Measuring the Autistic Women’s Experience (AWE)
Abstract
:1. Introduction
1.1. Relevance and Study Aim
1.2. Gender Differences in Autism
1.3. Identification of Autism in Females
1.4. Current Study
2. Materials and Methods
2.1. Participants
2.2. Materials
2.2.1. Autistic Women’s Experience (AWE)
2.2.2. Autism Spectrum Quotient (AQ)
2.3. Procedure
2.4. Statistical Analyses
3. Results
3.1. AWE
3.2. Intercorrelations and Reliability
3.3. Group Comparisons
3.4. ROC Analyses
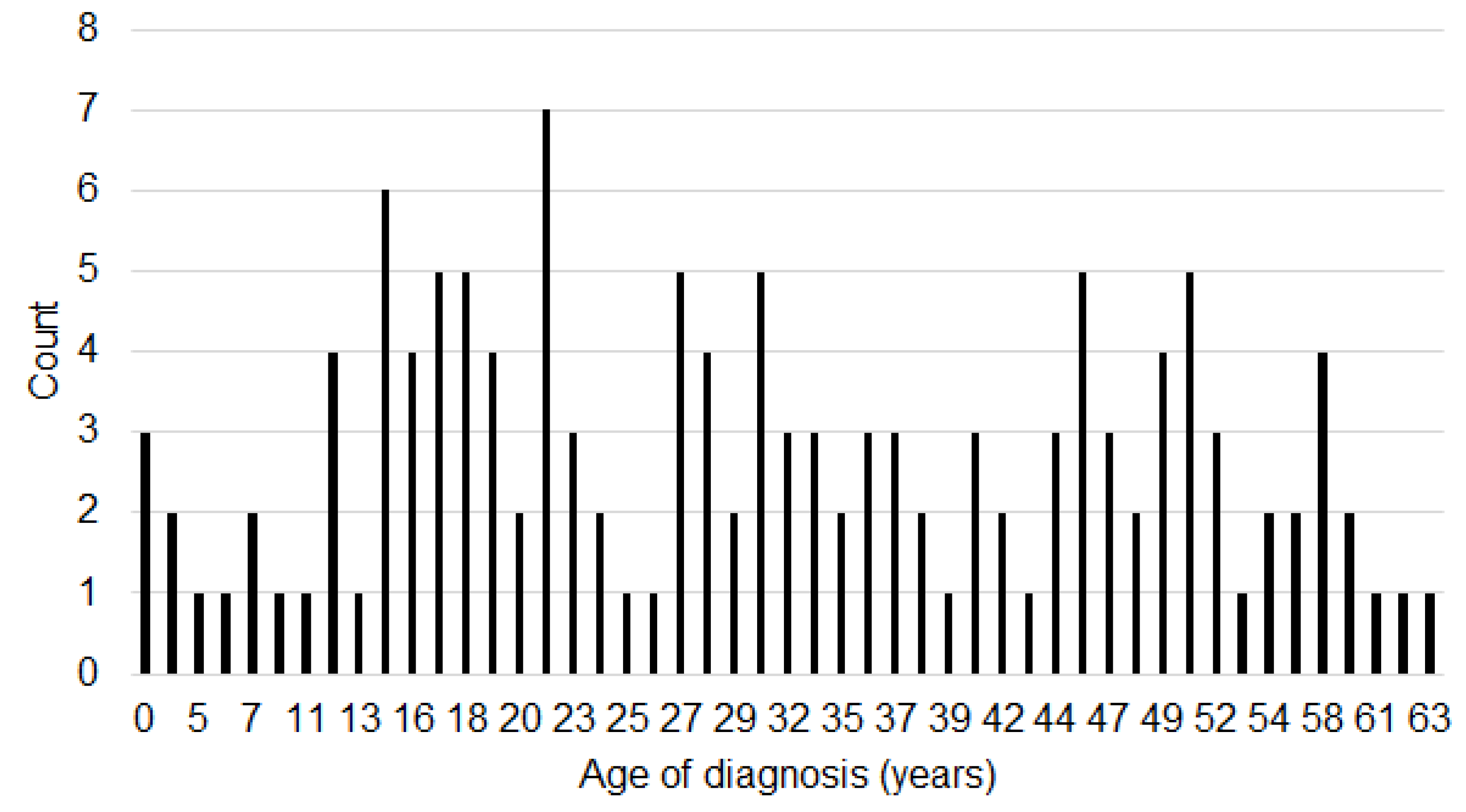
3.5. Early Versus Late Diagnosis
4. Discussion
4.1. Summary of the Main Findings
4.2. Gender-Specific Findings
4.3. Sensing Boundaries
4.4. Clinical Recommendations
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Item | t | DF | Sig. (2-Tailed) | Mean Difference | SE of Difference |
|---|---|---|---|---|---|
| AQ1 | 4.226 | 315 | 0.000 | 0.381 | 0.090 |
| AQ2 | 8.203 | 315 | 0.000 | 0.708 | 0.086 |
| AQ3 | 6.890 | 315 | 0.000 | 0.686 | 0.100 |
| AQ4 | 6.288 | 315 | 0.000 | 0.748 | 0.119 |
| AQ5 | 5.713 | 315 | 0.000 | 0.691 | 0.121 |
| AQ6 | 2.893 | 313 | 0.004 | 0.409 | 0.141 |
| AQ7 | 6.143 | 314 | 0.000 | 0.637 | 0.104 |
| AQ8 | 5.269 | 315 | 0.000 | 0.555 | 0.105 |
| AQ9 | 4.351 | 315 | 0.000 | 0.535 | 0.123 |
| AQ10 | 8.635 | 315 | 0.000 | 1.023 | 0.118 |
| AQ11 | 13.620 | 315 | 0.000 | 1.333 | 0.098 |
| AQ12 | 3.913 | 315 | 0.000 | 0.380 | 0.097 |
| AQ13 | 9.103 | 315 | 0.000 | 1.110 | 0.122 |
| AQ14 | 1.435 | 315 | 0.152 | 0.179 | 0.125 |
| AQ15 | 9.162 | 315 | 0.000 | 0.919 | 0.100 |
| AQ16 | 6.435 | 315 | 0.000 | 0.746 | 0.116 |
| AQ17 | 6.960 | 315 | 0.000 | 0.765 | 0.110 |
| AQ18 | 2.013 | 315 | 0.045 | 0.232 | 0.115 |
| AQ19 | 4.926 | 315 | 0.000 | 0.593 | 0.120 |
| AQ20 | 5.368 | 315 | 0.000 | 0.530 | 0.099 |
| AQ21 | 1.985 | 315 | 0.048 | 0.287 | 0.145 |
| AQ22 | 11.570 | 315 | 0.000 | 1.233 | 0.107 |
| AQ23 | 5.571 | 315 | 0.000 | 0.643 | 0.115 |
| AQ24 | 3.238 | 315 | 0.001 | 0.380 | 0.117 |
| AQ25 | 7.569 | 315 | 0.000 | 0.845 | 0.112 |
| AQ26 | 10.457 | 315 | 0.000 | 1.156 | 0.111 |
| AQ27 | 8.301 | 315 | 0.000 | 0.886 | 0.107 |
| AQ28 | 6.583 | 315 | 0.000 | 0.681 | 0.103 |
| AQ29 | 0.508 | 315 | 0.612 | 0.066 | 0.130 |
| AQ30 | −1.521 | 315 | 0.129 | −0.173 | 0.114 |
| AQ31 | 5.705 | 315 | 0.000 | 0.559 | 0.098 |
| AQ32 | 7.850 | 315 | 0.000 | 0.845 | 0.108 |
| AQ33 | 7.658 | 315 | 0.000 | 0.936 | 0.122 |
| AQ34 | 8.451 | 315 | 0.000 | 0.940 | 0.111 |
| AQ35 | 8.008 | 315 | 0.000 | 0.894 | 0.112 |
| AQ36 | 9.588 | 315 | 0.000 | 0.974 | 0.102 |
| AQ37 | 8.329 | 315 | 0.000 | 0.869 | 0.104 |
| AQ38 | 7.633 | 315 | 0.000 | 0.874 | 0.114 |
| AQ39 | 7.579 | 315 | 0.000 | 0.817 | 0.108 |
| AQ40 | 3.144 | 315 | 0.002 | 0.405 | 0.129 |
| AQ41 | 6.374 | 315 | 0.000 | 0.839 | 0.132 |
| AQ42 | 5.344 | 315 | 0.000 | 0.647 | 0.121 |
| AQ43 | 5.192 | 315 | 0.000 | 0.531 | 0.102 |
| AQ44 | 12.125 | 315 | 0.000 | 1.074 | 0.089 |
| AQ45 | 12.166 | 315 | 0.000 | 1.151 | 0.095 |
| AQ46 | 9.043 | 315 | 0.000 | 1.052 | 0.116 |
| AQ47 | 9.970 | 315 | 0.000 | 0.957 | 0.096 |
| AQ48 | 3.319 | 315 | 0.001 | 0.385 | 0.116 |
| AQ49 | −1.286 | 315 | 0.199 | −0.174 | 0.136 |
| AQ50 | 5.274 | 315 | 0.000 | 0.654 | 0.124 |
| NEW1 | 7.786 | 315 | 0.000 | 0.823 | 0.106 |
| NEW2 | 7.812 | 315 | 0.000 | 0.899 | 0.115 |
| NEW3 | 3.057 | 315 | 0.002 | 0.362 | 0.118 |
| NEW4 | 11.879 | 315 | 0.000 | 1.155 | 0.097 |
| NEW5 | 2.493 | 315 | 0.013 | 0.290 | 0.116 |
| NEW6 | 8.610 | 315 | 0.000 | 1.028 | 0.119 |
| NEW7 | 4.357 | 315 | 0.000 | 0.593 | 0.136 |
| NEW8 | 4.247 | 315 | 0.000 | 0.400 | 0.094 |
| NEW9 | 11.369 | 315 | 0.000 | 1.299 | 0.114 |
| NEW10 | 2.126 | 315 | 0.034 | 0.269 | 0.127 |
| NEW11 | 6.044 | 315 | 0.000 | 0.677 | 0.112 |
| NEW12 | 9.974 | 315 | 0.000 | 1.140 | 0.114 |
| NEW13 | −2.125 | 315 | 0.034 | −0.291 | 0.137 |
| NEW14 | 3.176 | 315 | 0.002 | 0.417 | 0.131 |
| NEW15 | 8.498 | 315 | 0.000 | 1.039 | 0.122 |
| NEW16 | 13.917 | 315 | 0.000 | 1.553 | 0.112 |
| NEW17 | 11.652 | 315 | 0.000 | 1.177 | 0.101 |
| NEW18 | 1.377 | 315 | 0.169 | 0.160 | 0.116 |
| NEW19 | 10.555 | 315 | 0.000 | 1.056 | 0.100 |
| NEW20 | 4.151 | 315 | 0.000 | 0.471 | 0.113 |
| NEW21 | 6.671 | 315 | 0.000 | 0.815 | 0.122 |
| NEW22 | 3.950 | 315 | 0.000 | 0.492 | 0.125 |
| NEW23 | 4.098 | 315 | 0.000 | 0.600 | 0.147 |
| NEW24 | 2.949 | 315 | 0.003 | 0.325 | 0.110 |
