Remote Symptom Monitoring to Enhance the Delivery of Palliative Cancer Care in Low-Resource Settings: Emerging Approaches from Africa
Abstract
1. Introduction
2. Remote Symptom Monitoring for Palliative Cancer Care in Sub-Saharan Africa
3. Case Studies
3.1. Case Study 1: Patient-Reported Outcome Side Effect (PROSE) Platform in Nigeria
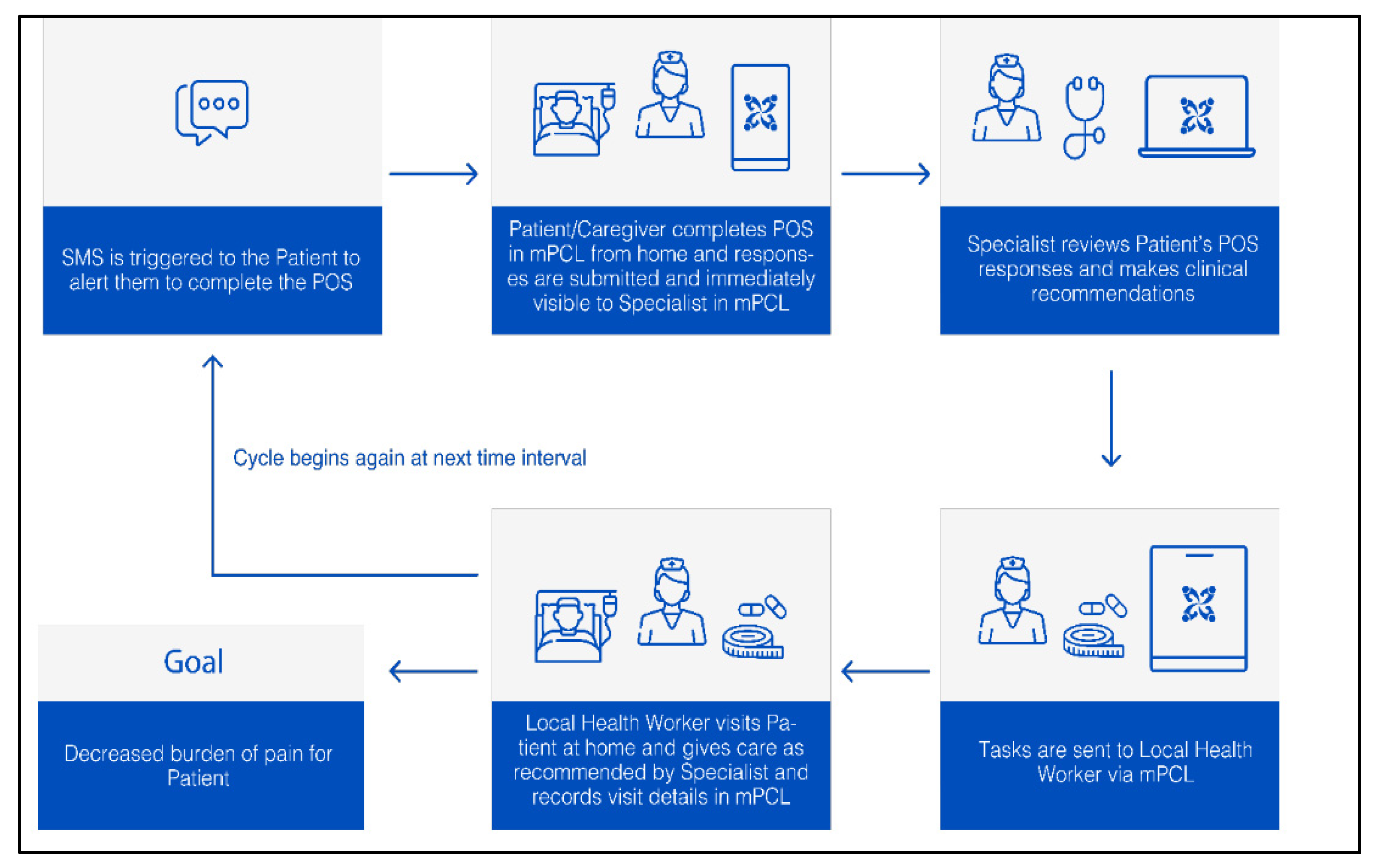
3.2. Case Study 2: m-Palliative Care Link for Palliative Care Coordination among Patients with Cancer in Tanzania
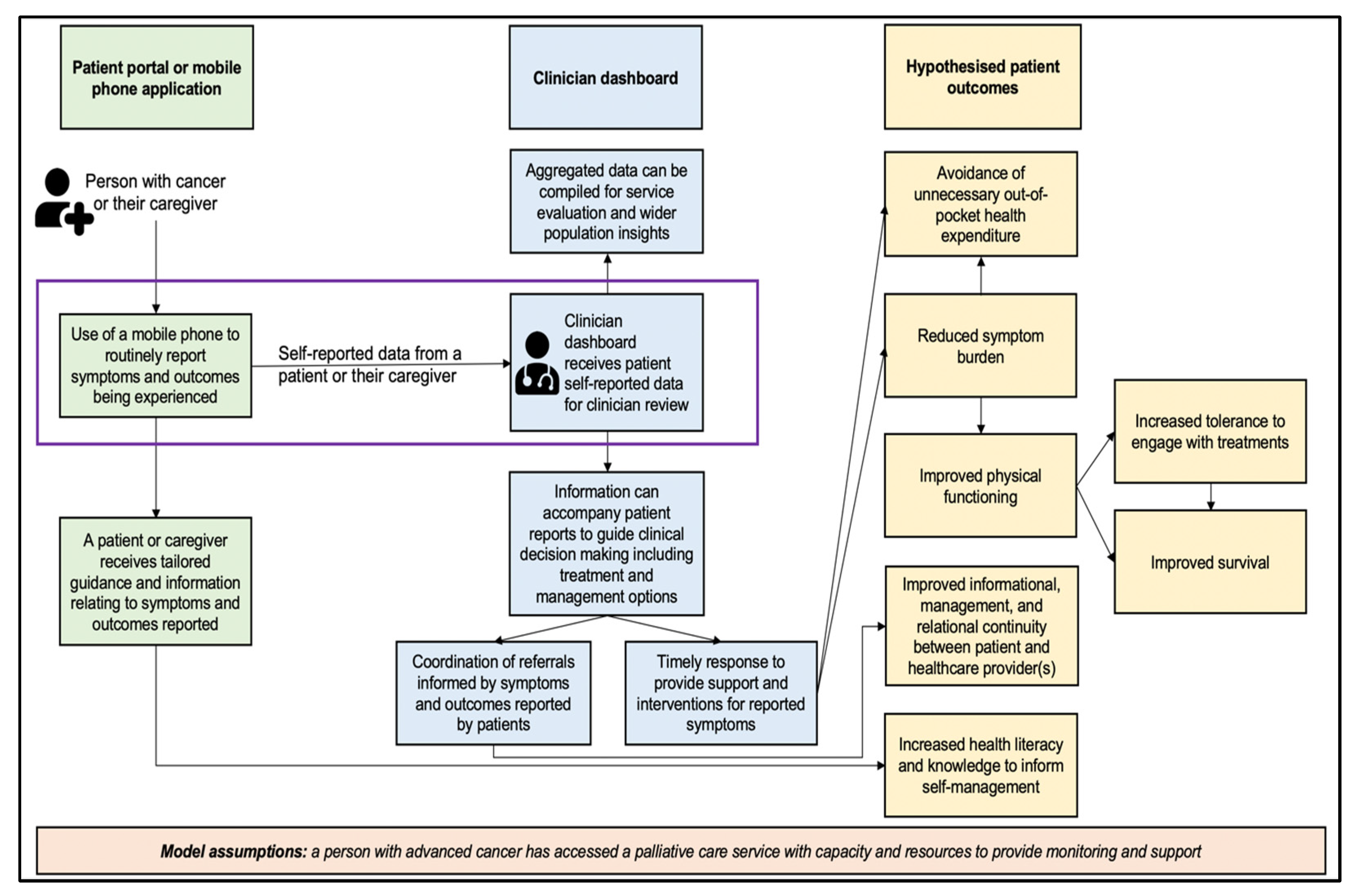
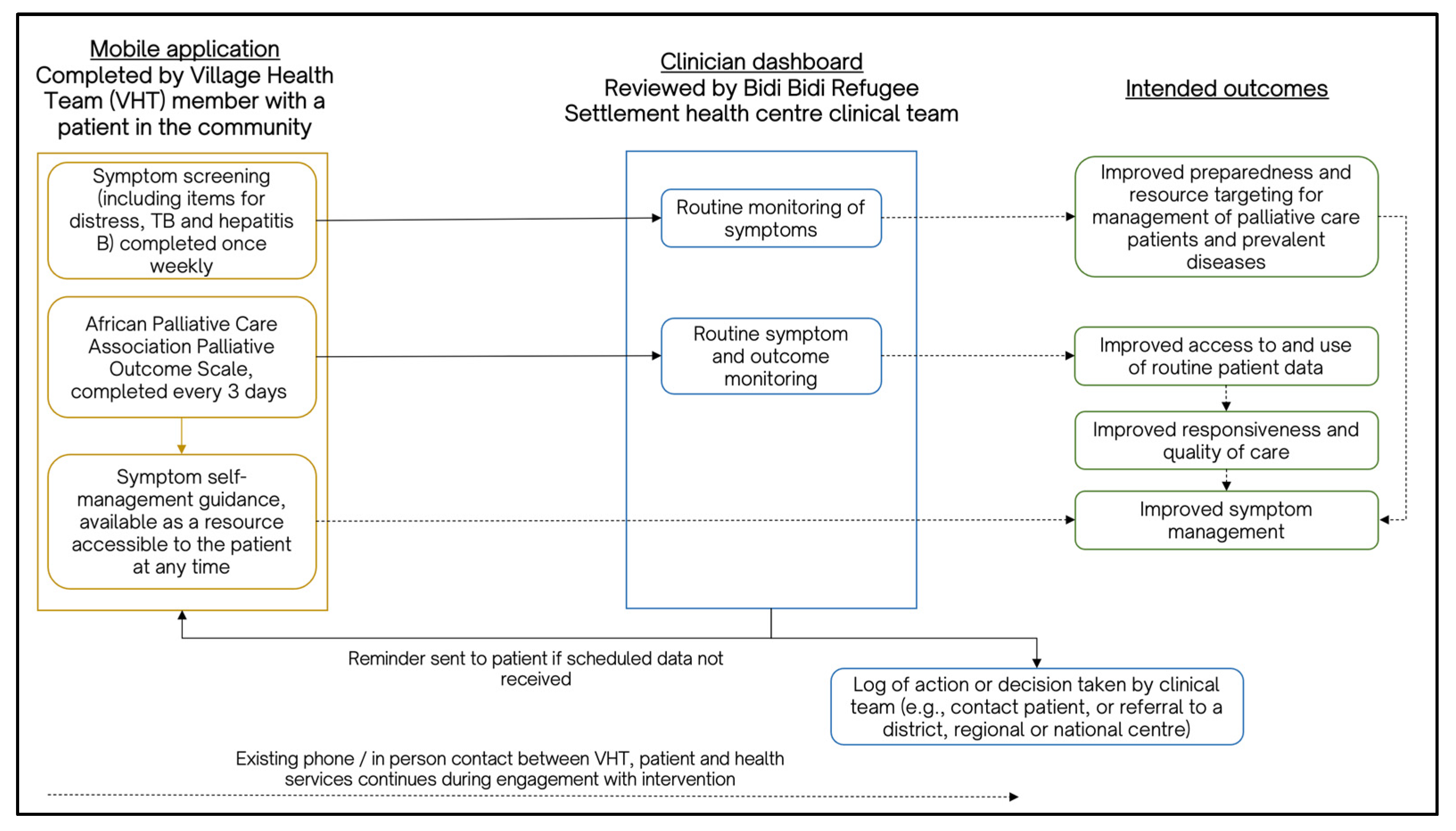
3.3. Case Study 3: African Palliative Care Association (APCA) Mobile Phone Application and Clinician Dashboard for Supporting Continuity of Care for People Living with Cancer in Refugee Settlements in Uganda
4. Developing the Evidence Base to Inform Future Research on Remote Symptom Monitoring for Cancer Care in SSA
4.1. Develop New Care Models and Accompanying Clinical Workflows
4.2. Determine the Economics of Digital Health Approaches for Palliative Cancer Care in SSA
4.3. Determine Capacity and Capability to Implement and Sustain Platforms to Support Remote Monitoring in Palliative Cancer Care
4.4. Consider the Potential of New Technologies and Their Relevance and Utility for Palliative Cancer Care in SSA
