Conscious Sedation for Dental Treatments in Subjects with Intellectual Disability: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
- Population: subjects with intellectual disability in need of dental treatment.
- Intervention: drug- or gas-mediated conscious sedation performed for dental treatment.
- Comparison: no comparison, no drug intervention, different drugs comparison or different dosages.
- Outcome: sedative, behavioral, anxiolytic efficacy, and safety of the sedative interventions.
- -
- Sedative efficacy. Measurement scales include the Ramsay Sedation Scale (scored 1–6 based on the response of the patient), the Richmond Agitation Sedation Scale (scores from +4 to −5), the State Behavioral Scale (scored from −3 to +2), the Bispectral Index Monitoring (range of scores from 0 to 100) and the Classification of Emotional Status designed by Breitkopf and Buttner (scored from 1–4).
- -
- Behavioral efficacy. Measurement scales include the Houpt Behavior Rating Scale (subdivided into 4 scales, each sub-scale then defined separately), the Frankl Behavior Rating Scale, the FLACC, the Venham Scale (scores from 0–3), the Visual Analogue Scale (scale from 0–10).
- -
- Anxiolytic efficacy. Measurement scales include pulse rate, the Children’s Fear Survey Schedule Dental Subscale (defined by 15 scores based on the item that the child is fearful of), and the Spielberger State Anxiety Inventory (psychological inventory based on a 4-point Likert scale consisting of 40 questions).
- -
- Safety. Evaluation includes side effects defined as any undesired harmful effects or reactions to the sedative agents during or after administration.
2.1. Eligibility Criteria
- Type of study: all types of clinical studies except for case series or case studies;
- Publication languages: papers published in English, Italian, and French;
- Time of publication: no time restrictions were applied;
- Type of intervention: conscious sedation with N2O or sedative drugs in subjects with intellectual disabilities undergoing dental treatments;
- Outcomes: sedative, behavioral, anxiolytic effectiveness, and safety of the intervention used to improve collaboration during dental treatments.
- The exclusion criteria were:
- Studies for which the full text is not available.
2.2. Information Sources and Search Strategy
- For PubMed, the string used was: (“Neurodevelopmental Disorders” [Mesh] OR “Disabled Persons” [Mesh] OR special needs) AND (“Dentistry” [Mesh] OR “Oral Health” [Mesh] OR “Mouth” [Mesh] OR “Dental Health Services” [Mesh] OR “Dent*” [Title/Abstract]) AND (“Dental Anxiety” [MeSH Terms] OR Behavior*[Title/Abstract] OR Collaboration[Title/Abstract] OR Succes*[Title/Abstract]) AND (“Benzodiazepines” [Mesh] OR “Tranquilizing Agents” [Pharmacological Action] OR “Tranquilizing Agents” [Mesh] OR “Imidazoles” [Mesh] OR “nitrous oxide” OR ketamine OR “Psychotropic Drugs” [Mesh] OR “conscious sedation” OR n2o OR “moderate sedation” OR “mild sedation”).
- For Embase: (‘mental disease’/exp OR ‘mental disease’ OR ‘disabled person’/exp OR ‘disabled person’) AND (‘dentistry’/exp OR ‘dentistry’ OR ‘mouth’/exp OR ‘mouth’ OR ‘dental health’/exp OR ‘dental health’ OR ‘stomatognathic system’/exp OR ‘stomatognathic system’) AND (‘dental anxiety’/exp OR ‘dental anxiety’ OR ‘collaboration’/exp OR ‘collaboration’ OR ‘treatment success’/exp OR ‘treatment success’) AND (‘benzodiazepine’/exp OR ‘benzodiazepine’ OR ‘tranquilizer’/exp OR ‘tranquilizer’ OR ‘imidazole derivative’/exp OR ‘imidazole derivative’ OR ‘imidazole’/exp OR ‘imidazole’ OR ‘nitrous oxide’/exp OR ‘nitrous oxide’ OR ‘ketamine’/exp OR ‘ketamine’ OR ‘psychotropic agent’/exp OR ‘psychotropic agent’ OR ‘conscious sedation’/exp OR ‘conscious sedation’ OR ‘benzodiazepine derivative’/exp/mj OR ‘anxiolytic agent’/exp/mj OR ketamine OR ‘nitrous oxide’ OR ‘psychotropic agent’/exp/mj OR n2o OR ‘conscious sedation’).
- For Scopus: (TITLE-ABS-KEY(mental disease) OR TITLE-ABS-KEY(disabled person) OR TITLE-ABS-KEY(neurodevelopmental disorders) OR TITLE-ABS-KEY(Disabled) OR TITLE-ABS-KEY(Special needs)) AND (TITLE-ABS-KEY(dentistry) OR TITLE-ABS-KEY(oral health) OR TITLE-ABS-KEY(mouth) OR TITLE-ABS-KEY(dental health) OR TITLE-ABS-KEY(dent*) OR TITLE-ABS-KEY(stomatognathic system)) AND (TITLE-ABS-KEY(dental anxiety) OR TITLE-ABS-KEY(behav*) OR TITLE-ABS-KEY(collaboration) OR TITLE-ABS-KEY(success*) OR TITLE-ABS-KEY(treatment success) OR TITLE-ABS-KEY(dental fear)) AND (TITLE-ABS-KEY(benzodiazepine) OR TITLE-ABS-KEY(tranquilizing agents) OR TITLE-ABS-KEY(tranquilizing drug) OR TITLE-ABS-KEY(imidazole) OR TITLE-ABS-KEY(nitrous oxide) OR TITLE-ABS-KEY(n2o) OR TITLE-ABS-KEY(ketamine) OR TITLE-ABS-KEY(psychotropic drugs) OR TITLE-ABS-KEY(conscious sedation) OR TITLE-ABS-KEY(moderate sedation) OR TITLE-ABS-KEY(mild sedation) OR TITLE-ABS-KEY(psychotropic agent)).
