Levels of Total Mercury and Health Risk Assessment of Consuming Freshwater Stingrays (Chondrichthyes: Potamotrygoninae) of the Brazilian Amazon
Abstract
:1. Introduction
2. Materials and Methods
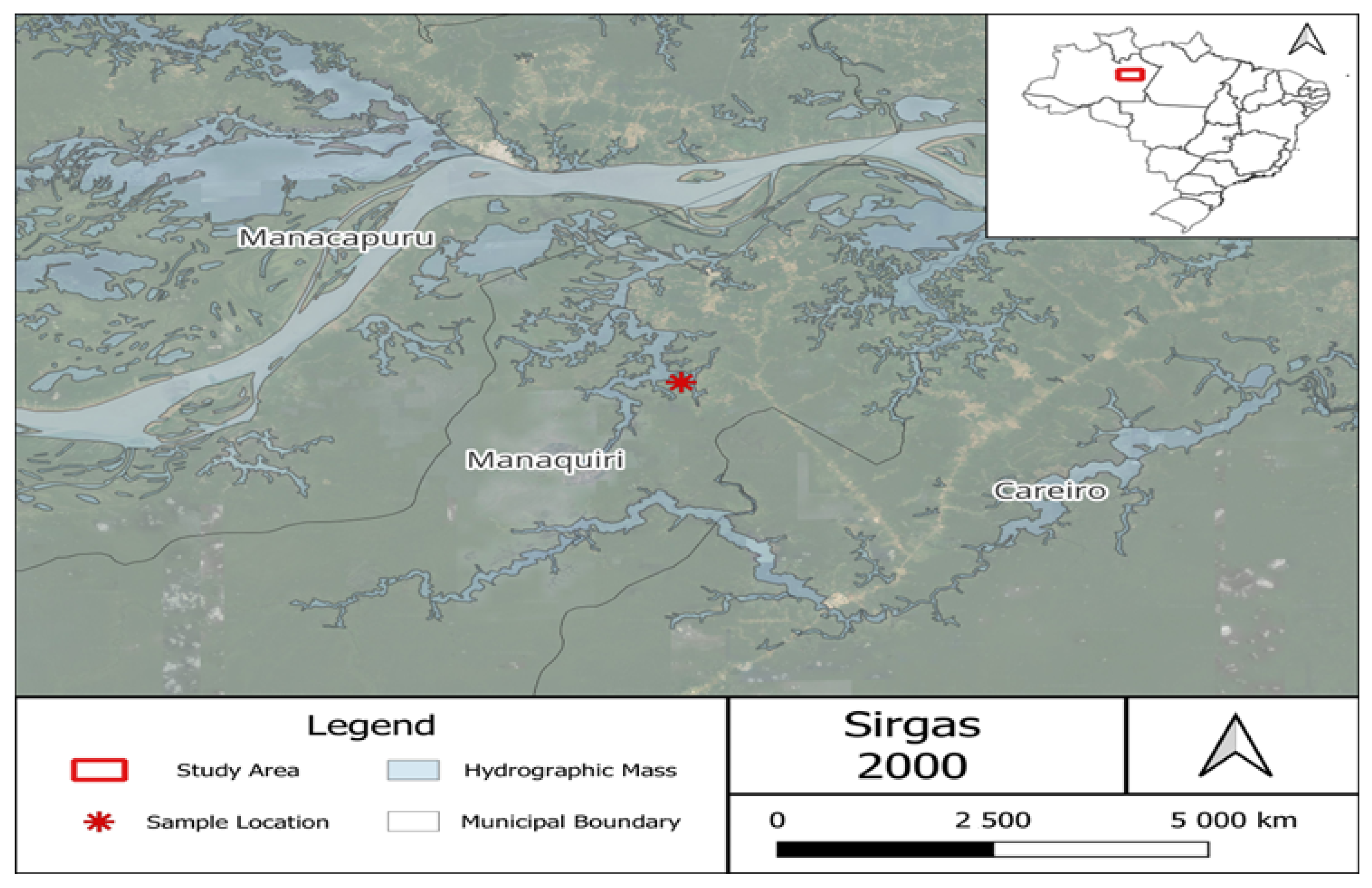
2.1. Study Area
2.2. Capture and Identification of the Animals
2.3. Mercury Quantification (Hg)
2.4. Risk Assessment Calculation
2.5. Water Analysis
2.6. Statistical Analyses
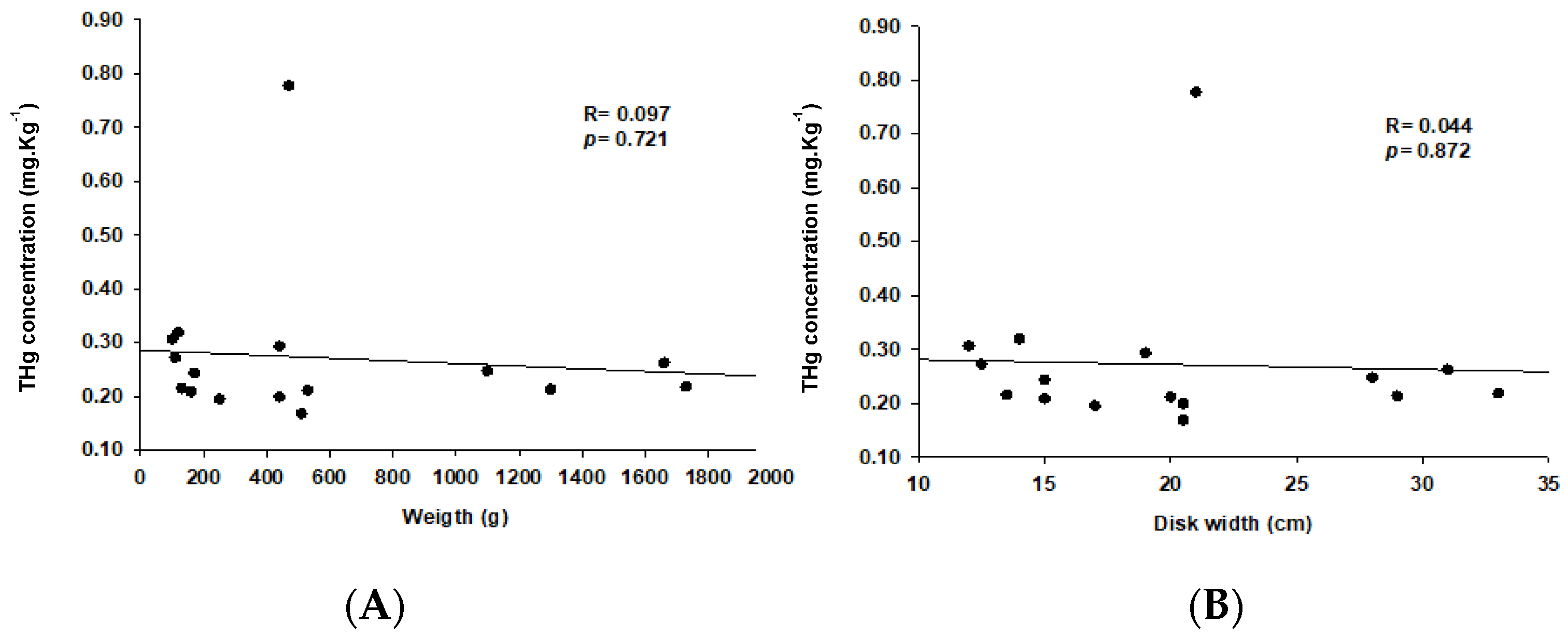
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caicedo-Rivas, G.; Salas-Moreno, M.; Marrugo-Negrete, J. Health Risk Assessment for Human Exposure to Heavy Metals via Food Consumption in Inhabitants of Middle Basin of the Atrato River in the Colombian Pacific. Int. J. Environ. Res. Public Health 2023, 20, 435. [Google Scholar] [CrossRef] [PubMed]
- Bello, T.C.S.; Buralli, R.J.; Cunha, M.P.L.; Dórea, J.G.; Diaz-Quijano, F.A.; Guimarães, J.R.D.; Marques, R.C. Mercury Exposure in Women of Reproductive Age in Rondônia State, Amazon Region, Brazil. Int. J. Environ. Res. Public Health 2023, 20, 5225. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.A.; Ferrari, R.G.; Alves, R.; Kaic, D.; Hauser-Davis, R.A.; Neves, S.L.; Conte-Junior, C.A. Mercurial contamination: A consumer health risk assessment concerning seafood from a eutrophic estuary in Southeastern Brazil. Front. Mar. Sci. 2022, 9, 765323. [Google Scholar] [CrossRef]
- Mussy, M.H.; Almeida, R.; Carvalho, D.P.; Lautgartte, L.C.; Holanda, I.B.B.; Almeida, M.G.; Souza-Filho, I.F.; Rezende, C.E.; Mal, O.; Bastos, W.R. Evaluating total mercury and methylmercury biomagnification using stable isotopes of carbon and nitrogen in fish from the Madeira River basin, Brazilian Amazon. Environ. Sci. Pollut. Res. 2023, 30, 33543–33554. [Google Scholar] [CrossRef]