4.5. Explore the Unknown Feasibility of Emerging Experimental Methodologies for Digital Health in Oncology Care in SSA
4.6. Monitor Inequalities in Access to and Benefits from Remote Monitoring Approaches
4.7. Measure Health System Benefit(s) and Integration
4.8. Build and Foster Partnerships
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pramesh, C.S.; Badwe, R.A.; Bhoo-Pathy, N.; Booth, C.M.; Chinnaswamy, G.; Dare, A.J.; de Andrade, V.P.; Hunter, D.J.; Gopal, S.; Gospodarowicz, M.; et al. Priorities for cancer research in low- and middle-income countries: A global perspective. Nat. Med. 2022, 28, 649–657. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guideline: Recommendations on Digital Interventions for Health System Strengthening; WHO: Geneva, Switzerland, 2019.
- Prager, G.W.; Braga, S.; Bystricky, B.; Qvortrup, C.; Criscitiello, C.; Esin, E.; Sonke, G.S.; Argilés Martínez, G.; Frenel, J.S.; Karamouzis, M.; et al. Global cancer control: Responding to the growing burden, rising costs and inequalities in access. ESMO Open 2018, 3, e000285. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Kayamba, V.; Peek, R.M., Jr.; Heimburger, D. Cancer Control in Low- and Middle-Income Countries: Is It Time to Consider Screening? J. Glob. Oncol. 