Enhanced Bioremediation of Aged Polycyclic Aromatic Hydrocarbons in Soil Using Immobilized Microbial Consortia Combined with Strengthening Remediation Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Samples and PAHs Degrading Strains
2.2. Construction and Optimization of PAHs Degrading Bacterial Consortia
2.3. Immobilization of the Bacterial Consortium H6 Onto Biochar and SEM Observation
2.4. Bioremediation Experiment
2.5. Analysis of PAHs Concentrations
2.6. Analysis of Soil Enzyme Activities
2.7. Statistical Analysis
3. Results and Discussion
3.1. PAH Degrading Strains and Antagonistic Effect
3.2. Degradation Efficiency of PAHs Mixtures by single Strain and Bacterial Consortia in Liquid Medium
3.3. Bioremediation of PAHs in Contaminated Soil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lomza, P.; Poszytek, K.; Sklodowska, A.; Drewniak, L. Evaluation of bioremediation of soil highly contaminated by petroleum hydrocarbons. New Biotechnol. 2016, 33, S141. [Google Scholar] [CrossRef]
- Gou, Y.; Zhao, Q.; Yang, S.; Qiao, P.; Cheng, Y.; Song, Y.; Sun, Z.; Zhang, T.; Wang, L.; Liu, Z. Enhanced degradation of polycyclic aromatic hydrocarbons in aged subsurface soil using integrated persulfate oxidation and anoxic biodegradation. Chem. Eng. J. 2020, 394, 125040. [Google Scholar] [CrossRef]
- Chakrabarti, C.; Khimani, M.; Patel, V.; Parekh, P.; Pillai, S.; Mata, J.; Vekariya, R.L.; Bhadja, P.; Muddassir, M. Solubilization of polycyclic aromatic hydrocarbons (PAHs) in PEO-PPO-PEO type linear and star block copolymers. J. Mol. Liq. 2021, 325, 115177. [Google Scholar] [CrossRef]
- Medaura, M.C.; Guivernau, M.; Moreno-Ventas, X.; Prenafeta-Boldú, F.X.; Viñas, M. Bioaugmentation of Native Fungi, an Efficient Strategy for the Bioremediation of an Aged Industrially Polluted Soil With Heavy Hydrocarbons. Front. Microbiol. 2021, 12, 626436. [Google Scholar] [CrossRef]