- For Cochrane: (neurodevelopmental disorders OR disabled person OR special needs OR mental disease OR retarded person OR handicap OR impaired person) AND (dentistry OR oral health OR mouth OR dental health OR stomatognathic system OR dent*) AND (dental anxiety OR behavior OR collaboration OR cooperation OR compliance OR treatment success OR success) AND (benzodiazepines OR tranquilizing agents OR imidazoles OR nitrous oxide OR ketamine OR psychotropic drugs OR psychotropic medications OR sedatives OR conscious sedation OR n2o OR moderate sedation OR mild sedation OR anxiolytic agents OR narcotics).
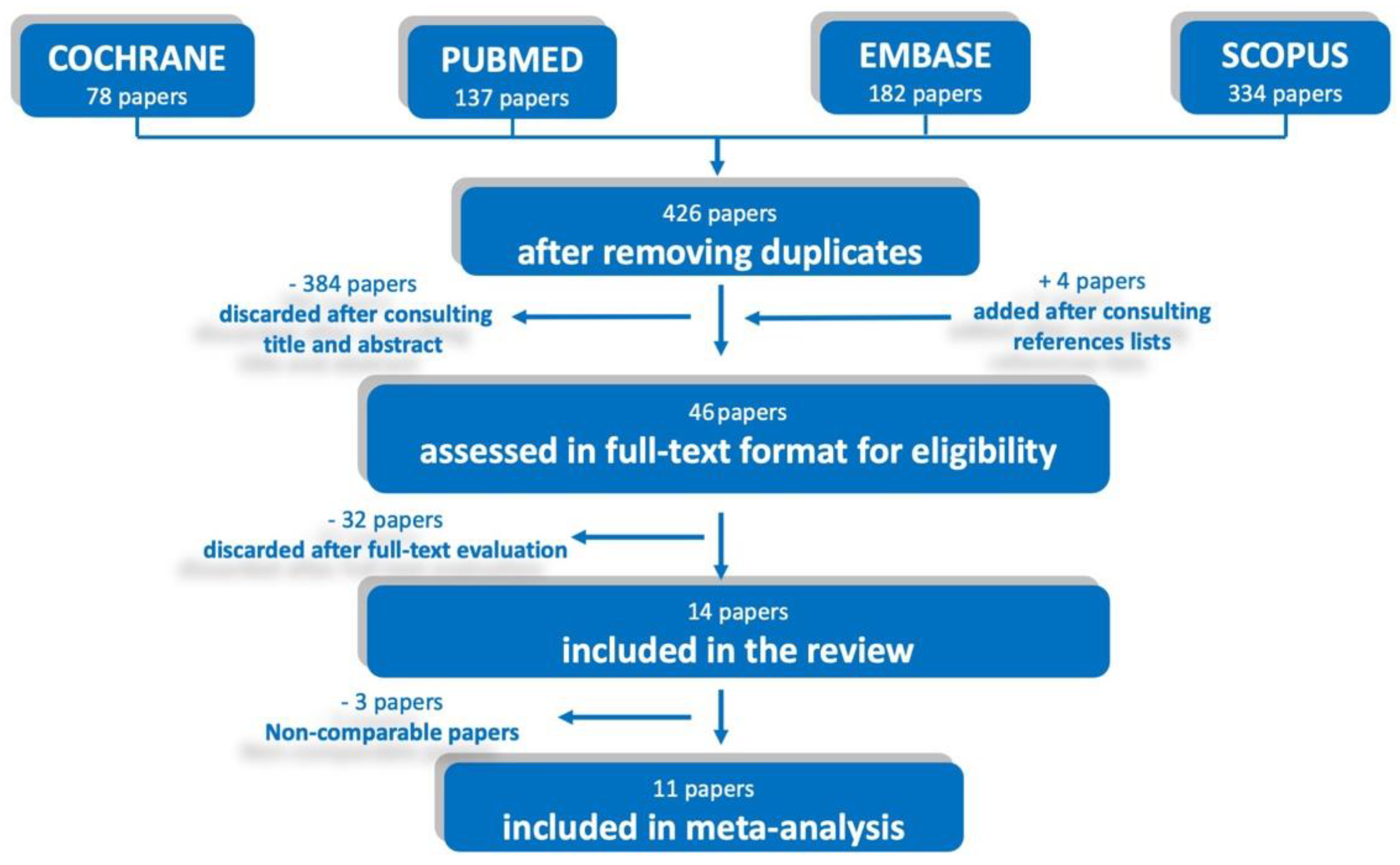
2.3. Study Selection
2.4. Data Collection and Synthesis
2.5. Outcome Variables
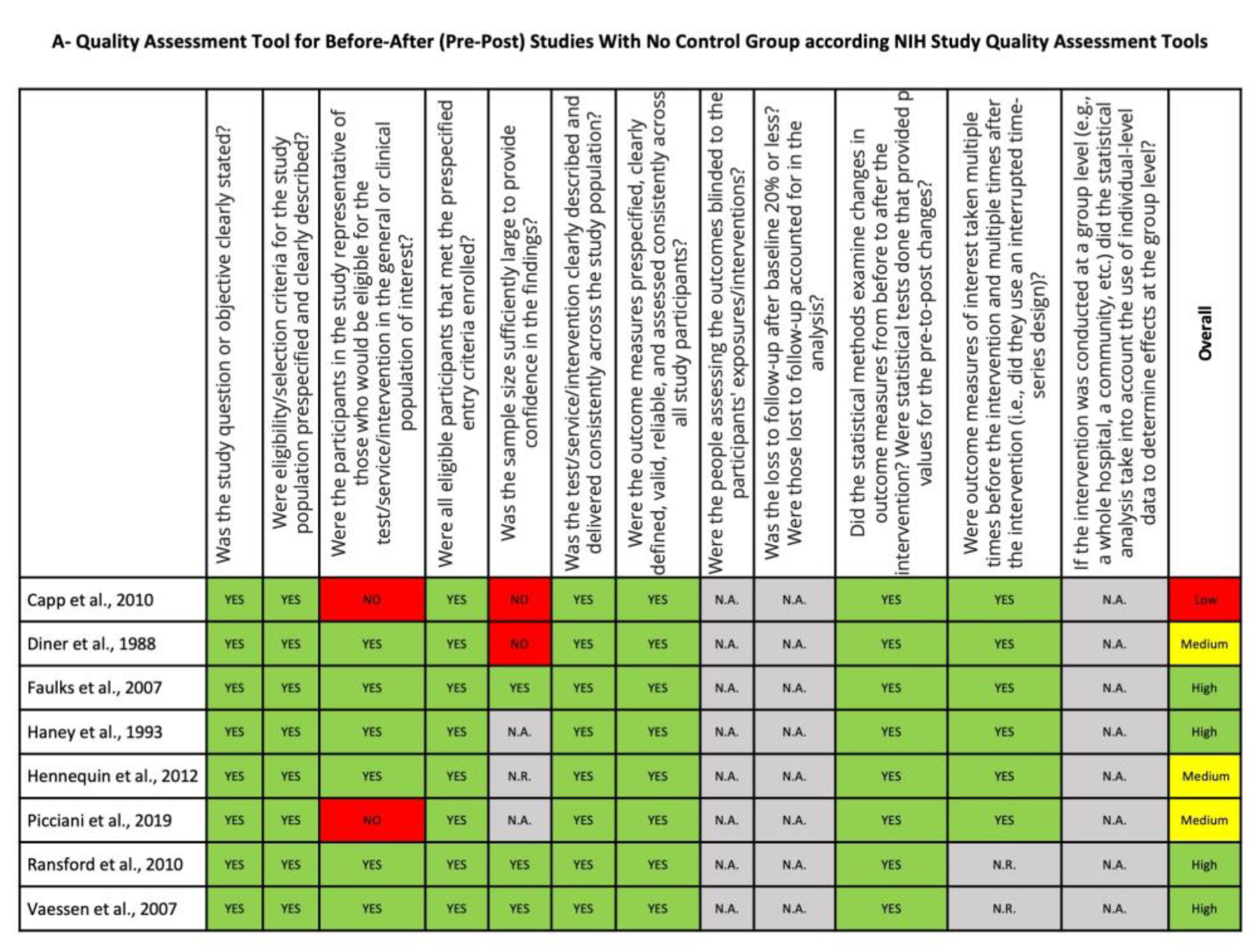
2.6. Risk of Bias Assessment and Quality Assessment
2.7. Data Analysis
3. Results
3.1. Studies Characteristics
3.2. Samples
3.3. Sedative Interventions
3.4. Primary Outcomes
3.5. Secondary Outcomes
3.6. Risk of Bias and Quality Assessment
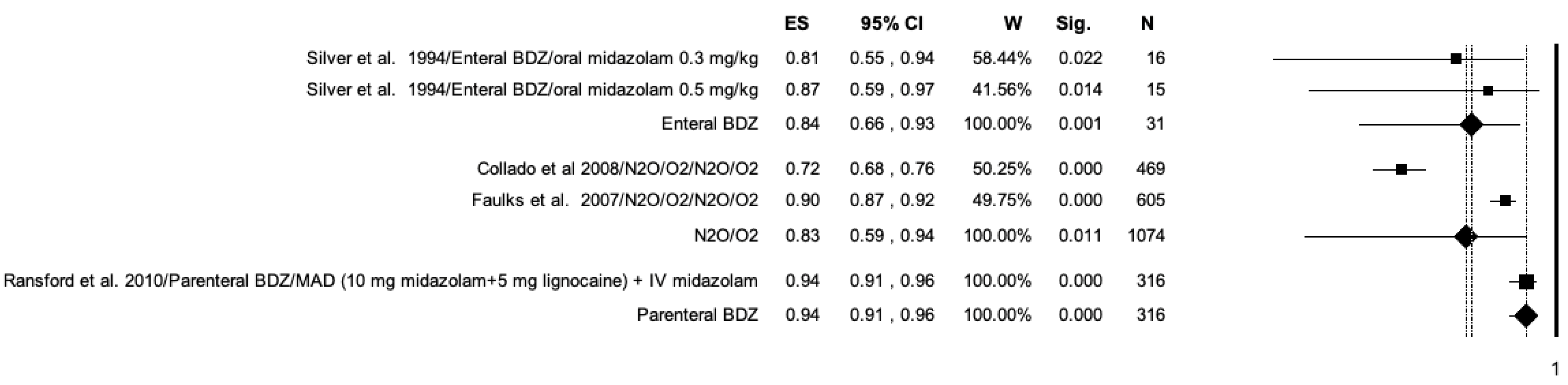
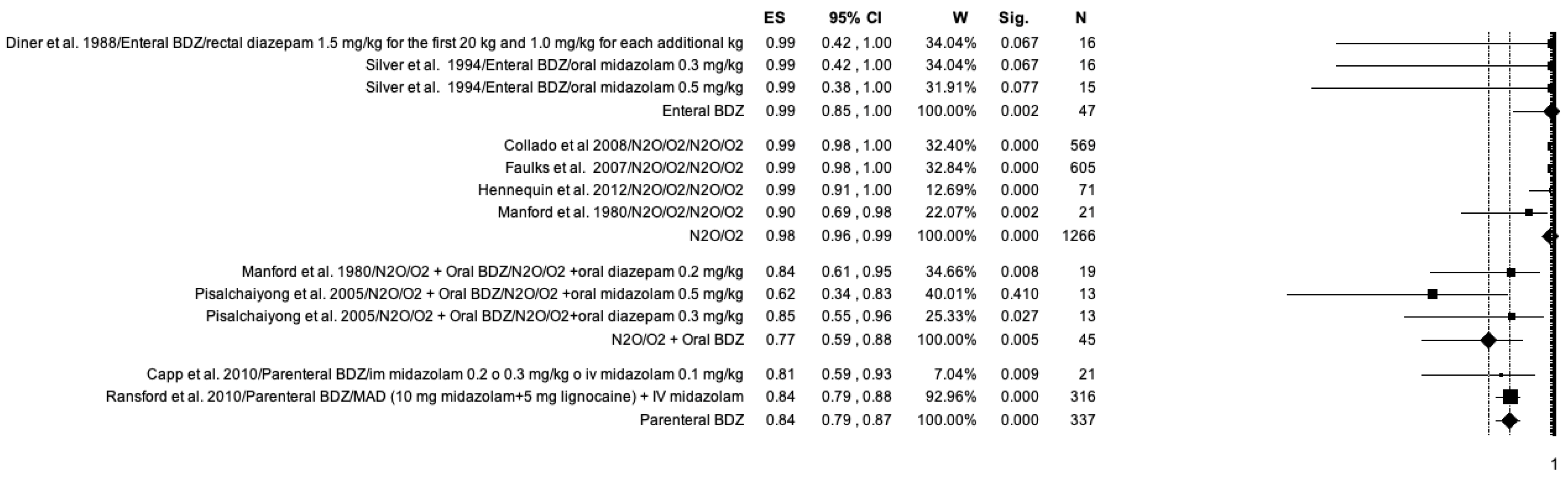
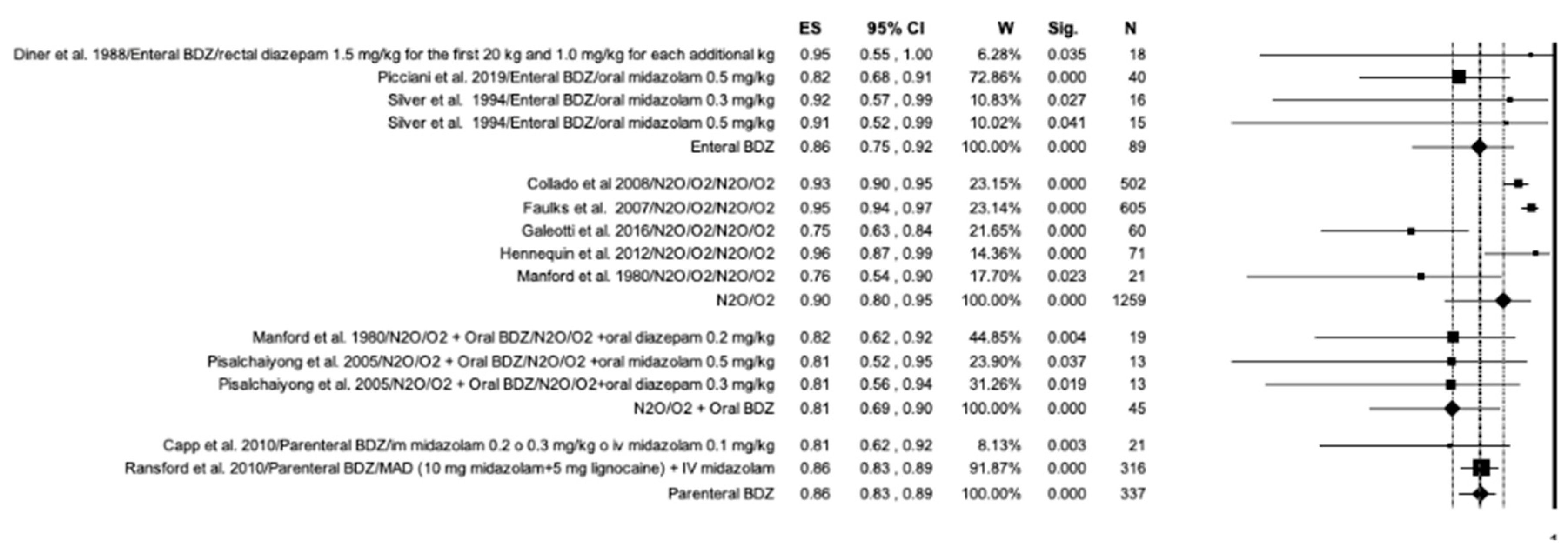
3.7. Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Glassman, P. A Review of Guidelines for Sedation, Anesthesia, and Alternative Interventions for People with Special Needs. Spec. Care Dent. 2009, 29, 9–16. [Google Scholar] [CrossRef]