| NEW25 | 0.930 | 315 | 0.353 | 0.119 | 0.128 |
| NEW26 | 8.864 | 315 | 0.000 | 0.904 | 0.102 |
| NEW27 | 9.176 | 315 | 0.000 | 0.993 | 0.108 |
| NEW28 | 6.640 | 315 | 0.000 | 0.772 | 0.116 |
| NEW29 | 4.871 | 315 | 0.000 | 0.518 | 0.106 |
| NEW30 | 1.613 | 315 | 0.108 | 0.190 | 0.118 |
| NEW31 | 7.164 | 315 | 0.000 | 0.788 | 0.110 |
| NEW32 | 10.533 | 315 | 0.000 | 0.957 | 0.091 |
| NEW33 | 8.458 | 315 | 0.000 | 1.025 | 0.121 |
| NEW34 | 9.630 | 315 | 0.000 | 0.971 | 0.101 |
| NEW35 | 5.814 | 315 | 0.000 | 0.529 | 0.091 |
| NEW36 | 10.393 | 315 | 0.000 | 1.254 | 0.121 |
| NEW37 | 6.525 | 315 | 0.000 | 0.738 | 0.113 |
| NEW38 | 0.941 | 315 | 0.348 | 0.105 | 0.112 |
| NEW39 | 5.143 | 315 | 0.000 | 0.581 | 0.113 |
| NEW40 | 4.255 | 315 | 0.000 | 0.479 | 0.113 |
| NEW41 | 6.970 | 315 | 0.000 | 0.827 | 0.119 |
| NEW42 | 7.728 | 315 | 0.000 | 0.938 | 0.121 |
| NEW43 | 3.222 | 315 | 0.001 | 0.343 | 0.106 |
| NEW44 | 7.644 | 315 | 0.000 | 0.900 | 0.118 |
| NEW45 | 6.599 | 315 | 0.000 | 0.733 | 0.111 |
| NEW46 | 6.137 | 315 | 0.000 | 0.593 | 0.097 |
| NEW47 | 1.958 | 315 | 0.051 | 0.193 | 0.099 |
| NEW48 | 1.785 | 315 | 0.075 | 0.164 | 0.092 |
| NEW49 | 9.379 | 315 | 0.000 | 1.037 | 0.111 |
| NEW50 | 8.465 | 315 | 0.000 | 1.072 | 0.127 |
| NEW51 | 4.288 | 315 | 0.000 | 0.539 | 0.126 |
| NEW52 | 6.425 | 315 | 0.000 | 0.662 | 0.103 |
References
- Begeer, S.; Mandell, D.; Wijnker-Holmes, B.; Venderbosch, S.; Rem, D.; Stekelenburg, F.; Koot, H.M. Sex differences in the timing of identification among children and adults with autism spectrum disorders. J. Autism Dev. Disord. 2013, 43, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Gesi, C.; Migliarese, G.; Torriero, S.; Capellazzi, M.; Omboni, A.C.; Cerveri, G.; Mencacci, C. Gender Differences in Misdiagnosis and Delayed Diagnosis among Adults with Autism Spectrum Disorder with No Language or Intellectual Disability. Brain Sci. 2021, 11, 912. [Google Scholar] [CrossRef]
- Green, R.M.; Travers, A.M.; Howe, Y.; McDougle, C.J. Women and Autism Spectrum Disorder: Diagnosis and Implications for Treatment of Adolescents and Adults. Curr. Psychiatry Rep. 2019, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- Kok, F.M. The Female Side of ADHD and ASD. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2021. [Google Scholar]
- Rutherford, M.; McKenzie, K.; Johnson, T.; Catchpole, C.; O’Hare, A.; McClure, I.; Forsyth, K.; McCartney, D.; Murray, A. Gender ratio in a clinical population sample, age of diagnosis and duration of assessment in children and adults with autism spectrum disorder. Autism 2016, 20, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Shattuck, P.T.; Durkin, M.; Maenner, M.; Newschaffer, C.; Mandell, D.S.; Wiggins, L.; Lee, L.C.; Rice, C.; Giarelli, E.; Kirby, R.; et al. Timing of identification among children with an autism spectrum disorder: Findings from a population-based surveillance study. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Louwerse, A.; Eussen, M.L.J.M.; Van der Ende, J.; de Nijs, P.F.A.; Van Gool, A.R.; Dekker, L.P.; Verheij, C.; Verheij, F.; Verhulst, F.C.; Greaves-Lord, K. ASD Symptom Severity in Adolescence of Individuals Diagnosed with PDD-NOS in Childhood: Stability and the Relation with Psychiatric Comorbidity and Societal Participation. J. Autism Dev. Disord. 2015, 45, 3908–3918. [Google Scholar] [CrossRef] [PubMed]
- Ayres, M.; Parr, J.R.; Rodgers, J.; Mason, D.; Avery, L.; Flynn, D. A systematic review of quality of life of adults on the autism spectrum. Autism 2018, 22, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Mason, D.; McConachie, H.; Garland, D.; Petrou, A.; Rodgers, J.; Parr, J.R. Predictors of quality of life for autistic adults. Autism Res. 2018, 11, 1138–1147. [Google Scholar] [CrossRef]
- Jadav, N.; Bal, V.H. Associations between co-occurring conditions and age of autism diagnosis: Implications for mental health training and adult autism research. Autism Res. 2022, 15, 2112–2125. [Google Scholar] [CrossRef]
- Bargiela, S.; Steward, R.; Mandy, W. The Experiences of Late-diagnosed Women with Autism Spectrum Conditions: An Investigation of the Female Autism Phenotype. J. Autism Dev. Disord. 2016, 46, 3281–3294. [Google Scholar] [CrossRef]