- Barnier, C.; Ouvrard, S.; Robin, C.; Morel, J.L. Desorption kinetics of PAHs from aged industrial soils for availability assessment. Sci. Total. Environ. 2014, 470–471, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Kuppusam, S.; Thavamani, P.; Venkateswarl, K.; Le, Y.B.; Naidu, R.; Megharaj, M. Remediation approaches for polycyclic aromatic hydrocarbons (PAHs) contaminated soils: Technological constraints, emerging trends and future directions. Chemosphere 2016, 168, 944–968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, G.; Liao, X. Negative role of biochars in the dissipation and vegetable uptake of polycyclic aromatic hydrocarbons (PAHs) in an agricultural soil: Cautions for application of biochars to remediate PAHs-contaminated soil. Ecotoxicol. Environ. Saf. 2021, 213, 112075. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zeng, G.; Tang, L.; Wang, J.; Wan, J.; Liu, Y.; Yu, J.; Yi, H.; Ye, S.; Deng, R. Sorption, transport and biodegradation—An insight into bioavailability of persistent organic pollutants in soil. Sci. Total. Environ. 2018, 610–611, 1154–1163. [Google Scholar] [CrossRef]
- Bezza, F.A.; Chirwa, E.M.N. The role of lipopeptide biosurfactant on microbial remediation of aged polycyclic aromatic hydrocarbons (PAHs)-contaminated soil. Chem. Eng. J. 2017, 309, 563–576. [Google Scholar] [CrossRef]
- Guo, G.; Tian, F.; Ding, K.; Wang, L.; Liu, T.; Yang, F. Effect of a bacterial consortium on the degradation of polycyclic aromatic hydrocarbons and bacterial community composition in Chinese soils. Int. Biodeterior. Biodegrad. 2017, 123, 56–62. [Google Scholar] [CrossRef]
- Xiong, B.; Zhang, Y.; Hou, Y.; Arp, H.P.H.; Reid, B.J.; Cai, C. Enhanced biodegradation of PAHs in historically contaminated soil by M. gilvum inoculated biochar. Chemosphere 2017, 182, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Janbandhu, A.; Fulekar, M. Biodegradation of phenanthrene using adapted microbial consortium isolated from petrochemical contaminated environment. J. Hazard. Mater. 2011, 187, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, C.; Wang, H.; Chen, W.; Huang, Q. Characterization of a phenanthrene-degrading microbial consortium enriched from petrochemical contaminated environment. Int. Biodeterior. Biodegrad. 2016, 115, 286–292. [Google Scholar] [CrossRef]
