [Google Scholar] [CrossRef]
- Knight-Jones, T.; Hang’Ombe, M.; Songe, M.; Sinkala, Y.; Grace, D. Microbial Contamination and Hygiene of Fresh Cow’s Milk Produced by Smallholders in Western Zambia. Int. J. Environ. Res. Public Health 2016, 13, 737. [Google Scholar] [CrossRef]
- Martin, N.H.; Torres-Frenzel, P.; Wiedmann, M. Invited review: Controlling dairy product spoilage to reduce food loss and waste. J. Dairy Sci. 2021, 104, 1251–1261. [Google Scholar] [CrossRef]
- Parseelan, A.; Muthu, S.; Kannan, P.; Ayyasamy, E.; Narayanan, R. Aerobic Plate Count of Milk and Dairy Products Marketed in Different Zones of Chennai. Int. J. Livest. Res. 2019, 9, 97–102. [Google Scholar] [CrossRef]
- Martini, M.; Salari, F.; Altomonte, I.; Ragona, G.; Piazza, A.; Gori, R.; Casati, D.; Brajon, G. Effects of pasteurization and storage conditions on donkey milk nutritional and hygienic characteristics. J. Dairy Res. 2018, 85, 445–448. [Google Scholar] [CrossRef]
- Lau, S.; Trmcic, A.; Martin, N.H.; Wiedmann, M.; Murphy, S.I. Development of a Monte Carlo simulation model to predict pasteurized fluid milk spoilage due to post-pasteurization contamination with gram-negative bacteria. J. Dairy Sci. 2022, 105, 1978–1998. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Roy, S.; Nabi, A.; Solaiman, S.; Rahman, M.; Huq, M.; Siddiquee, N.A.; Ahmed, N. Microbiological quality assessment of milk at different stages of the dairy value chain in a developing country setting. Int. J. Food Microbiol. 2018, 278, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Cui, C.; Li, X.; Yan, J.; Sun, E.; Wang, C.; Guo, H.; Hao, Y. Prevalence, antimicrobial susceptibility, and antibiotic resistance gene transfer of Bacillus strains isolated from pasteurized milk. J. Dairy Sci. 2023, 106, 75–83. [Google Scholar] [CrossRef]
- Jia, Z.; Huang, L.; Wei, Z.; Yao, Y.; Fang, T.; Li, C. Dynamic kinetic analysis of growth of Listeria monocytogenes in pasteurized cow milk. J. Dairy Sci. 2021, 104, 2654–2667. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Z.; Zhang, C.; Wang, L.; Pang, L.; Zhang, D.; Man, C.; Jiang, Y. Assessment of the production of Bacillus cereus protease and its effect on the quality of ultra-high temperature-sterilized whole milk. J. Dairy Sci. 2021, 104, 6577–6587. [Google Scholar] [CrossRef]
- Xing, Q.; Fu, X.; Liu, Z.; Cao, Q.; You, C. Contents and evolution of potential furfural compounds in milk-based formula, ultra-high temperature milk and pasteurised yoghurt. Int. Dairy J. 2021, 120, 105086. [Google Scholar] [CrossRef]
- Aouadhi, C.; MAa Roufi, A.; Mejri, S. Incidence and characterisation of aerobic spore-forming bacteria originating from dairy milk in Tunisia. Int. J. Dairy Technol. 2014, 67, 95–102. [Google Scholar] [CrossRef]
- Patil, M.P.; Nagvekar, A.S.; Ingole, S.D.; Bharucha, S.V.; Palve, V.T. Somatic cell count and alkaline phosphatase activity in milk for evaluation of mastitis in buffalo. Vet. World 2015, 8, 363–366. [Google Scholar] [CrossRef]

| Item * | Milk | Mean ± SD ^ | Median | Min | Max | 25th Perc. | 75th Perc. | Threshold Values | Over-Limit Ratio |
|---|---|---|---|---|---|---|---|---|---|
| APC log10 CFU/mL | Raw | 4.55 ± 1.43 | 4.37 | <1 | 8.15 | 3.15 | 5.53 | 6.30 # | 9.89% (43/435) |
| Pasteurized | 2.83 ± 1.32 | <1 | <1 | 8.15 | <1 | 2 | 5.00 & | 2.22% (10/451) | |
| AB log10 CFU/mL | Raw | 2.00 ± 0.82 | <1 | <1 | 5.04 | <1 | 1.71 | _ | _ |
| Pasteurized | 1.67 ± 0.76 | <1 | <1 | 5.30 | <1 | <1 | _ | _ | |
| TAB log10 CFU/mL | Raw | 1.49 ± 0.44 | <1 | <1 | 2.48 | <1 | <1 | _ | _ |
| Pasteurized | 1.61 ± 0.76 | <1 | <1 | 3.81 | <1 | <1 | _ | _ | |
| APA log10 mU/L | Raw | 5.59 ± 0.40 | 5.65 | 1.96 | 6.68 | 5.51 | 5.82 | _ | _ |
| Pasteurized | 2.22 ± 0.38 | <1.78 | <1.78 | 3.53 | <1.78 | 2.08 | 2.54 @ | 9.71% (43/443) |
| Item * | Mean ± SD ^ | Median | Min | Max | 25th Perc. | 75th Perc. |
|---|---|---|---|---|---|---|
| APC log10 CFU/mL | 1.45 ± 0.45 | <1 | <1 | 2.32 | <1 | <1 |
| AB log10 CFU/mL | 1.08 ± 0.15 | <1 | <1 | 1.34 | <1 | <1 |
| TAB log10 CFU/mL | <1 | <1 | <1 | <1 | <1 | <1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Z.; Li, Y.; Yan, L.; Yang, S.; Yang, D. Correlation Analysis of Microbial Contamination and Alkaline Phosphatase Activity in Raw Milk and Dairy Products. Int. J. Environ. Res. Public Health 2023, 20, 1825. https://doi.org/10.3390/ijerph20031825
Peng Z, Li Y, Yan L, Yang S, Yang D. Correlation Analysis of Microbial Contamination and Alkaline Phosphatase Activity in Raw Milk and Dairy Products. International Journal of Environmental Research and Public Health. 2023; 20(3):1825. https://doi.org/10.3390/ijerph20031825
Chicago/Turabian StylePeng, Zixin, Ying Li, Lin Yan, Shuran Yang, and Dajin Yang. 2023. "Correlation Analysis of Microbial Contamination and Alkaline Phosphatase Activity in Raw Milk and Dairy Products" International Journal of Environmental Research and Public Health 20, no. 3: 1825. https://doi.org/10.3390/ijerph20031825
APA StylePeng, Z., Li, Y., Yan, L., Yang, S., & Yang, D. (2023). Correlation Analysis of Microbial Contamination and Alkaline Phosphatase Activity in Raw Milk and Dairy Products. International Journal of Environmental Research and Public Health, 20(3), 1825. https://doi.org/10.3390/ijerph20031825