2019, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Agodirin, O.; Olatoke, S.; Rahman, G.; Olaogun, J.; Olasehinde, O.; Katung, A.; Kolawole, O.; Ayandipo, O.; Etonyeaku, A.; Habeeb, O.; et al. Presentation intervals and the impact of delay on breast cancer progression in a black African population. BMC Public Health 2020, 20, 962. [Google Scholar] [CrossRef] [PubMed]
- Wassie, M.; Fentie, B. Prevalence of late-stage presentation and associated factors of cervical cancer patients in Tikur Anbesa Specialized Hospital, Ethiopia: Institutional based cross-sectional study. Infect. Agents Cancer 2021, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Tekalign, T.; Teshome, M. Prevalence and determinants of late-stage presentation among cervical cancer patients, a systematic review and meta-analysis. PLoS ONE 2022, 17, e0267571. [Google Scholar] [CrossRef]
- Rogers, J.L.; Perry, L.M.; Hoerger, M. Summarizing the Evidence Base for Palliative Oncology Care: A Critical Evaluation of the Meta-analyses. Clin. Med. Insights Oncol. 2020, 14, 1179554920915722. [Google Scholar] [CrossRef]
- Worldwide Hospice Palliative Care Alliance. Global Atlas of Palliative Care; Worldwide Hospice Palliative Care Alliance: London, UK, 2020; Available online: https://cdn.who.int/media/docs/default-source/integrated-health-services-(ihs)/csy/palliative-care/whpca_global_atlas_p5_digital_final.pdf?sfvrsn=1b54423a_3 (accessed on 9 November 2023).