- Li, M.; Yin, H.; Zhu, M.; Yu, Y.; Lu, G.; Dang, Z. Co-metabolic and biochar-promoted biodegradation of mixed PAHs by highly efficient microbial consortium QY1. J. Environ. Sci. 2021, 107, 65–76. [Google Scholar] [CrossRef]
- Laothamteep, N.; Kawano, H.; Vejarano, F.; Suzuki-Minakuchi, C.; Shintani, M.; Nojiri, H.; Pinyakong, O. Effects of environmental factors and coexisting substrates on PAH degradation and transcriptomic responses of the defined bacterial consortium OPK. Environ. Pollut. 2021, 277, 116769. [Google Scholar] [CrossRef]
- Wanapaisan, P.; Laothamteep, N.; Vejarano, F.; Chakraborty, J.; Shintani, M.; Muangchinda, C.; Morita, T.; Suzuki-Minakuchi, C.; Inoue, K.; Nojiri, H.; et al. Synergistic degradation of pyrene by five culturable bacteria in a mangrove sediment-derived bacterial consortium. J. Hazard. Mater. 2018, 342, 561–570. [Google Scholar] [CrossRef]
- Zafra, G.; Absalón, E.; Anducho-Reyes, M.; Fernandez, F.J.; Cortés-Espinosa, D.V. Construction of PAH-degrading mixed microbial consortia by induced selection in soil. Chemosphere 2017, 172, 120–126. [Google Scholar] [CrossRef]
- Liang, X.; Guo, C.; Liu, S.; Dang, Z.; Wei, Y.; Yi, X.; Abel, S. Cosolubilization of phenanthrene and pyrene in surfactant micelles: Experimental and atomistic simulations studies. J. Mol. Liq. 2018, 263, 1–9. [Google Scholar] [CrossRef]
- Liu, S.; Guo, C.; Dang, Z.; Liang, X. Comparative proteomics reveal the mechanism of Tween80 enhanced phenanthrene biodegradation by Sphingomonas sp. GY2B. Ecotoxicol. Environ. Saf. 2017, 137, 256–264. [Google Scholar] [CrossRef]
- Bezza, F.A.; Chirwa, E.M.N. Biosurfactant-enhanced bioremediation of aged polycyclic aromatic hydrocarbons (PAHs) in creosote contaminated soil. Chemosphere 2016, 144, 635–644. [Google Scholar] [CrossRef]
- Chong, Z.-Y.; Liao, X.-Y.; Yan, X.-L.; Sun, L.; Zhao, D.; Liang, T. Enhanced Desorption of PAHs from Manufactured Gas Plant Soils Using Different Types of Surfactants. Pedosphere 2014, 24, 209–219. [Google Scholar] [CrossRef]