- Hull, L.; Mandy, W.; Lai, M.C.; Baron-Cohen, S.; Allison, C.; Smith, P.; Petrides, K.V. Development and Validation of the Camouflaging Autistic Traits Questionnaire (CAT-Q). J. Autism Dev. Disord. 2019, 49, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Leedham, A.; Thompson, A.R.; Smith, R.; Freeth, M. ‘I was exhausted trying to figure it out’: The experiences of females receiving an autism diagnosis in middle to late adulthood. Autism 2020, 24, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Cage, E.; Monaco, J.D.; Newell, V. Experiences of Autism Acceptance and Mental Health in Autistic Adults. J. Autism Dev. Disord. 2018, 48, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Lombardo, M.V.; Auyeung, B.; Chakrabarti, B.; Baron-Cohen, S. Sex/Gender Differences and Autism: Setting the Scene for Future Research. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, J.D.; Marrus, N.; Maloney, S.E.; Yip, B.; Sandin, S.; Turner, T.N.; Selmanovic, D.; Kroll, K.L.; Gutmann, D.H.; Constantino, J.N.; et al. Can the “female protective effect” liability threshold model explain sex differences in autism spectrum disorder? Neuron 2022, 110, 3243–3262. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Tsompanidis, A.; Auyeung, B.; Nørgaard-Pedersen, B.; Hougaard, D.M.; Abdallah, M.; Cohen, A.; Pohl, A. Foetal oestrogens and autism. Mol. Psychiatry 2020, 25, 2970–2978. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Auyeung, B.; Nørgaard-Pedersen, B.; Hougaard, D.M.; Abdallah, M.W.; Melgaard, L.; Cohen, A.S.; Chakrabarti, B.; Ruta, L.; Lombardo, M. V Elevated fetal steroidogenic activity in autism. Mol. Psychiatry 2015, 20, 369–376. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Wheelwright, S.; Skinner, R.; Martin, J.; Clubley, E. The Autism-Spectrum Quotient (AQ): Evidence from Asperger Syndrome/High-Functioning Autism, Males and Females, Scientists and Mathematicians. J. Autism Dev. Disord. 2001, 31, 5–17. [Google Scholar] [CrossRef]
- Clayton, J.A.; Tannenbaum, C. Reporting sex, gender, or both in clinical research? JAMA J. Am. Med. Assoc. 2016, 316, 1863–1864. [Google Scholar] [CrossRef]
- Golden, L.C.; Voskuhl, R. The importance of studying sex differences in disease: The example of multiple sclerosis. J. Neurosci. Res. 2017, 95, 633–643. [Google Scholar] [CrossRef]
- Cumin, J.; Pelaez, S.; Mottron, L. Positive and differential diagnosis of autism in verbal women of typical intelligence: A Delphi study. Autism 2021, 26, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Baird, G.; Simonoff, E.; Pickles, A.; Chandler, S.; Loucas, T.; Meldrum, D.; Charman, T. Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: The Special Needs and Autism Project (SNAP). Lancet 2006, 368, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Fombonne, E. Epidemiology of pervasive developmental disorders. Pediatr. Res. 2009, 65, 591–598. [Google Scholar] [CrossRef]
- Rivet, T.T.; Matson, J.L. Review of gender differences in core symptomatology in autism spectrum disorders. Res. Autism Spectr. Disord. 2011, 5, 957–976. [Google Scholar] [CrossRef]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.; Stapley, S.; Newlove-Delgado, T.; Salmon, A.; White, R.; Warren, F.; Pearson, A.; Ford, T. Time trends in autism diagnosis over 20 years: A UK population-based cohort study. J. Child Psychol. Psychiatry 2022, 63, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Ratto, A.B. Commentary: What’s so special about girls on the autism spectrum?—A commentary on Kaat et al. (2020). J. Child Psychol. Psychiatry Allied Discip. 2021, 62, 107–109. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5); American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- Wood-Downie, H.; Wong, B.; Kovshoff, H.; Cortese, S.; Hadwin, J.A. Research Review: A systematic review and meta-analysis of sex/gender differences in social interaction and communication in autistic and nonautistic children and adolescents. J. Child Psychol. Psychiatry 2021, 62, 922–936. [Google Scholar] [CrossRef]
- Hull, L.; Petrides, K.V.; Mandy, W. The Female Autism Phenotype and Camouflaging: A Narrative Review. Rev. J. Autism Dev. Disord. 2020, 7, 306–317. [Google Scholar] [CrossRef]
- Raymaker, D.M.; Teo, A.R.; Steckler, N.A.; Lentz, B.; Scharer, M.; Delos Santos, A.; Kapp, S.K.; Hunter, M.; Joyce, A.; Nicolaidis, C. “Having All of Your Internal Resources Exhausted Beyond Measure and Being Left with No Clean-Up Crew”: Defining Autistic Burnout. Autism Adulthood 2020, 2, 132–143. [Google Scholar] [CrossRef]