- Court, L.; Olivier, J. Approaches to integrating palliative care into African health systems: A qualitative systematic review. Health Policy Plan. 2020, 35, 1053–1069. [Google Scholar] [CrossRef]
- Namukwaya, E.; Deogratius Mwaka, A.; Namisango, E.; Mwesiga, M.D.; Downing, J. Current State of Palliative Care in Uganda. In Palliative Care for Chronic Cancer Patients in the Community: Global Approaches and Future Applications; Silbermann, M., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 267–278. [Google Scholar]
- Fraser, B.A.; Powell, R.A.; Mwangi-Powell, F.A.; Namisango, E.; Hannon, B.; Zimmermann, C.; Rodin, G. Palliative Care Development in Africa: Lessons from Uganda and Kenya. J. Glob. Oncol. 2017, 1–10. [Google Scholar] [CrossRef]
- Downing, J.; Grant, L.; Leng, M.; Namukwaya, E. Understanding Models of Palliative Care Delivery in Sub-Saharan Africa: Learning from Programs in Kenya and Malawi. J. Pain Symptom Manag. 2015, 50, 362–370. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brant, J.M.; Silbermann, M. Global Perspectives on Palliative Care for Cancer Patients: Not All Countries Are the Same. Curr. Oncol. Rep. 2021, 23, 60. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.A.; Kendall, M.; Mitchell, G.; Moine, S.; Amblàs-Novellas, J.; Boyd, K. Palliative care from diagnosis to death. BMJ 2017, 356, j878. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, W.; Addai, B.W.; Adewole, I.; Ainsworth, V.; Alaro, J.; Alatise, O.I.; Ali, Z.; Anderson, B.O.; Anorlu, R.; Avery, S.; et al. Cancer in sub-Saharan Africa: A Lancet Oncology Commission. Lancet Oncol. 2022, 23, e251–e312. [Google Scholar] [CrossRef] [PubMed]
- Merriman, A.; Mwebesa, E.; Zirimenya, L. Improving access to palliative care for patients with cancer in Africa: 25 years of Hospice Africa. Ecancermedicalscience 2019, 13, 946. [Google Scholar] [CrossRef] [PubMed]
- Mutebi, M.; Adewole, I.; Orem, J.; Abdella, K.; Coker, O.; Kolawole, I.; Komen, A.; Munema, A.; Ndlovu, N.; O’Brien, M.; et al. Toward Optimization of Cancer Care in Sub-Saharan Africa: Development of National Comprehensive Cancer Network Harmonized Guidelines for Sub-Saharan Africa. JCO Glob. Oncol. 2020, 6, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Holeman, I.; Evans, J.; Kane, D.; Grant, L.; Pagliari, C.; Weller, D. Mobile health for cancer in low to middle-income countries: Priorities for research and development. Eur. J. Cancer Care 2014, 23, 750–756. [Google Scholar] [CrossRef]
- Nkhoma, K.B.; Ebenso, B.; Akeju, D.; Adejoh, S.; Bennett, M.; Chirenje, M.; Dandadzi, A.; Nabirye, E.; Namukwaya, E.; Namisango, E.; et al. Stakeholder perspectives and requirements to guide the development of digital technology for palliative cancer services: A multi-country, cross-sectional, qualitative study in Nigeria, Uganda and Zimbabwe. BMC Palliat. Care 2021, 20, 4. [Google Scholar] [CrossRef]
- Nigeria Federal Ministry of Health. National Health ICT Strategic Framework 2015–2020; Federal Ministry of Health: Abuja, Nigeria, 2016. Available online: https://www.health.gov.ng/doc/HealthICTStrategicFramework.pdf (accessed on 9 November 2023).
- Ministry of Health. Uganda National eHealth Policy; Ministry of Health: Kampala, Uganda, 2016. Available online: https://health.go.ug/sites/default/files/National%20eHealth%20Policy%202016_1.pdf (accessed on 9 November 2023).
- GSMA. The Mobile Economy Sub-Saharan Africa 2020; GSM Association: London, UK, 2021; Available online: https://www.gsma.com/mobileeconomy/wp-content/uploads/2021/09/GSMA_ME_SSA_2021_English_Web_Singles.pdf (accessed on 9 November 2023).