- Kebede, G.; Tafese, T.; Abda, E.M.; Kamaraj, M.; Assefa, F. Factors Influencing the Bacterial Bioremediation of Hydrocarbon Contaminants in the Soil: Mechanisms and Impacts. J. Chem. 2021, 2021, 9823362. [Google Scholar] [CrossRef]
- Kronenberg, M.; Trably, E.; Bernet, N.; Patureau, D. Biodegradation of polycyclic aromatic hydrocarbons: Using microbial bioelectrochemical systems to overcome an impasse. Environ. Pollut. 2017, 231, 509–523. [Google Scholar] [CrossRef]
- Dellagnezze, B.; Vasconcellos, S.; Angelim, A.; Melo, V.; Santisi, S.; Cappello, S.; Oliveira, V. Bioaugmentation strategy employing a microbial consortium immobilized in chitosan beads for oil degradation in mesocosm scale. Mar. Pollut. Bull. 2016, 107, 107–117. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, J.; Zhang, H.; Zheng, C.; Wei, R.; Gao, Y.; Yang, L. Efficient nitrate removal by Pseudomonas mendocina GL6 immobilized on biochar. Bioresour. Technol. 2021, 320, 124324. [Google Scholar] [CrossRef]
- Huang, R.-Y.; Tian, W.-J.; Liu, Q.; Yu, H.-B.; Jin, X.; Zhao, Y.-G.; Zhou, Y.-H.; Feng, G. Enhanced biodegradation of pyrene and indeno(1,2,3-cd)pyrene using bacteria immobilized in cinder beads in estuarine wetlands. Mar. Pollut. Bull. 2016, 102, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-T.; Lee, J.-F.; Liu, K.-H.; Liao, Y.-F.; Yang, V. Immobilization of fungal laccase onto a nonionic surfactant-modified clay material: Application to PAH degradation. Environ. Sci. Pollut. Res. 2016, 23, 4024–4035. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.; Luth, M.; Kenney, R.; Crowley, D. Evaluation of pinewood biochar as a carrier of bacterial strain Enterobacter cloacae UW5 for soil inoculation. Appl. Soil Ecol. 2014, 84, 192–199. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total. Environ. 2021, 763, 144204. [Google Scholar] [CrossRef] [PubMed]
- Ping, L.; Zhang, C.; Zhu, Y.; Wu, M.; Hu, X.; Li, Z.; Zhao, H. Biodegrading of pyrene by a newly isolated Pseudomonas putida PL2. Biotechnol. Bioprocess Eng. 2011, 16, 1000–1008. [Google Scholar] [CrossRef]
- Fuentes, M.S.; Saez, J.M.; Benimeli, C.S.; Amoroso, M.J. Lindane Biodegradation by Defined Consortium of Indigenous Streptomyces Strains. Water Air Soil Poll. 2011, 222, 217–231. [Google Scholar] [CrossRef]