- Constantino, J.N.; Todd, R.D. Intergenerational transmission of subthreshold autistic traits in the general population. Biol. Psychiatry 2005, 57, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Sedgewick, F.; Hill, V.; Yates, R.; Pickering, L.; Pellicano, E. Gender Differences in the Social Motivation and Friendship Experiences of Autistic and Non-autistic Adolescents. J. Autism Dev. Disord. 2016, 46, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Tierney, S.; Burns, J.; Kilbey, E. Looking behind the mask: Social coping strategies of girls on the autistic spectrum. Res. Autism Spectr. Disord. 2016, 23, 73–83. [Google Scholar] [CrossRef]
- Dean, M.; Kasari, C.; Shih, W.; Frankel, F.; Whitney, R.; Landa, R.; Lord, C.; Orlich, F.; King, B.; Harwood, R. The peer relationships of girls with ASD at school: Comparison to boys and girls with and without ASD. J. Child Psychol. Psychiatry Allied Discip. 2014, 55, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Bourson, L.; Prevost, C. Characteristics of restricted interests in girls with ASD compared to boys: A systematic review of the literature. Eur. Child Adolesc. Psychiatry 2022, 1, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Van Wijngaarden-Cremers, P.J.M.; Van Eeten, E.; Groen, W.B.; Van Deurzen, P.A.; Oosterling, I.J.; Van Der Gaag, R.J. Gender and age differences in the core triad of impairments in autism spectrum disorders: A systematic review and meta-analysis. J. Autism Dev. Disord. 2014, 44, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, G.; Masi, G.; Toni, C.; Dell’Osso, L.; Marazziti, D.; Perugi, G. Clinical features, developmental course, and psychiatric comorbidity of adult autism spectrum disorders. CNS Spectr. 2014, 19, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, A.; Fulton, K.; Lai, M.C.; Pellicano, E.; Mandy, W. Recognition of Girls on the Autism Spectrum by Primary School Educators: An Experimental Study. Autism Res. 2020, 13, 1358–1372. [Google Scholar] [CrossRef]
- Kumazaki, H.; Muramatsu, T.; Kosaka, H.; Fujisawa, T.X.; Iwata, K.; Tokoda, A.; Tsuchiya, K.; Mimura, M. Sex differences in cognitive and symptom profiles in children with high functioning autism spectrum disorders. Res. Autism Spectr. Disord. 2015, 13–14, 1–7. [Google Scholar] [CrossRef]
- Lai, M.C.; Lombardo, M.V.; Pasco, G.; Ruigrok, A.N.V.; Wheelwright, S.J.; Sadek, S.A.; Chakrabarti, B.; Baron-Cohen, S. A Behavioral Comparison of Male and Female Adults with High Functioning Autism Spectrum Conditions. PLoS ONE 2011, 6, e20835. [Google Scholar] [CrossRef]
- Duvekot, J.; van der Ende, J.; Verhulst, F.C.; Slappendel, G.; van Daalen, E.; Maras, A.; Greaves-Lord, K. Factors influencing the probability of a diagnosis of autism spectrum disorder in girls versus boys. Autism 2017, 21, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Wood-Downie, H.; Wong, B.; Kovshoff, H.; Mandy, W.; Hull, L.; Hadwin, J.A. Sex/Gender Differences in Camouflaging in Children and Adolescents with Autism. J. Autism Dev. Disord. 2021, 51, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Kopp, S.; Gillberg, C. The Autism Spectrum Screening Questionnaire (ASSQ)-Revised Extended Version (ASSQ-REV): An instrument for better capturing the autism phenotype in girls? A preliminary study involving 191 clinical cases and community controls. Res. Dev. Disabil. 2011, 32, 2875–2888. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, R.A.; Bartels, M.; Cath, D.C.; Boomsma, D.I. Factor structure, reliability and criterion validity of the autism-spectrum quotient (AQ): A study in Dutch population and patient groups. J. Autism Dev. Disord. 2008, 38, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, A.; Baron-Cohen, S.; Wheelwright, S.; Tojo, Y. The Autism-Spectrum Quotient (AQ) in Japan: A cross-cultural comparison. J. Autism Dev. Disord. 2006, 36, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Bezemer, M.L.; Blijd-Hoogewys, E.M.A.; Meek-Heekelaar, M. The Predictive Value of the AQ and the SRS-A in the Diagnosis of ASD in Adults in Clinical Practice. J. Autism Dev. Disord. 2021, 51, 2402–2415. [Google Scholar] [CrossRef] [PubMed]