- Purohit, B.M.; Acharya, S.; Bhat, M. Oral Health Status and Treatment Needs of Children Attending Special Schools in South India: A Comparative Study. Spec. Care Dent. 2010, 30, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Messer, L.B.; Calache, H. A Study of the Dental Treatment Needs of Children with Disabilities in Melbourne, Australia. Aust. Dent. J. 2001, 46, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Tiller, S.; Wilson, K.I.; Gallagher, J.E. Oral Health Status and Dental Service Use of Adults with Learning Disabilities Living in Residential Institutions and in the Community. Community Dent. Health 2001, 18, 167–171. [Google Scholar] [PubMed]
- Gabre, P.; Martinsson, T.; Gahnberg, L. Longitudinal Study of Dental Caries, Tooth Mortality and Interproximal Bone Loss in Adults with Intellectual Disability. Eur. J. Oral Sci. 2001, 109, 20–26. [Google Scholar] [CrossRef]
- Anders, P.L.; Davis, E.L. Oral Health of Patients with Intellectual Disabilities: A Systematic Review. Spec. Care Dent. 2010, 30, 110–117. [Google Scholar] [CrossRef]
- Varela, I.; Fernández-Feijoo, J.; García, E.; Diniz-Freitas, M.; Martínez, I.; Roca, J.; Diz, P.; Limeres, J. Development of a New Tool for Predicting the Behavior of Individuals with Intellectual Disability in the Dental Office: A Pilot Study. Disabil. Health J. 2022, 15, 101229. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, X.; Wang, Y.; Huang, R.; Yang, R.; Zou, J. Postoperative Complications in Chinese Children Following Dental General Anesthesia: A Cross-Sectional Study. Medicine 2020, 99, e23065. [Google Scholar] [CrossRef]
- Lyratzopoulos, G.; Blain, K.M. Inhalation Sedation with Nitrous Oxide as an Alternative to Dental General Anaesthesia for Children. J. Public Health Med. 2003, 25, 303–312. [Google Scholar] [CrossRef]
- Guideline on Behavior Guidance for the Pediatric Dental Patient. Pediatr. Dent. 2015, 37, 57–70.
- Pedlar, J.; Frame, J.W. Control of Pain and Anxiety: Selection of and Preparation for Sedation or Anaesthesia; Elsevier Ltd.: Amsterdam, The Netherlands, 2007; ISBN 9780443100734. [Google Scholar]
- Hosey, M.T. UK National Clinical Guidelines in Paediatric Dentistry. Managing Anxious Children: The Use of Conscious Sedation in Paediatric Dentistry. Int. J. Paediatr. Dent. 2002, 12, 359–372. [Google Scholar] [CrossRef]
- Fiorillo, L. Conscious Sedation in Dentistry. Medicina 2019, 55, 778. [Google Scholar] [CrossRef] [PubMed]
- Coke, J.M.; Edwards, M.D. Minimal and Moderate Oral Sedation in the Adult Special Needs Patient. Dent. Clin. North Am. 2009, 53, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Becker, D.E.; Rosenberg, M. Nitrous Oxide and the Inhalation Anesthetics. Anesth. Prog. 2008, 55, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Vallogini, G.; Festa, P.; Matarazzo, G.; Gentile, T.; Garret-bernardin, A.; Zanette, G.; Galeotti, A. Conscious Sedation in Dentistry for the Management of Pediatric Patients with Autism: A Narrative Review of the Literature. Children 2022, 9, 460. [Google Scholar] [CrossRef] [PubMed]
- Haney, K.L.; McWhorter, A.G.; Seale, N.S. An Assessment of the Success of Meperidine and Promethazine Sedation in Medically Compromised Children. ASDC J. Dent. Child 1993, 60, 288–294. [Google Scholar]
- Vaessen, H.H.B.; Schouten, A.N.J.; van der Hoeve, H.; Knape, J.T.A. The Feasibility of Office-Based Propofol Sedation for Dental Care in Patients with Intellectual Disability by Sedation Practitioners. Spec. Care Dent. 2017, 37, 93–98. [Google Scholar] [CrossRef]
- Oei-Lim, L.B.; Vermeulen-Cranch, D.M.; Bouvy-Berends, E.C. Conscious Sedation with Propofol in Dentistry. Br. Dent. J. 1991, 170, 340–342. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The Well-Built Clinical Question: A Key to Evidence-Based Decisions. ACP J. Club 1995, 123, A12–A13. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-Bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-Bias Assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Study Quality Assessment Tools NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 15 December 2022).