- Sun, S.; Wang, H.; Yan, K.; Lou, J.; Ding, J.; Snyder, S.A.; Wu, L.; Xu, J. Metabolic interactions in a bacterial co-culture accelerate phenanthrene degradation. J. Hazard. Mater. 2021, 403, 123825. [Google Scholar] [CrossRef] [PubMed]
- Garbeva, P.; Silby, M.W.; Raaijmakers, J.M.; Levy, S.B.; de Boer, W. Transcriptional and antagonistic responses of Pseudomonas fluorescens Pf0-1 to phylogenetically different bacterial competi- tors. ISME J. 2011, 5, 973–985. [Google Scholar] [CrossRef]
- Ren, D.; Madsen, J.S.; Sørensen, S.J.; Burmølle, M. High prevalence of biofilm synergy among bacterial soil isolates in cocultures indicates bacterial interspecific cooperation. ISME J. 2015, 9, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Werner, D.; Luthy, R.G. Modeling PAH mass transfer in a slurry of contaminated soil or sediment amended with organic sorbents. Water Res. 2008, 42, 2931–2942. [Google Scholar] [CrossRef]
- Li, K.; Landriault, M.; Fingas, M.; Llompart, M. Accelerated solvent extraction (ASE) of environmental organic compounds in soils using a modified supercritical fluid extractor. J. Hazard. Mater. 2003, 102, 93–104. [Google Scholar] [CrossRef]
- Andreoni, V.; Cavalca, L.; Rao, M.A.; Nocerino, G.; Bernasconi, S.; Dell’Amico, E.; Colombo, M.; Gianfreda, L. Bacterial communities and enzyme activities of PAHs polluted soils. Chemosphere 2004, 57, 401–412. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, R.; Lin, X.; Liu, W.; Chen, R.; Li, X. Interactive Effect of Biosurfactant and Microorganism to Enhance Phytoremediation for Removal of Aged Polycyclic Aromatic Hydrocarbons from Contaminated Soils. J. Health Sci. 2010, 56, 257–266. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Z.; Sun, S.; Wang, Q.; Zhong, W. Enhanced degradation of bioremediation residues in petroleum-contaminated soil using a two-liquid-phase bioslurry reactor. Chemosphere 2009, 77, 161–168. [Google Scholar] [CrossRef]