- English, M.C.W.; Gignac, G.E.; Visser, T.A.W.; Whitehouse, A.J.O.; Maybery, M.T. A comprehensive psychometric analysis of autism-spectrum quotient factor models using two large samples: Model recommendations and the influence of divergent traits on total-scale scores. Autism Res. 2020, 13, 45–60. [Google Scholar] [CrossRef]
- Belcher, H.L.; Uglik-Marucha, N.; Vitoratou, S.; Ford, R.M.; Morein-Zamir, S. Gender bias in autism screening: Measurement invariance of different model frameworks of the Autism Spectrum Quotient. BJPsych Open 2023, 9, e173. [Google Scholar] [CrossRef]
- Kloosterman, P.H.; Keefer, K.V.; Kelley, E.A.; Summerfeldt, L.J.; Parker, J.D.A. Evaluation of the factor structure of the Autism-Spectrum Quotient. Pers. Individ. Dif. 2011, 50, 310–314. [Google Scholar] [CrossRef]
- Lau, W.Y.P.; Gau, S.S.F.; Chiu, Y.N.; Wu, Y.Y.; Chou, W.J.; Liu, S.K.; Chou, M.C. Psychometric properties of the Chinese version of the Autism Spectrum Quotient (AQ). Res. Dev. Disabil. 2013, 34, 294–305. [Google Scholar] [CrossRef]
- Hurst, R.M.; Mitchell, J.T.; Kimbrel, N.A.; Kwapil, T.K.; Nelson-Gray, R.O. Examination of the reliability and factor structure of the Autism Spectrum Quotient (AQ) in a non-clinical sample. Pers. Individ. Dif. 2007, 43, 1938–1949. [Google Scholar] [CrossRef]
- Freeth, M.; Sheppard, E.; Ramachandran, R.; Milne, E. A cross-cultural comparison of autistic traits in the UK, India and Malaysia. J. Autism Dev. Disord. 2013, 43, 2569–2583. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Cassidy, S.; Auyeung, B.; Allison, C.; Achoukhi, M.; Robertson, S.; Pohl, A.; Lai, M.C. Attenuation of typical sex differences in 800 adults with autism vs. 3,900 controls. PLoS ONE 2014, 9, e102251. [Google Scholar] [CrossRef] [PubMed]
- Ruzich, E.; Allison, C.; Chakrabarti, B.; Smith, P.; Musto, H.; Ring, H.; Baron-Cohen, S. Sex and STEM Occupation Predict Autism-Spectrum Quotient (AQ) Scores in Half a Million People. PLoS ONE 2015, 10, e0141229. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.M.; Warrier, V.; Allison, C.; Baron-Cohen, S. Testing the empathizing–systemizing theory of sex differences and the extreme male brain theory of autism in half a million people. Proc. Natl. Acad. Sci. USA 2018, 115, 12152–12157. [Google Scholar] [CrossRef] [PubMed]
- Allely, C.S. Understanding and recognising the female phenotype of autism spectrum disorder and the “camouflage” hypothesis: A systematic PRISMA review. Adv. Autism 2019, 5, 14–37. [Google Scholar] [CrossRef]
- Taylor, E.; Holt, R.; Tavassoli, T.; Ashwin, C.; Baron-Cohen, S. Revised scored Sensory Perception Quotient reveals sensory hypersensitivity in women with autism. Mol. Autism 2020, 11, 18. [Google Scholar] [CrossRef]
- Tavassoli, T.; Hoekstra, R.A.; Baron-Cohen, S. The Sensory Perception Quotient (SPQ): Development and validation of a new sensory questionnaire for adults with and without autism. Mol. Autism 2014, 5, 29. [Google Scholar] [CrossRef]
- Brett, J.; Staniszewska, S.; Mockford, C.; Herron-Marx, S.; Hughes, J.; Tysall, C.; Suleman, R. Mapping the impact of patient and public involvement on health and social care research: A systematic review. Health Expect. 2014, 17, 637–650. [Google Scholar] [CrossRef]
- Piening, S.; van Balkom, I.D.C.; Stapert, A.F.; Henning, D.; Greaves-Lord, K.; Davids, R.C.D.; Castelein, S.; Groen, Y. Perspectives on Autism Spectrum Disorder Diagnosis, Symptoms, Treatment and Gender Roles: A Qualitative Study of Similarities and Differences between Sexes. Int. J. Environ. Res. Public Health 2023, 20. in press. [Google Scholar]
- Clark, L.A.; Watson, D. Constructing validity: New developments in creating objective measuring instruments. Psychol. Assess. 2019, 31, 1412–1427. [Google Scholar] [CrossRef] [PubMed]
- Costello, A.B.; Osborne, J. Best practices in exploratory factor analysis: Four recommendations for getting the most from your analysis. Res. Eval. Pract. Assess. Res. Eval. 2005, 10, 7. [Google Scholar]