- Yadav, K.; Ginsburg, O.; Basu, P.; Mehrotra, R. Telemedicine and Cancer Care in Low- and Middle-Income Countries during the SARS-CoV-2 Pandemic. JCO Glob. Oncol. 2021, 1633–1638. [Google Scholar] [CrossRef]
- Grewal, U.S.; Shankar, A.; Saini, D.; Seth, T.; Roy, S.; Aden, D.; Bhandari, D.; Singh, P. Tele-health and cancer care in the era of COVID-19: New opportunities in low and middle income countries (LMICs). Cancer Treat. Res. Commun. 2021, 27, 100313. [Google Scholar] [CrossRef]
- Wang, T.; Molassiotis, A.; Chung, B.P.M.; Tan, J.Y. Unmet care needs of advanced cancer patients and their informal caregivers: A systematic review. BMC Palliat. Care 2018, 17, 96. [Google Scholar] [CrossRef] [PubMed]
- Waller, A.; Girgis, A.; Johnson, C.; Lecathelinais, C.; Sibbritt, D.; Forstner, D.; Liauw, W.; Currow, D.C. Improving Outcomes for People with Progressive Cancer: Interrupted Time Series Trial of a Needs Assessment Intervention. J. Pain Symptom Manag. 2012, 43, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Powell, R.A.; Harding, R.; Namisango, E.; Katabira, E.; Gwyther, L.; Radbruch, L.; Murray, S.A.; El-Ansary, M.; Leng, M.; Ajayi, I.O.; et al. Palliative care research in Africa: Consensus building for a prioritized agenda. J. Pain Symptom Manag. 2014, 47, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Harding, R.; Selman, L.; Powell, R.A.; Namisango, E.; Downing, J.; Merriman, A.; Ali, Z.; Gikaara, N.; Gwyther, L.; Higginson, I. Research into palliative care in sub-Saharan Africa. Lancet Oncol. 2013, 14, e183–e188. [Google Scholar] [CrossRef] [PubMed]
- Hasson, F.; Nicholson, E.; Muldrew, D.; Bamidele, O.; Payne, S.; McIlfatrick, S. International palliative care research priorities: A systematic review. BMC Palliat. Care 2020, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Deal, A.M.; Kris, M.G.; Scher, H.I.; Hudis, C.A.; Sabbatini, P.; Rogak, L.; Bennett, A.V.; Dueck, A.C.; Atkinson, T.M.; et al. Symptom Monitoring with Patient-Reported Outcomes during Routine Cancer Treatment: A Randomized Controlled Trial. J. Clin. Oncol. 2016, 34, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Stover, A.M.; Schrag, D.; Chung, A.; Jansen, J.; Henson, S.; Carr, P.; Ginos, B.; Deal, A.; Spears, P.A.; et al. Clinical Utility and User Perceptions of a Digital System for Electronic Patient-Reported Symptom Monitoring during Routine Cancer Care: Findings from the PRO-TECT Trial. JCO Clin. Cancer Inf. 2020, 4, 947–957. [Google Scholar] [CrossRef]
- Howell, D.; Li, M.; Sutradhar, R.; Gu, S.; Iqbal, J.; O’Brien, M.A.; Seow, H.; Dudgeon, D.; Atzema, C.; Earle, C.C.; et al. Integration of patient-reported outcomes (PROs) for personalized symptom management in “real-world” oncology practices: A population-based cohort comparison study of impact on healthcare utilization. Support Care Cancer 2020, 28, 4933–4942. [Google Scholar] [CrossRef]
- Rocque, G.B.; Dionne-Odom, J.N.; Stover, A.M.; Daniel, C.L.; Azuero, A.; Huang, C.-H.S.; Ingram, S.A.; Franks, J.A.; Caston, N.E.; Dent, D.A.N.; et al. Evaluating the implementation and impact of navigator-supported remote symptom monitoring and management: A protocol for a hybrid type 2 clinical trial. BMC Health Serv. Res. 2022, 22, 538. [Google Scholar] [CrossRef]
- Agom, D.A.; Onyeka, T.C.; Iheanacho, P.N.; Ominyi, J. Barriers to the Provision and Utilization of Palliative Care in Africa: A Rapid Scoping Review. Indian J. Palliat. Care 2021, 27, 3–17. [Google Scholar] [CrossRef]
- Iragorri, N.; de Oliveira, C.; Fitzgerald, N.; Essue, B. The Out-of-Pocket Cost Burden of Cancer Care—A Systematic Literature Review. Curr. Oncol. 2021, 28, 1216–1248. [Google Scholar] [CrossRef] [PubMed]
- Farrant, L.; Harding, R.; Anderson, D.; Greeff, L.; Kassanjee, R.; Krause, R.; Mohamed, Z.; Parkes, J.; Gwyther, L. Symptom prevalence and burden, and the risk of depression among patients with advanced cancer attending two South African oncology units. Ecancermedicalscience 2022, 16, 1349. [Google Scholar] [CrossRef] [PubMed]
- Namukwaya, E.; Nabirye, E.; Dandadzi, A.; Akeju, D.; Adejoh, S.; Namisango, E.; Nkhoma, K.; Ebenso, B.; Allsop, M.J. “From the Time You Start with Them Until the Lord Calls You”: A Qualitative Study on the Experiences and Expectations of People Living with Advanced Cancer Interacting with Palliative Care Services in Uganda, Nigeria and Zimbabwe. J. Pain Symptom Manag. 2022, 64, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Selman, L.; Higginson, I.J.; Agupio, G.; Dinat, N.; Downing, J.; Gwyther, L.; Mashao, T.; Mmoledi, K.; Moll, A.P.; Sebuyira, L.M.; et al. Meeting information needs of patients with incurable progressive disease and their families in South Africa and Uganda: Multicentre qualitative study. BMJ 2009, 338, b1326. [Google Scholar] [CrossRef] [PubMed]