- Perucci, P.; Casucci, C.; Dumontet, S. An improved method to evaluate the o-diphenol oxidase activity of soil. Soil Biol. Biochem. 2000, 32, 1927–1933. [Google Scholar] [CrossRef]
- Wang, L.; Li, F.; Zhan, Y.; Zhu, L. Shifts in microbial community structure during in situ surfactant-enhanced bioremediation of polycyclic aromatic hydrocarbon-contaminated soil. Environ. Sci. Pollut. Res. 2016, 23, 14451–14461. [Google Scholar] [CrossRef]
- Gong, Z.; Li, P.; Wang, X.; Zhang, H.; Song, Y.; Li, B. Co-metabolic degradation of pyrene in soil. Yingyong Shengtai Xuebao 2001, 12, 447–450. [Google Scholar] [PubMed]
- Gao, C.-H.; Cao, H.; Cai, P.; Sørensen, S.J. The initial inoculation ratio regulates bacterial coculture interactions and metabolic capacity. ISME J. 2021, 15, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Kurt, Z.; Tsementzi, D.; Weigand, M.R.; Kim, M.; Janet Hatt, K.; Tndukar, M.; Pavlostathis, S.G.; Spain, J.C. Microbial community degradation of widely used quaternary ammonium disinfectants. Appl. Environ. Microbiol. 2014, 80, 5892–5900. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, H.; Qi, M.; Wang, X.; Adebanjo, O.O.; Lu, Z. Metabolite Cross-Feeding between Rhodococcus ruber YYL and Bacillus cereus MLY1 in the Biodegradation of Tetrahydrofuran under pH Stress. Appl. Environ. Microbiol. 2019, 85, e01196-19. [Google Scholar] [CrossRef] [PubMed]
- Macchi, M.; Festa, S.; Vega-Vela, N.E.; Morelli, I.S.; Coppotelli, B.M. Assessing interactions, predicting function, and increasing degradation potential of a PAH-degrading bacterial consortium by effect of an inoculant strain. Environ. Sci. Pollut. Res. 2019, 26, 25932–25944. [Google Scholar] [CrossRef]
- Kumari, S.; Regar, R.K.; Manickam, N. Improved polycyclic aromatic hydrocarbon degradation in a crude oil by individual and a consortium of bacteria. Bioresour. Technol. 2018, 254, 174–179. [Google Scholar] [CrossRef]
- Isaac, P.; Martínez, F.L.; Bourguignon, N.; Sánchez, L.A.; Ferrero, M.A. Improved PAHs removal performance by a defined bacterial consortium of indigenous Pseudomonas and actinobacteria from Patagonia, Argentina. Int. Biodeterior. Biodegrad. 2015, 101, 23–31. [Google Scholar] [CrossRef]