- Murray, A.L.; Allison, C.; Smith, P.L.; Baron-Cohen, S.; Booth, T.; Auyeung, B. Investigating diagnostic bias in autism spectrum conditions: An item response theory analysis of sex bias in the AQ-10. Autism Res. 2017, 10, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Lombardo, M.V.; Chakrabarti, B.; Ruigrok, A.N.V.; Bullmore, E.T.; Suckling, J.; Auyeung, B.; Happé, F.; Szatmari, P.; Baron-cohen, S.; et al. Neural self-representation in autistic women and association with ‘ compensatory camouflaging. Autism 2019, 23, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, M.; Bölte, S.; Poustka, F. Autism spectrum disorders: Sex differences in autistic behaviour domains and coexisting psychopathology. Dev. Med. Child Neurol. 2007, 49, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.J.; Rudolph, K.D. A Review of Sex Differences in Peer Relationship Processes: Potential Trade-offs for the Emotional and Behavioral Development of Girls and Boys. Psychol. Bull. 2006, 132, 98. [Google Scholar] [CrossRef] [PubMed]
- Fiene, L.; Ireland, M.J.; Brownlow, C. The Interoception Sensory Questionnaire (ISQ): A Scale to Measure Interoceptive Challenges in Adults. J. Autism Dev. Disord. 2018, 48, 3354–3366. [Google Scholar] [CrossRef]
- Lupindo, B.M.; Maw, A.; Shabalala, N. Late diagnosis of autism: Exploring experiences of males diagnosed with autism in adulthood. Curr. Psychol. 2022, 1, 1–17. [Google Scholar] [CrossRef]
- Murphy, J.; Geary, H.; Millgate, E.; Catmur, C.; Bird, G. Direct and indirect effects of age on interoceptive accuracy and awareness across the adult lifespan. Psychon. Bull. Rev. 2017, 25, 1193–1202. [Google Scholar] [CrossRef]
- Eagly, A.H.; Wood, W. Social role theory. In Handbook of Theories of Social Psychology; Van Lange, P.A.M., Kruglanski, A.W., Higgins, E.T., Eds.; SAGE Publications Ltd.: London, UK, 2012; Volume 2, pp. 458–476. [Google Scholar]
- Dewinter, J.; De Graaf, H.; Begeer, S. Sexual Orientation, Gender Identity, and Romantic Relationships in Adolescents and Adults with Autism Spectrum Disorder. J. Autism Dev. Disord. 2017, 47, 2927–2934. [Google Scholar] [CrossRef]
- Allison, C.; Auyeung, B.; Baron-Cohen, S. Toward Brief “Red Flags” for Autism Screening: The Short Autism Spectrum Quotient and the Short Quantitative Checklist in 1,000 Cases and 3,000 Controls. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 202–212.e7. [Google Scholar] [CrossRef] [PubMed]
- Grove, R.; Hoekstra, R.A.; Wierda, M.; Begeer, S. Exploring sex differences in autistic traits: A factor analytic study of adults with autism. Autism 2017, 21, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, R.A.; Vinkhuyzen, A.A.E.; Wheelwright, S.; Bartels, M.; Boomsma, D.I.; Baron-Cohen, S.; Posthuma, D.; Van Der Sluis, S. The construction and validation of an abridged version of the autism-spectrum quotient (AQ-short). J. Autism Dev. Disord. 2011, 41, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.; Skirrow, P.; Hare, D.J. Asperger through the looking glass: An exploratory study of self-understanding in people with Asperger’s syndrome. J. Autism Dev. Disord. 2012, 42, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.M.; Attwood, T.; Garnett, M.; Stokes, M.A. Am I Autistic? Utility of the Girls Questionnaire for Autism Spectrum Condition as an Autism Assessment in Adult Women. Autism Adulthood 2020, 2, 216–226. [Google Scholar] [CrossRef]
- Mundfrom, D.J.; Shaw, D.G.; Ke, T.L. Minimum Sample Size Recommendations for Conducting Factor Analyses. Int. J. Test. 2005, 5, 159–168. [Google Scholar] [CrossRef]
- Putnick, D.L.; Bornstein, M.H.; Shriver, E.K. Measurement invariance conventions and reporting: The state of the art and future directions for psychological research. Dev. Rev. 2016, 41, 71–90. [Google Scholar] [CrossRef]
- Brugha, T.; Tyrer, F.; Leaver, A.; Lewis, S.; Seaton, S.; Morgan, Z.; Tromans, S.; van Rensburg, K. Testing adults by questionnaire for social and communication disorders, including autism spectrum disorders, in an adult mental health service population. Int. J. Methods Psychiatr. Res. 2020, 29, e1814. [Google Scholar] [CrossRef]
- Ketelaars, C.; Horwitz, E.; Sytema, S.; Bos, J.; Wiersma, D.; Minderaa, R.; Hartman, C.A. Brief report: Adults with mild autism spectrum disorders (ASD): Scores on the autism spectrum quotient (AQ) and comorbid psychopathology. J. Autism Dev. Disord. 2008, 38, 176–180. [Google Scholar] [CrossRef]
- American Psychological Association. Publication Manual of the American Psychological Association, 7th ed.; American Psychological Association: Washington, DC, USA, 2020. [Google Scholar]
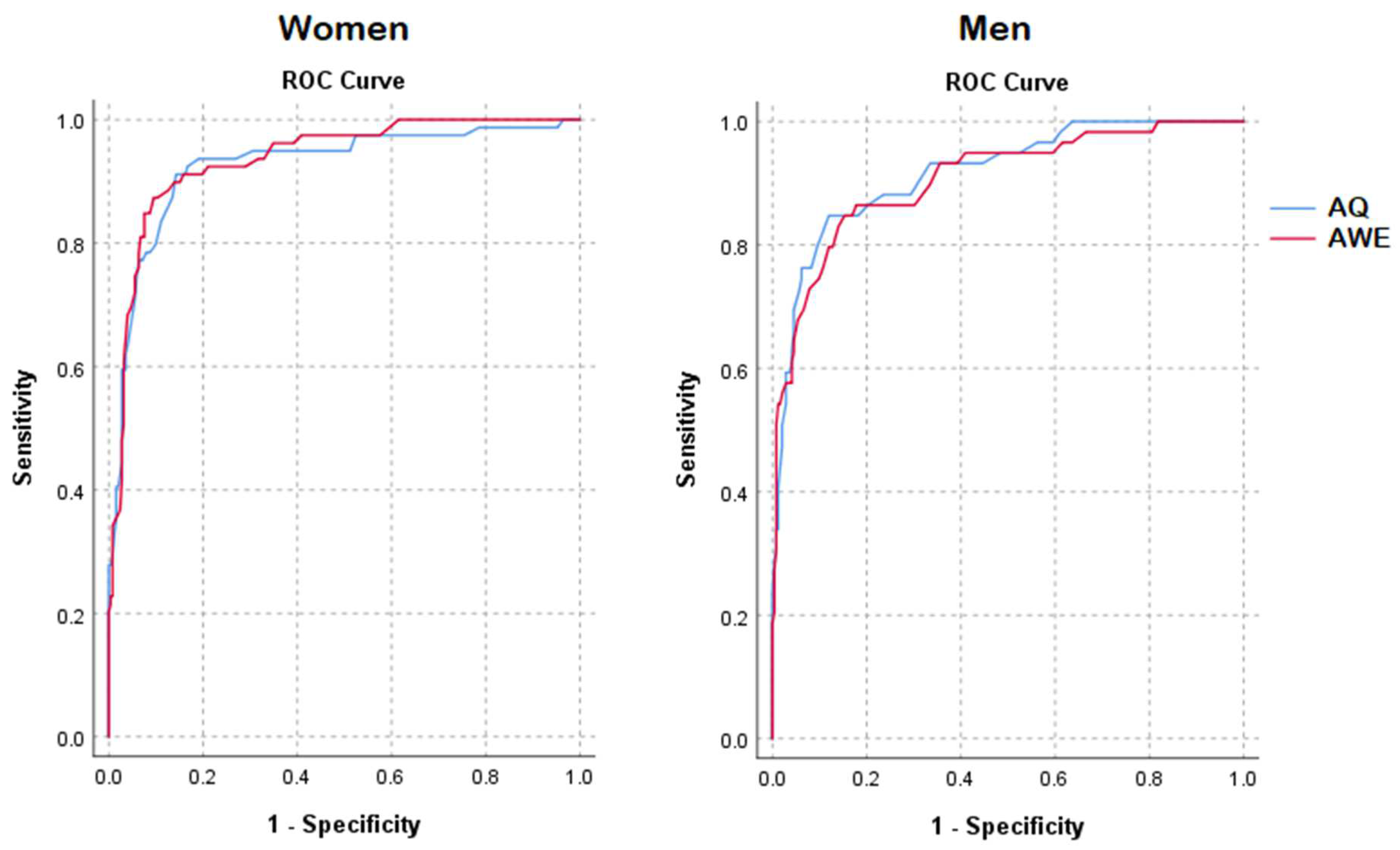
| Women | Men | |||
|---|---|---|---|---|