- Abate, Y.; Solomon, K.; Azmera, Y.M.; de Fouw, M.; Kaba, M. Barrier analysis for continuity of palliative care from health facility to household among adult cancer patients in Addis Ababa, Ethiopia. BMC Palliat. Care 2023, 22, 57. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, O.A.; Nkhoma, K.; Maddocks, M.; Harding, R. What constitutes a palliative care need in people with serious illnesses across Africa? A mixed-methods systematic review of the concept and evidence. Palliat. Med. 2021, 35, 1052–1070. [Google Scholar] [CrossRef] [PubMed]
- Omigbodun, A.; Agboola, A.D.; Fayehun, O.A.; Fayehun, O.A.; Ajisola, M.; Oladejo, A.; Popoola, O.; Lilford, R. Trends in Clinical Stage at Presentation for Four Common Adult Cancers in Ibadan, Nigeria. medRxiv 2023. [Google Scholar] [CrossRef]
- Lebimoyo, A.; Ola, B.; Adewuya, A.; Atilola, O.; Popoola, A. Mental Health and Quality of Life among Patients with Gynaecological Cancers in Lagos, Nigeria. Malays. J. Psychiatry 2020, 29, 41–57. [Google Scholar]
- Olasehinde, O.; Alatise, O.; Omisore, A.; Wuraola, F.; Odujoko, O.; Romanoff, A.; Akinkuolie, A.; Arowolo, O.; Adisa, A.; Knapp, G.; et al. Contemporary management of breast cancer in Nigeria: Insights from an institutional database. Int. J. Cancer 2021, 148, 2906–2914. [Google Scholar] [CrossRef]
- GSMA. Tanzania’s Digitalisation Journey: Opportunities for Value Creation; GSMA Head Office: London, UK, 2023. [Google Scholar]
- Hazin, R.; Qaddoumi, I. Teleoncology: Current and future applications for improving cancer care globally. Lancet Oncol. 2010, 11, 204–210. [Google Scholar] [CrossRef]
- Morse, R.S.; Lambden, K.; Quinn, E.; Ngoma, T.; Mushi, B.; Ho, Y.X.; Ngoma, M.; Mahuna, H.; Sagan, S.B.; Mmari, J.; et al. A Mobile App to Improve Symptom Control and Information Exchange Among Specialists and Local Health Workers Treating Tanzanian Cancer Patients: Human-Centered Design Approach. JMIR Cancer 2021, 7, e24062. [Google Scholar] [CrossRef] [PubMed]
- Harding, R.; Selman, L.; Agupio, G.; Dinat, N.; Downing, J.; Gwyther, L.; Mashao, T.; Mmoledi, K.; Moll, T.; Sebuyira, L.M.; et al. Validation of a core outcome measure for palliative care in Africa: The APCA African Palliative Outcome Scale. Health Qual Life Outcomes 2010, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Ngoma, M.; Mushi, B.; Morse, R.S.; Ngoma, T.; Mahuna, H.; Lambden, K.; Quinn, E.; Sagan, S.B.; Ho, Y.X.; Lucas, F.L.; et al. mPalliative Care Link: Examination of a Mobile Solution to Palliative Care Coordination among Tanzanian Patients with Cancer. JCO Glob. Oncol. 2021, 7, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.X.; Morse, R.S.; Lambden, K.; Mushi, B.P.; Ngoma, M.; Mahuna, H.; Ngoma, T.; Miesfeldt, S. How a Digital Case Management Platform Affects Community-Based Palliative Care of Sub-Saharan African Cancer Patients: Clinician-Users’ Perspectives. Appl. Clin. Inform. 2022, 13, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Okunade, K.; Nkhoma, K.B.; Salako, O.; Akeju, D.; Ebenso, B.; Namisango, E.; Soyannwo, O.; Namukwaya, E.; Dandadzi, A.; Nabirye, E.; et al. Understanding data and information needs for palliative cancer care to inform digital health intervention development in Nigeria, Uganda and Zimbabwe: Protocol for a multicountry qualitative study. BMJ Open 2019, 9, e032166. [Google Scholar] [CrossRef] [PubMed]
- Marston, J.; Coghlan, R.; Doherty, M.; Khan, F.; Leng, M.; Munday, D.; Petrova, M.; Powell, R.A.; Schwarz, L. Briefing Note: Palliative Care in Refugee Camps and Humanitarian Crises. 2020, International Association of Hospice and Palliative Care. Available online: http://globalpalliativecare.org/covid-19/uploads/briefing-notes/briefing-note-refugee-camps-and-humanitarian-crises.pdf (accessed on 9 November 2023).
- Donovan, K.A.; Grassi, L.; McGinty, H.L.; Jacobsen, P.B. Validation of the distress thermometer worldwide: State of the science. Psychooncology 2014, 23, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Kavuma, M. The Usability of Electronic Medical Record Systems Implemented in Sub-Saharan Africa: A Literature Review of the Evidence. JMIR Hum. Factors 2019, 6, e9317. [Google Scholar] [CrossRef]