- Collado, V.; Faulks, D.; Nicolas, E.; Hennequin, M. Conscious Sedation Procedures Using Intravenous Midazolam for Dental Care in Patients with Different Cognitive Profiles: A Prospective Study of Effectiveness and Safety. PLoS ONE 2013, 8, e71240. [Google Scholar] [CrossRef] [PubMed]
- Hennequin, M.; Collado, V.; Faulks, D.; Koscielny, S.; Onody, P.; Nicolas, E. A Clinical Trial of Efficacy and Safety of Inhalation Sedation with a 50% Nitrous Oxide/Oxygen Premix (KalinoxTM) in General Practice. Clin. Oral Investig. 2012, 16, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Collado, V.; Nicolas, E.; Faulks, D.; Tardieu, C.; Manière, M.C.; Droz, D.; Onody, P.; Hennequin, M. Evaluation of Safe and Effective Administration of Nitrous Oxide after a Postgraduate Training Course. BMC Clin. Pharmacol. 2008, 8, 3. [Google Scholar] [CrossRef]
- Faulks, D.; Hennequin, M.; Albecker-Grappe, S.; Manière, M.C.; Tardieu, C.; Berthet, A.; Wolikow, M.; Droz, D.; Koscielny, S.; Onody, P. Sedation with 50% Nitrous Oxide/Oxygen for Outpatient Dental Treatment in Individuals with Intellectual Disability. Dev. Med. Child Neurol. 2007, 49, 621–625. [Google Scholar] [CrossRef]
- Manford, M.L.M.; Roberts, G.J. Dental Treatment in Young Handicapped Patients. An Assessment of Relative Analgesia as an Alternative to General Anaesthesia. Anaesthesia 1980, 35, 1157–1168. [Google Scholar] [CrossRef]
- Ransford, N.J.; Manley, M.C.G.; Lewis, D.A.; Thompson, S.A.; Wray, L.J.; Boyle, C.A.; Longman, L.P. Intranasal/Intravenous Sedation for the Dental Care of Adults with Severe Disabilities: A Multicentre Prospective Audit. Br. Dent. J. 2010, 208, 565–569. [Google Scholar] [CrossRef]
- Silver, T.; Wilson, C.; Webb, M. Evaluation of Two Dosages of Oral Midazolam as a Conscious Sedation for Physically and Neurologically Compromised Pediatric Dental Patients. Pediatr. Dent. 1994, 16, 350–359. [Google Scholar]
- Capp, P.L.; de Faria, M.E.J.; Siqueira, S.R.D.T.; Cillo, M.T.P.; Prado, E.G.B.; de Siqueira, J.T.T. Special Care Dentistry: Midazolam Conscious Sedation for Patients with Neurological Diseases. Eur. J. Paediatr. Dent. 2010, 11, 162–164. [Google Scholar]
- Picciani, B.L.S.; dos Santos, B.M.; Silva-Júnior, G.O.; Marinho, M.A.; Papa, E.G.; Faria, M.D.B.; Bastos, L.F.; de Gouvêa, C.V.D. Contribution of Benzodiazepines in Dental Care of Patients with Special Needs. J. Clin. Exp. Dent. 2019, 11, e1170. [Google Scholar] [CrossRef] [PubMed]
- Diner, M.H.; Fortin, R.C.; Marcoux, P.; Legault, V. Behavioral Influences of Rectal Diazepam in Solution on Dental Patients with Mentally and Physically Handicapping Conditions. Spec. Care Dent. 1988, 8, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, A.; Garret Bernardin, A.; D’Antò, V.; Ferrazzano, G.F.; Gentile, T.; Viarani, V.; Cassabgi, G.; Cantile, T. Inhalation Conscious Sedation with Nitrous Oxide and Oxygen as Alternative to General Anesthesia in Precooperative, Fearful, and Disabled Pediatric Dental Patients: A Large Survey on 688 Working Sessions. Biomed Res. Int. 2016, 2016, 7289310. [Google Scholar] [CrossRef] [PubMed]
- Pisalchaiyong, T.; Trairatvorakul, C.; Jirakijja, J.; Yuktarnonda, W. Comparison of the Effectiveness of Oral Diazepam and Midazolam for the Sedation of Autistic Patients during Dental Treatment. Pediatr. Dent. 2005, 27, 198–206. [Google Scholar] [PubMed]
- Bennett, C.R. Conscious Sedation: An Alternative to General Anesthesia. J. Dent. Res. 1984, 63, 832–833. [Google Scholar] [CrossRef]
- Ashley, P.F.; Chaudhary, M.; Lourenço-Matharu, L. Sedation of Children Undergoing Dental Treatment. Cochrane Database Syst. Rev. 2018, 12, CD003877. [Google Scholar] [CrossRef]
- Glassman, P.; Caputo, A.; Dougherty, N.; Lyons, R.; Messieha, Z.; Miller, C.; Peltier, B.; Romer, M. Special Care Dentistry Association Consensus Statement on Sedation, Anesthesia, and Alternative Techniques for People with Special Needs. Spec. Care Dent. 2009, 29, 2–8. [Google Scholar] [CrossRef]
- Stanková, M.; Buček, A.; Dostálová, T.; Ginzelová, K.; Pacáková, Z.; Seydlová, M. Patients with Special Needs within Treatment under General Anesthesia–Meta-Analysis. Prague Med. Rep. 2011, 112, 216–225. [Google Scholar]
- Jamieson, W.J.; Vargas, K. Recall Rates and Caries Experience of Patients Undergoing General Anesthesia for Dental Treatment. Pediatr. Dent. 2007, 29, 253–260. [Google Scholar]
- Yost, Q.; Nelson, T.; Sheller, B.; McKinney, C.M.; Tressel, W.; Chim, A.N. Children with Autism Spectrum Disorder Are Able to Maintain Dental Skills: A Two-Year Case Review of Desensitization Treatment. Pediatr. Dent. 2019, 41, 397–403. [Google Scholar]



| Authors | Year | Journal | Country | Databases | Type of Study |
|---|---|---|---|---|---|
| Capp et al. [33] | 2010 | European Journal of Paediatric Dentistry | Brazil | PMD, SC, EMB | Prospective clinical study |
| Collado et al. [28] | 2008 | BMC Clinical Pharmacology | France | PMD, SC, EMB | Multicenter Prospective clinical study |