| GP (n = 238) | ASD (n = 79) | GP (n = 236) | ASD (n = 59) | |
| Age | 37 (SD 15) | 36 (SD 14) | 49 (SD 20) | 42 (SD 14) |
| Age of diagnosis (mean in years) | - | 33 (SD 18) | - | 30 (SD 14) |
| Age of diagnosis (min–max) | 6–59 | 4–63 | ||
| Diagnosed before/after age 18 years | 16/55 (8 missing) | 14/40 (5 missing) | ||
| Living situation | ||||
| Single | 63 (26.5%) | 30 (38.0%) | 59 (25.0%) | 36 (61.0%) |
| Living together | 151 (63.4%) | 29 (36.7%) | 161 (68.2%) | 17 (28.8%) |
| Assisted living | 1 (0.4%) | 4 (5.1%) | 1 (0.4%) | 2 (3.4%) |
| Different | 23 (9.7%) | 16 (20.7%) | 15 (6.4%) | 3 (5.1%) |
| Highest level of education attained | ||||
| Elementary education | 0 | 2 (2.5%) | 3 (1.3%) | 1 (1.7%) |
| Elementary education + other than the following | 2 (0.8%) | 0 | 2 (0.8%) | 1 (1.7%) |
| Junior secondary vocational/general education | 7 (2.9%) | 7 (8.9%) | 15 (6.4%) | 5 (8.5%) |
| Senior secondary vocational/general education or pre-university education | 49 (20.6%) | 23 (29.1%) | 63 (26.7%) | 18 (30.5%) |
| Higher professional education (BSc) | 121 (50.8%) | 39 (49.4%) | 116 (49.2%) | 19 (32.2%) |
| Academic education (MSc) | 55 (23.1%) | 5 (6.3%) | 36 (15.3%) | 6 (10.2%) |
| Other | 4 (1.7%) | 3 (3.8%) | 1 (0.4%) | 8 (13.6%) |
| Educational program | ||||
| No | 164 (68.9%) | 64 (81.0%) | 188 (79.7%) | 55 (93.2%) |
| Full-time | 54 (22.7%) | 14 (17.7%) | 37 (15.7%) | 2 (3.4%) |
| Part-time | 20 (8.4%) | 1 (1.3%) | 11 (4.7%) | 1 (1.7%) |
| Job status | ||||
| No | 38 (16.0%) | 45 (57.0%) | 96 (40.7%) | 38 (64.4%) |
| Full-time | 48 (20.2%) | 8 (10.1%) | 83 (35.2%) | 11 (18.6%) |
| Part-time | 152 (63.9%) | 26 (32.9%) | 57 (24.2%) | 9 (15.3%) |
| Item Numbers a | Domain Labels and Items b | Domain Loadings |
|---|---|---|
| Social functioning and communication (SFC) | ||
| NEW42 | I often think out a social event completely in advance * | 0.819 |
| NEW39 | During a conversation, I am continuously consciously observing how the other person reacts to me * | 0.787 |
| NEW27 | I don’t have to think about how I’m going to act toward others | 0.767 |
| NEW49 | I usually consciously watch others to see what I should say * | 0.724 |
| NEW29 | I get upset when someone else’s mood isn’t good * | 0.702 |
| NEW50 | I often think out a conversation in advance * | 0.672 |
| NEW44 | I become quiet in a conversation with several people * | 0.602 |
| NEW28 | I go to social events when I don’t want to because I’m afraid I won’t belong otherwise * | 0.592 |
| NEW31 | After a conversation, I often think for a long time about what someone actually meant * | 0.573 |
| AQ46 | New situations make me anxious * | 0.554 |
| NEW8 | I usually adapt to the other person in a friendship * | 0.542 |
| AQ26 | I frequently find that I don’t know how to keep a conversation going * | 0.534 |
| NEW34 | I automatically know the best thing to say in a conversation | 0.533 |
| NEW46 | I am satisfied with my relationships with my friends, family, or partner even if they are not perfect | 0.507 |
| NEW2 | I go to social events when I don’t want to because it’s expected of me * | 0.505 |
| AQ33 | When I talk on the phone, I’m not sure when it’s my turn to speak * | 0.482 |
| NEW19 | I feel that I really fit in during a conversation | 0.478 |
| AQ22 | I find it hard to make new friends * | 0.443 |
| NEW17 | I am good at keeping up friendships | 0.386 |
| Initiative and social motivation (ISM) | ||
| AQ44 | I enjoy social occasions | 0.789 |
| NEW4 | Contact with other people gives me energy | 0.781 |
| AQ47 | I enjoy meeting new people | 0.768 |
| NEW35 | People and their behaviour don’t interest me much * | 0.583 |
| AQ13 | I would rather go to a library than a party * | 0.564 |
| AQ15 | I find myself drawn more strongly to people than to things | 0.550 |
| NEW32 | I usually feel a connection quickly when I’m talking to others | 0.539 |
| NEW20 | After a few days of doing nothing, I really have to do something | 0.483 |
| AQ34 | I enjoy doing things spontaneously | 0.473 |
| NEW37 | I feel comfortable in a busy environment with a lot of noise, light, and/or smell | 0.457 |
| NEW33 | I’ve been pretending to be social for years, but I’m actually not * | 0.395 |
| Social Intuition (SI) | ||
| AQ36 | I find it easy to work out what someone is thinking or feeling just by looking at their face | 0.646 |