- Pettit, L. Understanding EMRAM and how it can be used by policy-makers, hospital CIOs and their IT teams. World Hosp. Health Serv. 2013, 49, 7–9. [Google Scholar]
- Kabukye, J.K.; Kakungulu, E.; Keizer, N.D.; Cornet, R. Digital health in oncology in Africa: A scoping review and cross-sectional survey. Int. J. Med. Inform. 2022, 158, 104659. [Google Scholar] [CrossRef]
- Namisango, E.; Bhakta, N.; Wolfe, J.; McNeil, M.J.; Powell, R.A.; Kibudde, S.; Luyirika, E.B.K.; Mulema, V.; Feudtner, C.; Baker, J.N. Status of Palliative Oncology Care for Children and Young People in Sub-Saharan Africa: A Perspective Paper on Priorities for New Frontiers. JCO Glob. Oncol. 2021, 7, 1395–1405. [Google Scholar] [CrossRef]
- Silveira, A.; Sequeira, T.; Gonçalves, J.; Lopes Ferreira, P. Patient reported outcomes in oncology: Changing perspectives—A systematic review. Health Qual. Life Outcomes 2022, 20, 82. [Google Scholar] [CrossRef] [PubMed]
- Reid, E.A.; Abathun, E.; Diribi, J.; Mamo, Y.; Wondemagegnhu, T.; Hall, P.; Fallon, M.; Grant, L. Early palliative care in newly diagnosed cancer in Ethiopia: Feasibility randomised controlled trial and cost analysis. BMJ Support. Palliat. Care 2022. [Google Scholar] [CrossRef] [PubMed]
- Donkor, A.; Atuwo-Ampoh, V.D.; Yakanu, F.; Torgbenu, E.; Ameyaw, E.K.; Kitson-Mills, D.; Vanderpuye, V.; Kyei, K.A.; Anim-Sampong, S.; Khader, O.; et al. Financial toxicity of cancer care in low- and middle-income countries: A systematic review and meta-analysis. Support Care Cancer 2022, 30, 7159–7190. [Google Scholar] [CrossRef] [PubMed]
- Squitieri, L.; Bozic, K.J.; Pusic, A.L. The Role of Patient-Reported Outcome Measures in Value-Based Payment Reform. Value Health 2017, 20, 834–836. [Google Scholar] [CrossRef] [PubMed]
- Issahaku, Y.; Thoumi, A.; Abiiro, G.A.; Ogbouji, O.; Nonvignon, J. Is value-based payment for healthcare feasible under Ghana’s National Health Insurance Scheme? Health Res. Policy Syst. 2021, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Neumark, T.; Prince, R.J. Digital Health in East Africa: Innovation, Experimentation and the Market. Glob. Policy 2021, 12, 65–74. [Google Scholar] [CrossRef]
- Labrique, A.B.; Wadhwani, C.; Williams, K.A.; Lamptey, P.; Hesp, C.; Luk, R.; Aerts, A. Best practices in scaling digital health in low and middle income countries. Glob. Health 2018, 14, 103. [Google Scholar] [CrossRef]
- McCormack, V.; Newton, R. Research priorities for social inequalities in cancer in sub-Saharan Africa. In Reducing Social Inequalities in Cancer: Evidence and Priorities for Research; Vaccarella, S., Lortet-Tieulent, J., Saracci, R., Eds.; International Agency for Research on Cancer: Lyon, France, 2019. [Google Scholar]
- Mutatina, B.; Basaza, R.; Obuku, E.; Lavis, J.N.; Sewankambo, N. Identifying and characterising health policy and system-relevant documents in Uganda: A scoping review to develop a framework for the development of a one-stop shop. Health Res. Policy Syst. 2017, 15, 7. [Google Scholar] [CrossRef]
- Schwartz, J.I.; Guwatudde, D.; Nugent, R.; Mondo Kiiza, C. Looking at non-communicable diseases in Uganda through a local lens: An analysis using locally derived data. Glob. Health 2014, 10, 77. [Google Scholar] [CrossRef]
- Verma, N.; Mamlin, B.; Flowers, J.; Acharya, S.; Labrique, A.; Cullen, T. OpenMRS as a global good: Impact, opportunities, challenges, and lessons learned from fifteen years of implementation. Int. J. Med. Inform. 2021, 149, 104405. [Google Scholar] [CrossRef]
- Vijayan, V.; Connolly, J.P.; Condell, J.; McKelvey, N.; Gardiner, P. Review of Wearable Devices and Data Collection Considerations for Connected Health. Sensors 2021, 21, 5589. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Zopf, E.M.; Howden, E.J. Effect and feasibility of wearable physical activity trackers and pedometers for increasing physical activity and improving health outcomes in cancer survivors: A systematic review and meta-analysis. J. Sport Health Sci. 2022, 11, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Huhn, S.; Matzke, I.; Koch, M.; Gunga, H.-C.; Maggioni, M.A.; Sié, A.; Boudo, V.; Ouedraogo, W.A.; Compaoré, G.; Bunker, A.; et al. Using wearable devices to generate real-world, individual-level data in rural, low-resource contexts in Burkina Faso, Africa: A case study. Front. Public Health 2022, 10, 972177. [Google Scholar] [CrossRef] [PubMed]
- GSM Association, The Mobile Economy: Sub-Saharan Africa 2022. Available online: https://www.gsma.com/mobileeconomy/wp-content/uploads/2022/10/The-Mobile-Economy-Sub-Saharan-Africa-2022.pdf (accessed on 9 November 2023).