| Collado et al. [26] | 2013 | Plos One | France | Reference | NRCT |
| Diner et al. [35] | 1988 | Special Care in Dentistry | Canada | SC, EMB | Prospective clinical study |
| Faulks et al. [29] | 2007 | Developmental Medicine and Child Neurology | France | PMD, SC, EMB | Prospective clinical study |
| Galeotti et al. [36] | 2016 | BioMed Research International | Italy | PMD, SC, EMB | Observational study |
| Haney et al. [17] | 1993 | ASDC Journal of Dentistry for Children | USA | SC, EMB | Retrospective study |
| Hennequin et al. [27] | 2012 | Clinical Oral Investigations | France | SC, EMB | Multicenter Prospective clinical study |
| Manford et al. [30] | 1980 | Anaesthesia | UK | CHR, SC, EMB | Randomized control trial |
| Picciani et al. [34] | 2019 | Journal of Clinical and Experimental Dentistry | Brazil | SC, EMB | Prospective clinical study |
| Pisalchaiyong et al. [37] | 2005 | Pediatric Dentistry | Thailand | PMD, SC | Prospective randomized, cross-over study |
| Ransford et al. [31] | 2010 | British Dental Journal | United Kingdom | SC, EMB | Multicenter Prospective clinical study |
| Silver et al. [32] | 1994 | Pediatric Dentistry | UK | CHR, SC, | Randomized control trial |
| Vaessen et al. [18] | 2017 | Special Care in Dentistry | The Netherlands | SC, EMB | Retrospective study |
| Author (Years) | N of Patients | Mean Age (Range) | Evaluation Scale | Administered Drug/Operator | Results of Primary Outcome | Results of Secondary Outcome | ||
|---|---|---|---|---|---|---|---|---|
| N of Sedations | Sex (M/F) | Dental Procedures | ||||||
| Galeotti et al. (2016) [36] | 472 | 6.6 | Modified Venham Scale, Vital signs | N2O/O2 (at different concentrations) by dentist | Sedation efficacy: | 75% | Behavior assessment: n.a. | |
| (60 *) | (4–17) | Side effects: | n.a. | Vital signs: n.a. | ||||
| Deep sedation: | n.a. | |||||||
| 472 | n.a. | Oral examination, oral hygiene, restorative treatment, oral surgery | ||||||
| (60 *) | ||||||||
| Collado et al. (2013) [26] | 142 | 30.5 | Venham Scale, Ramsay score for sedation assessment, Vital signs | iv midazolam (8.8 mg +/-4.9 mg) and N2O/O2 (50/50%) if necessary, premedication with Midazolam (os/ra 0.3 to 0.5 mg/kg), if necessary, by dentist | Sedation efficacy: | 89% | Behavior assessment (Venham score 0): | |
| (98 *) | (8–57) | Side effects: | 16% |
| ||||
| Deep sedation: | 3% |
| ||||||
| 320 | 113/74 |
| ||||||
| (187 *) | Vital signs: | |||||||
| ||||||||
| ||||||||
| Oral examination, radiographs, impressions, scaling, restorative treatment, prosthetic treatment, oral surgery |
| |||||||
| ||||||||
| ||||||||
| ||||||||
| Influence of repeated sedation: | ||||||||
| Venham score 0 decreased at venous cannulation (p = 0.01), and during dental treatment (p < 0.01) | ||||||||
| Level of sedation: | ||||||||
| During treatment Ramsey score = 1.96 (±0.72) | ||||||||
| Hennequin et al. (2012) [27] | 549 | 22.8 | Venham Scale, VAS Scale | N2O/O2 (50/50%) by dentist | Sedation efficacy: | 87% | Behavior assessment: n.a. | |
| (n.a.*) | (1–80) | Side effects: | n.a. | Patient/dentist satisfaction: n.a. | ||||
| Oral examination, scaling, restorative treatment, oral surgery | Deep sedation: | 0% | ||||||
| 638 | 308/241 | |||||||
| (71*) | ||||||||
| Collado et al. (2008) [28] | 662 | n.a. | Venham Scale | N2O/O2 (50/50%) by dentist | Sedation efficacy: | 90% | Behavior assessment: | |
| (325 *) | (>5) | Side effects: | 28% | Cooperation increased from application of the mask to perioperative steps (p < 0.01) | ||||
| Oral examination, radiograph, oral hygiene, restorative treatment, oral surgery | Deep sedation: | 0% | ||||||
| 826 | n.a. | Role of operator on success: | ||||||
| (469 *) | Not-expert vs experts (failures 13% vs 9% (p < 0.01) | |||||||
| Faulks et al. (2007) [29] | 349 | 22 | Venham Scale | N2O/O2 (50/50%) by dentist | Sedation efficacy: | 91% | Behavior assessment: | |
| (3–81) | Side effects: | Venham scores: decrease from mask application to treatment performance (p < 0.01), and during local anesthesia (p < 0.01). Autistic patients showed poorer cooperation compared to other IDs (p < 0.01) | ||||||
| 605 | 192/157 | n.a. | 10%; nausea/vomiting > in longer sedation (p < 0.01) | |||||
| Deep sedation: | 0% | |||||||
| Pisalchaiyong et al. (2005) [37] | 13 | 8.7 | Rating scale for sleep, body movement and crying behavior | N2O/O2 (50/50%) plus diazepam (0.3 mg/kg) or N2O/O2 (50/50%) plus midazolam (0.5 mg/kg) by dentist and anesthesiologist | Sedation efficacy: | Behavior assessment: | ||
| (5–15) |
| 77% who received diazepam and 100% who received midazolam rated as “good” and “very good” | ||||||
| ||||||||
| 26 | 10/3 | |||||||
| Side effects: n.a. | ||||||||
| Preventive procedures, scaling, restorative treatment, prosthetics treatment, oral surgery | Deep sedation | |||||||
| ||||||||
| ||||||||