- Ren, W.; Ren, G.; Teng, Y.; Li, Z.; Li, L. Time-dependent effect of graphene on the structure, abundance, and function of the soil bacterial community. J. Hazard. Mater. 2015, 297, 286–294. [Google Scholar] [CrossRef]
- Sun, G.-D.; Xu, Y.; Jin, J.-H.; Zhong, Z.-P.; Liu, Y.; Luo, M.; Liu, Z.-P. Pilot scale ex-situ bioremediation of heavily PAHs-contaminated soil by indigenous microorganisms and bioaugmentation by a PAHs-degrading and bioemulsifier-producing strain. J. Hazard. Mater. 2012, 233–234, 72–78. [Google Scholar] [CrossRef]
- Lang, F.S.; Destain, J.; Delvigne, F.; Druart, P.; Ongena, M.; Thonart, P. Biodegradation of Polycyclic Aromatic Hydrocarbons in Mangrove Sediments Under Different Strategies: Natural Attenuation, Biostimulation, and Bioaugmentation with Rhodococcus erythropolis T902.1. Water Air Soil Pollut. 2016, 227, 297. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, Y.; Wu, X.; Xu, X.; Kong, S.; Tong, L.; Jiang, Z.; Li, B. Biodegradation of PAHs by Acinetobacter isolated from karst groundwater in a coal-mining area. Environ. Earth Sci. 2015, 73, 7479–7488. [Google Scholar] [CrossRef]
- Yang, X.P.; Wang, S.M.; Zhang, D.W.; Zhou, L.X. Isolation and nitrogen degradation characteristics of an aerobic heterotrophic nitrifying-denitrifying bacterium, Bacillus subtilis A1. Bioresour. Technol. 2011, 102, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhu, L.; Li, F. Influences and mechanisms of surfactants on pyrene biodegradation based on interactions of surfactant with a Klebsiella oxytoca strain. Bioresour. Technol. 2013, 142, 454–461. [Google Scholar] [CrossRef]
- Sarma, S.J.; Pakshirajan, K. Surfactant aided biodegradation of pyrene using immobilized cells of Mycobacterium frederiksbergense. Int. Biodeterior. Biodegrad. 2011, 65, 73–77. [Google Scholar] [CrossRef]
- Chen, B.; Yuan, M. Enhanced sorption of polycyclic aromatic hydrocarbons by soil amended with biochar. J. Soils Sediments 2011, 11, 62–71. [Google Scholar] [CrossRef]
- Lu, R.K. Soil Argrochemistry Analysis Protocals; China Agriculture Science Press: Beijing, China, 1999. [Google Scholar]