| AQ27 | I find it easy to “read between the lines” when someone is talking to me | 0.581 |
| AQ45 | I find it difficult to work out people’s intentions * | 0.540 |
| AQ31 | I know how to tell if someone listening to me is getting bored | 0.525 |
| AQ3 | If I try to imagine something, I find it very easy to create a picture in my mind | 0.521 |
| AQ20 | When I’m reading a story, I find it difficult to work out the characters’ intentions * | 0.510 |
| AQ8 | When I’m reading a story, I can easily imagine what the characters might look like | 0.492 |
| AQ42 | I find it difficult to imagine what it would be like to be someone else * | 0.487 |
| AQ35 | I am often the last to understand the point of a joke * | 0.480 |
| Sensing boundaries (SB) | ||
| NEW11 | I am in touch with my body and rest when I need to | 0.717 |
| NEW26 | I notice when I am exhausted or overloaded in time | 0.652 |
| NEW52 | When I’ve done too much, I only feel it afterwards * | 0.519 |
| NEW36 | I’ve been doing more than I can actually handle all my life * | 0.431 |
| NEW9 | I have enough energy to do normal daily activities | 0.411 |
| Attention to detail (AD) | ||
| AQ9 | I am fascinated by dates * | 0.742 |
| AQ19 | I am fascinated by numbers * | 0.719 |
| AQ41 | I like to collect information about categories of things (e.g., types of car, types of bird, types of train, types of plant, etc.) * | 0.448 |
| AQ23 | I notice patterns in things all the time * | 0.424 |
| NEW6 | I know everything about one subject, for example about animals, diets, historical periods or upbringing * | 0.376 |
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1. AWE | - | 0.920 ** | 0.835 ** | 0.714 ** | 0.743 ** | 0.557 ** |
| 2. SFC | - | 0.683 ** | 0.522 ** | 0.662 ** | 0.399 ** | |
| 3. ISM | - | 0.548 ** | 0.536 ** | 0.345 ** | ||
| 4. SI | - | 0.453 ** | 0.339 ** | |||
| 5. SB | - | 0.299 ** | ||||
| 6. AD | - |
| Women | Men | |||
|---|---|---|---|---|
| NT (n = 238) | ASD (n = 79) | NT (n = 236) | ASD (n = 59) | |
| AQ | 104.6 (14.5) | 139.0 (17.6) | 109.0 (13.5) | 138.9 (16.1) |
| AWE | 100.7 (18.6) | 143.7 (17.1) | 102.4 (16.9) | 138.3 (19.5) |
| SFC | 41.7 (8.0) | 58.7 (8.3) | 39.7 (8.4) | 53.5 (9.4) |
| ISM | 20.4 (6.2) | 30.3 (6.1) | 21.5 (5.7) | 29.5 (6.6) |
| SI | 16.0 (4.3) | 22.9 (5.0) | 17.3 (3.9) | 22.9 (4.7) |
| SB | 11.5 (3.4) | 16.3 (2.6) | 10.2 (2.7) | 15.5 (3.0) |
| AD | 11.2 (4.0) | 15.6 (3.6) | 13.7 (3.4) | 16.9 (3.8) |
| GP Women vs. GP Men | ASD Women vs. ASD Men | ASD Women vs. GP Women | ASD Men vs. GP Men | |
|---|---|---|---|---|
| AQ | −0.31 *** | 0.01 ns | 2.13 *** | 2.01 *** |
| AWE | 0.26 ns | 0.29 ns | 2.41 *** | 1.97 *** |
| SFC | 0.24 * | 0.59 *** | 2.09 *** | 1.55 *** |
| ISM | −0.18 * | 0.13 ns | 1.61 *** | 1.3 *** |
| SI | −0.32 *** | 0.00 ns | 1.48 *** | 1.3 *** |
| SB | 0.42 *** | 0.28 ns | 1.26 *** | 1.86 *** |
| AD | −0.67 *** | −0.35 * | 1.16 *** | 0.89 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groen, Y.; Ebert, W.M.; Dittner, F.M.; Stapert, A.F.; Henning, D.; Greaves-Lord, K.; Davids, R.C.D.; Castelein, S.; Baron Cohen, S.; Allison, C.; et al. Measuring the Autistic Women’s Experience (AWE). Int. J. Environ. Res. Public Health 2023, 20, 7148. https://doi.org/10.3390/ijerph20247148
Groen Y, Ebert WM, Dittner FM, Stapert AF, Henning D, Greaves-Lord K, Davids RCD, Castelein S, Baron Cohen S, Allison C, et al. Measuring the Autistic Women’s Experience (AWE). International Journal of Environmental Research and Public Health. 2023; 20(24):7148. https://doi.org/10.3390/ijerph20247148
Chicago/Turabian StyleGroen, Yvonne, W. Miro Ebert, Francien M. Dittner, Anne Fleur Stapert, Daria Henning, Kirstin Greaves-Lord, R. C. D. (Lineke) Davids, Stynke Castelein, Simon Baron Cohen, Carrie Allison, and et al. 2023. "Measuring the Autistic Women’s Experience (AWE)" International Journal of Environmental Research and Public Health 20, no. 24: 7148. https://doi.org/10.3390/ijerph20247148
APA StyleGroen, Y., Ebert, W. M., Dittner, F. M., Stapert, A. F., Henning, D., Greaves-Lord, K., Davids, R. C. D., Castelein, S., Baron Cohen, S., Allison, C., Van Balkom, I. D. C., & Piening, S. (2023). Measuring the Autistic Women’s Experience (AWE). International Journal of Environmental Research and Public Health, 20(24), 7148. https://doi.org/10.3390/ijerph20247148