| Haney et al. (1993) [17] | 143 | 6.4 | n.a. | Meperidine (1mg/lb) plus promethazine (0,5mg/lb) plus N2O/O2 (≤ 50/50%) by dentist | Sedation efficacy: | 68% | Role of operator on success: n.a. | |
| (* n.a.) | (2–18) | Side effects: | 3% | Level of sedation: | ||||
| ||||||||
| ||||||||
| 282 | n.a. |
| ||||||
| (120 *) | n.a. | |||||||
| Manford et al. (1980) [30] | 40 | n.a. | Customized behavioral scale | N2O/O2 or N2O/O2 plus iv diazepam (0.2 mg/kg) by dentist and anesthesiologist | Sedation efficacy: | Behavior assessment: n.a. (reported in graphs) | ||
| (5–22) |
| Treatment acceptance: n.a. | ||||||
| ||||||||
| 40 | n.a. | |||||||
| n.a. | Deep sedation: | |||||||
| ||||||||
| ||||||||
| Author (Years) | N of Patients | Mean Age (Range) | Evaluation Scale | Administered Drug/Operator | Results of Primary Outcome | Results of Secondary Outcome | ||
|---|---|---|---|---|---|---|---|---|
| N of Sedations | Sex (M/F) | Dental Procedures | ||||||
| Picciani et al. (2019) [34] | 40 | 18 | Vital signs | Midazolam (OS 0.5 mg/kg) by dentist and anesthesiologist | Sedation efficacy: | 82% | Vital signs: | |
| (6–73) |
| |||||||
| ||||||||
| 40 | 28/12 | |||||||
| Preventive procedure, impression, restorative treatment, oral surgery | ||||||||
| Vaessen et al. (2017) [18] | 124 | 52 | OAA/S, vital signs | Propofol (1%, TPC 1.5 μg ml) by dentist and anesthesiologist | Sedation efficacy: | 100% | Level of sedation: | |
| (18–75) | Side effects: | 37% | OAA/S: 4.1 | |||||
| Deep sedation: | 27% | Vital signs: n.a. | ||||||
| 124 | n.a. | |||||||
| Oral examination, radiograph, scaling, restorative treatment, oral surgery | ||||||||
| Capp et al. (2010) [33] | 40 | n.a. | Customized behavioral scale (A = allowed treatment, B = reacted to stimuli but allowed treatment, C = not allowed treatment) | Midazolam (im 0.2–0.3 mg/kg or iv 0.1 mg/kg) by dentist and anesthesiologist | Sedation efficacy: | 81% | Behavior assessment: | |
| (21 *) | (2–54) | Deep sedation: | 19% |
| ||||
| ||||||||
| 40 | n.a. |
| ||||||
| (21*) | Restorative treatment, oral surgery | |||||||
| Ransford et al. (2010) [31] | 289 | n.a. | Dental sedation teachers group scale for behavior and level of sedation; acceptability of treatment, vital signs | Midazolam (MAD or iv) by dentist and anesthesiologist | Sedation efficacy: | 76% | Treatment acceptance: | |
| (>18) | Side effects: | 6% |
| |||||
| Deep sedation: | 17%. |
| ||||||
| 316 | n.a. | n.a. |
| |||||
| Behavior assessment: | ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| Level of sedation: | ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| ||||||||
| Vital signs: n.a. | ||||||||
| Silver et al. (1994) [32] | 31 | 9 | Customized behavior scale (modified Frankl Scale deleting G4), vital signs | Midazolam (os 0.3 versus 0.5 mg/kg) by dentist and anesthesiologist | Sedation efficacy: | Behavior assessment: | ||
| (3–18) |
| T1 score 3 | T2 score 3 | |||||
|
|
| ||||||
| 31 | 16/15 | Side effects: |
|
| ||||
| n.a. |
| Vital signs: n.a. (reported in graphs) | ||||||
| ||||||||
| Deep sedation: | 0% | |||||||
| Diner et al. (1988) [35] | 42 | n.a. | Customized behavioral scale (evaluation of movements of head, arms, trunk, legs), vital signs | Diazepam (ra 1.5 mg/kg for the first 20 kg of weight + 1 mg/kg for additional kg) by dentist, hygienist and anesthesiologist | Sedation efficacy: | 80% | Behavior assessment: | |
| (4–31) | Deep sedation: | 0% | Improved = 80% | |||||
| Unchanged = 15% | ||||||||
| 20 | n.a. | Worsened = 5% | ||||||
| Vital signs: n.a. | ||||||||
| Preventive procedure, scaling | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salerno, C.; Cirio, S.; Zambon, G.; D’Avola, V.; Parcianello, R.G.; Maspero, C.; Campus, G.; Cagetti, M.G. Conscious Sedation for Dental Treatments in Subjects with Intellectual Disability: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 1779. https://doi.org/10.3390/ijerph20031779
Salerno C, Cirio S, Zambon G, D’Avola V, Parcianello RG, Maspero C, Campus G, Cagetti MG. Conscious Sedation for Dental Treatments in Subjects with Intellectual Disability: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2023; 20(3):1779. https://doi.org/10.3390/ijerph20031779
Chicago/Turabian StyleSalerno, Claudia, Silvia Cirio, Giulia Zambon, Valeria D’Avola, Roberta Gaia Parcianello, Cinzia Maspero, Guglielmo Campus, and Maria Grazia Cagetti. 2023. "Conscious Sedation for Dental Treatments in Subjects with Intellectual Disability: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 20, no. 3: 1779. https://doi.org/10.3390/ijerph20031779
APA StyleSalerno, C., Cirio, S., Zambon, G., D’Avola, V., Parcianello, R. G., Maspero, C., Campus, G., & Cagetti, M. G. (2023). Conscious Sedation for Dental Treatments in Subjects with Intellectual Disability: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 20(3), 1779. https://doi.org/10.3390/ijerph20031779