- Xu, L.; Sanders, L.; Li, K.; Chow, J.C.L. Chatbot for Health Care and Oncology Applications Using Artificial Intelligence and Machine Learning: Systematic Review. JMIR Cancer 2021, 7, e27850. [Google Scholar] [CrossRef] [PubMed]
- Kabukye, J.K.; Ilozumba, O.; Broerse, J.E.W.; de Keizer, N.; Cornet, R. Implementation of an Interactive Voice Response System for Cancer Awareness in Uganda: Mixed Methods Study. JMIR Mhealth Uhealth 2021, 9, e22061. [Google Scholar] [CrossRef] [PubMed]
- Hearn, J.; Wali, S.; Birungi, P.; Cafazzo, J.A.; Ssinabulya, I.; Akiteng, A.R.; Ross, H.J.; Seto, E.; Schwartz, J.I. A digital self-care intervention for Ugandan patients with heart failure and their clinicians: User-centred design and usability study. Digit. Health 2022, 8, 20552076221129064. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Evans, J.; Gupta, S. Barriers to Scale of Digital Health Systems for Cancer Care and Control in Last-Mile Settings. J. Glob. Oncol. 2017, 4, 1–3. [Google Scholar] [CrossRef]
- Glasgow, R.E.; McKay, H.G.; Piette, J.D.; Reynolds, K.D. The RE-AIM framework for evaluating interventions: What can it tell us about approaches to chronic illness management? Patient Educ. Couns. 2001, 44, 119–127. [Google Scholar] [CrossRef]
- Damschroder, L.J.; Aron, D.C.; Keith, R.E.; Kirsh, S.R.; Alexander, J.A.; Lowery, J.C. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement. Sci. 2009, 4, 50. [Google Scholar] [CrossRef]
- Nahum-Shani, I.; Dziak, J.J.; Wetter, D.W. MCMTC: A Pragmatic Framework for Selecting an Experimental Design to Inform the Development of Digital Interventions. Front. Digit. Health 2022, 4, 798025. [Google Scholar] [CrossRef]
- Jane Bates, M.; Gordon, M.R.P.; Gordon, S.B.; Tomeny, E.M.; Muula, A.S.; Davies, H.; Morris, C.; Manthalu, G.; Namisango, E.; Masamba, L.; et al. Palliative care and catastrophic costs in Malawi after a diagnosis of advanced cancer: A prospective cohort study. Lancet Glob. Health 2021, 9, e1750–e1757. [Google Scholar] [CrossRef] [PubMed]
- Venkataramanan, R.; Pradhan, A.; Kumar, A.; Purushotham, A.; Alajlani, M.; Arvanitis, T.N. Digital Inequalities in Cancer Care Delivery in India: An Overview of the Current Landscape and Recommendations for Large-Scale Adoption. Front. Digit. Health 2022, 4, 916342. [Google Scholar] [CrossRef] [PubMed]
- LeBaron, V.; Adhikari, A.; Bennett, R.; Chapagain Acharya, S.; Dhakal, M.; Elmore, C.E.; Fitzgibbon, K.; Gongal, R.; Kattel, R.; Koirala, G.; et al. A survey of cancer care institutions in Nepal to inform design of a pain management mobile application. BMC Palliat. Care 2021, 20, 171. [Google Scholar] [CrossRef] [PubMed]
- Mutebi, M.; Bhatia, R.; Salako, O.; Rubagumya, F.; Grover, S.; Hammad, N. Innovative Use of mHealth and Clinical Technology for Oncology Clinical Trials in Africa. JCO Glob. Oncol. 2020, 948–953. [Google Scholar] [CrossRef]
- Allsop, M.J.; Kabukye, J.; Powell, R.A.; Namisango, E. Routine Data and Minimum Datasets for Palliative Cancer Care in Sub-Saharan Africa: Their Role, Barriers and Facilitators. In Palliative Care for Chronic Cancer Patients in the Community: Global Approaches and Future Applications; Silbermann, M., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 179–192. [Google Scholar]
- Perla, R.; Provost, L.; Murray, S.K. The run chart: A simple analytical tool for learning from variation in healthcare processes. BMJ Qual. Saf. 2011, 20, 46–51. [Google Scholar] [CrossRef]
- Jordan, R.I.; Allsop, M.J.; ElMokhallalati, Y.; Jackson, C.E.; Edwards, H.L.; Chapman, E.J.; Deliens, L.; Bennett, M.I. Duration of palliative care before death in international routine practice: A systematic review and meta-analysis. BMC Med. 2020, 18, 368. [Google Scholar] [CrossRef]
- Royston, G.; Pakenham-Walsh, N.; Zielinski, C. Universal access to essential health information: Accelerating progress towards universal health coverage and other SDG health targets. BMJ Glob. Health 2020, 5, e002475. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salako, O.; Enyi, A.; Miesfeldt, S.; Kabukye, J.K.; Ngoma, M.; Namisango, E.; LeBaron, V.; Sisimayi, C.; Ebenso, B.; Lorenz, K.A.; et al. Remote Symptom Monitoring to Enhance the Delivery of Palliative Cancer Care in Low-Resource Settings: Emerging Approaches from Africa. Int. J. Environ. Res. Public Health 2023, 20, 7190. https://doi.org/10.3390/ijerph20247190
Salako O, Enyi A, Miesfeldt S, Kabukye JK, Ngoma M, Namisango E, LeBaron V, Sisimayi C, Ebenso B, Lorenz KA, et al. Remote Symptom Monitoring to Enhance the Delivery of Palliative Cancer Care in Low-Resource Settings: Emerging Approaches from Africa. International Journal of Environmental Research and Public Health. 2023; 20(24):7190. https://doi.org/10.3390/ijerph20247190
Chicago/Turabian StyleSalako, Omolola, Adaorah Enyi, Susan Miesfeldt, Johnblack K. Kabukye, Mamsau Ngoma, Eve Namisango, Virginia LeBaron, Chenjerai Sisimayi, Bassey Ebenso, Karl A. Lorenz, and et al. 2023. "Remote Symptom Monitoring to Enhance the Delivery of Palliative Cancer Care in Low-Resource Settings: Emerging Approaches from Africa" International Journal of Environmental Research and Public Health 20, no. 24: 7190. https://doi.org/10.3390/ijerph20247190
APA StyleSalako, O., Enyi, A., Miesfeldt, S., Kabukye, J. K., Ngoma, M., Namisango, E., LeBaron, V., Sisimayi, C., Ebenso, B., Lorenz, K. A., Wang, Y., Ryan Wolf, J., van den Hurk, C., & Allsop, M. (2023). Remote Symptom Monitoring to Enhance the Delivery of Palliative Cancer Care in Low-Resource Settings: Emerging Approaches from Africa. International Journal of Environmental Research and Public Health, 20(24), 7190. https://doi.org/10.3390/ijerph20247190