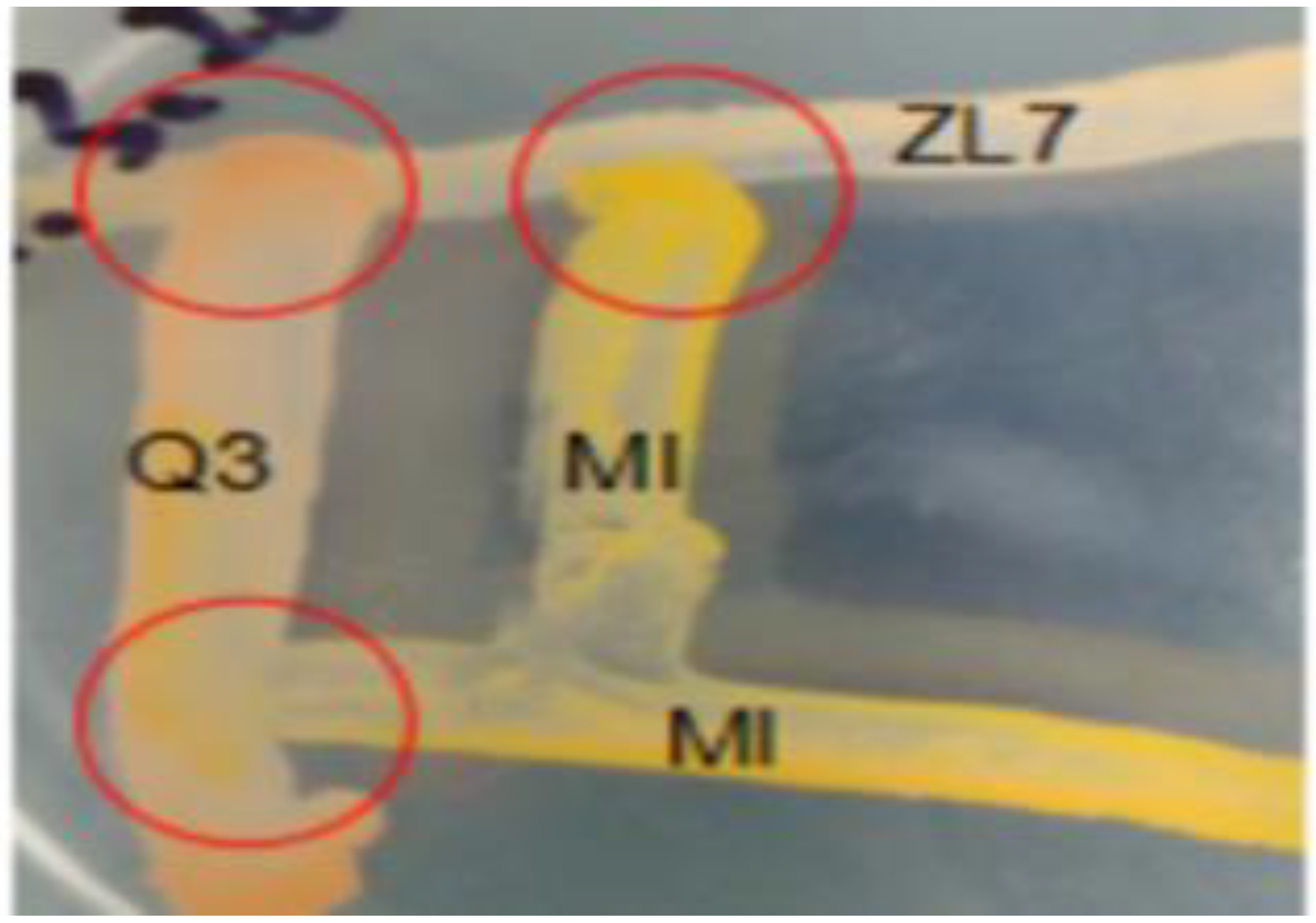


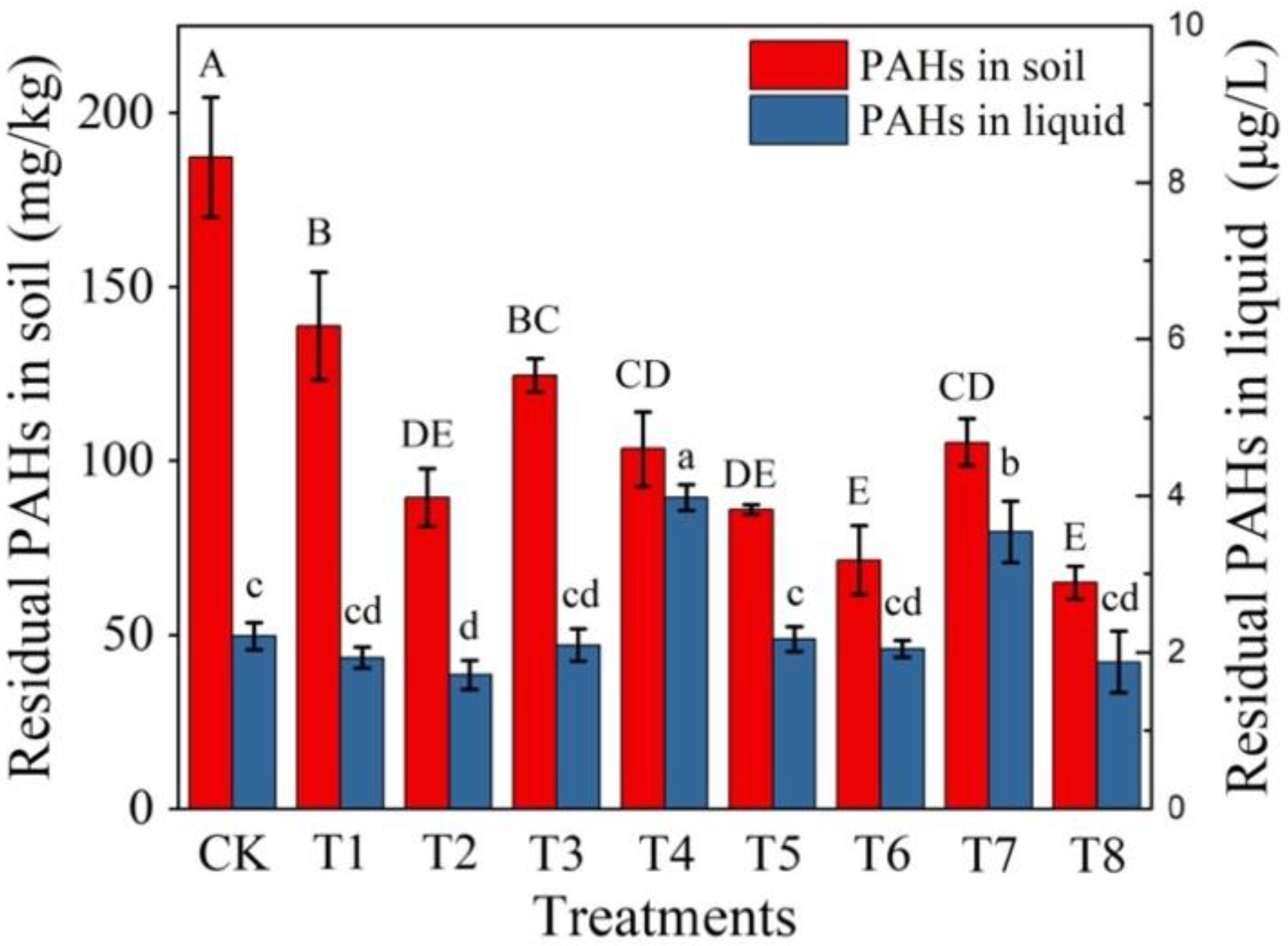
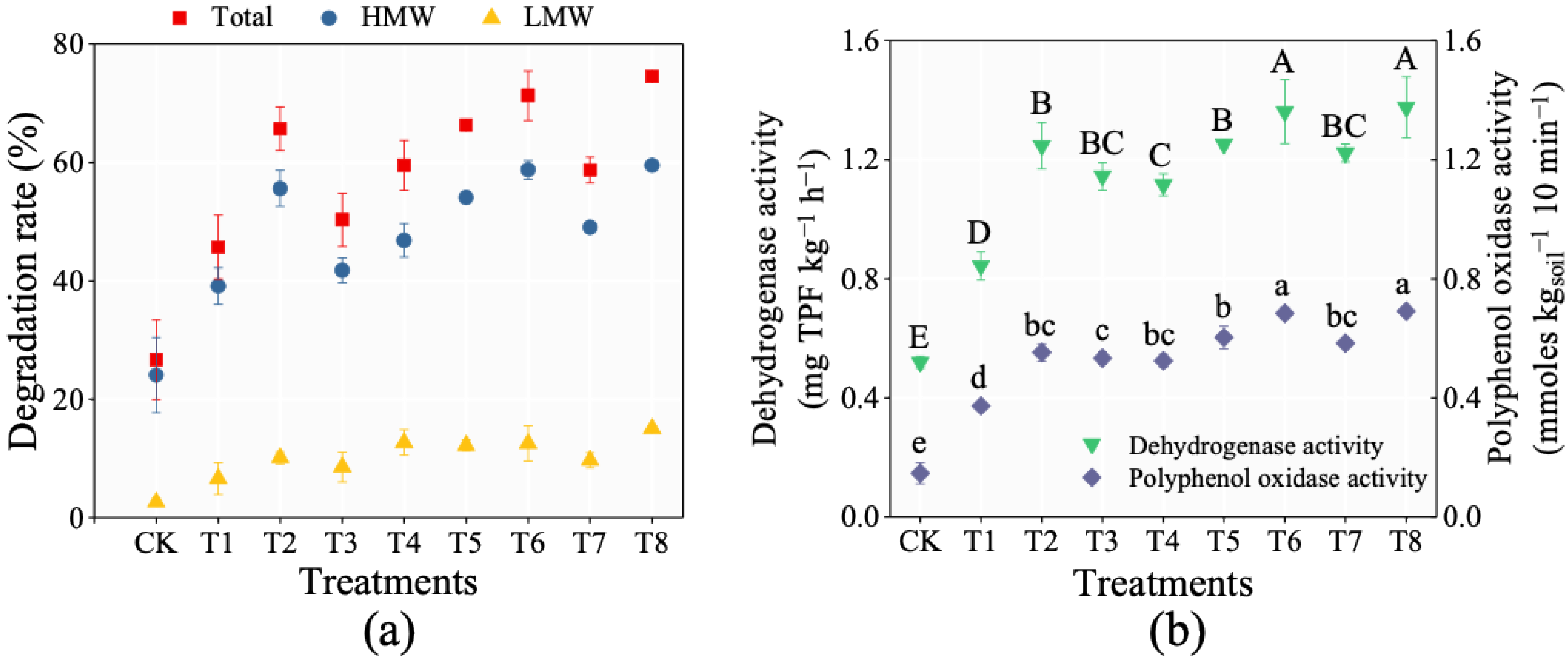
| Treatment Groups | Experimental Design |
|---|---|
| CK | Coke plant (CP) soil |
| T1 | Bacterial consortium H6 |
| T2 | Immobilized bacterial consortium H6 on biochar |
| T3 | Glucose + bacterial consortium H6 |
| T4 | SDBS + bacterial consortium H6 |
| T5 | Glucose + immobilized bacterial consortium H6 on biochar |
| T6 | SDBS + immobilized bacterial consortium H6 on biochar |
| T7 | SDBS + glucose + bacterial consortium H6 |
| T8 | SDBS + glucose + immobilized bacterial consortium H6 on biochar |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Gao, X.; Wang, S.; Zhang, Y.; Coulon, F.; Cai, C. Enhanced Bioremediation of Aged Polycyclic Aromatic Hydrocarbons in Soil Using Immobilized Microbial Consortia Combined with Strengthening Remediation Strategies. Int. J. Environ. Res. Public Health 2023, 20, 1766. https://doi.org/10.3390/ijerph20031766
Zhou H, Gao X, Wang S, Zhang Y, Coulon F, Cai C. Enhanced Bioremediation of Aged Polycyclic Aromatic Hydrocarbons in Soil Using Immobilized Microbial Consortia Combined with Strengthening Remediation Strategies. International Journal of Environmental Research and Public Health. 2023; 20(3):1766. https://doi.org/10.3390/ijerph20031766
Chicago/Turabian StyleZhou, Haixuan, Xiurong Gao, Suhang Wang, Youchi Zhang, Frederic Coulon, and Chao Cai. 2023. "Enhanced Bioremediation of Aged Polycyclic Aromatic Hydrocarbons in Soil Using Immobilized Microbial Consortia Combined with Strengthening Remediation Strategies" International Journal of Environmental Research and Public Health 20, no. 3: 1766. https://doi.org/10.3390/ijerph20031766
APA StyleZhou, H., Gao, X., Wang, S., Zhang, Y., Coulon, F., & Cai, C. (2023). Enhanced Bioremediation of Aged Polycyclic Aromatic Hydrocarbons in Soil Using Immobilized Microbial Consortia Combined with Strengthening Remediation Strategies. International Journal of Environmental Research and Public Health, 20(3), 1766. https://doi.org/10.3390/ijerph20031766