- Azevedo, L.S.; Pestana, I.A.; Nascimento, L.; Oliveira, R.C.; Bastos, W.R.; Di Benedetto, A.P.M. Risk of exposure to Hg and pesticides residues in a traditional fishing community in the Amazon: A probabilistic approach based on the dietary pattern. Environ. Sci. Pollut. Res. 2022, 29, 34164–34173. [Google Scholar] [CrossRef]
- Beltran-Pedreros, S.; Zuanon, J.; Leite, R.G.; Peleja, J.R.P.; Mendonça, A.B.; Forsberg, B.R. Mercury bioaccumulation in fish of commercial importance from different trophic categories in an Amazon floodplain lake. Neotr. Ichth. 2011, 9, 901–908. [Google Scholar] [CrossRef]
- Harayashiki, C.A.Y.; Reichelt-Brushett, A.; Cowden, K.; Benkendorff, K. Effects of Oral Exposure to Inorganic Mercury on the Feeding Behavior and Biochemical Markers in Yellowfin Bream (Acanthopagrus australis). Mar. Environ. Res. 2018, 134, 1–15. [Google Scholar] [CrossRef]
- Villen-Perez, S.; Anaya, L.F.; Cruz, D.C.; Fearnside, P.M. Mining threatens isolated indigenous peoples in the Brazilian Amazon. Global Environ. Change Hum. Policy Dimens. 2022, 72, 102398. [Google Scholar] [CrossRef]
- Meadows-Oliver, M. Environmental Toxicants: Lead and Mercury. J. Pediatric. Health Care 2012, 26, 213–215. [Google Scholar] [CrossRef]
- Hacon, S.S.; Dorea, J.G.; Fonseca, M.F.; Oliveira, B.A.; Mourão, D.S.; Ruiz, C.M.V.; Bastos, W.R. The influence of changes in lifestyle and mercury exposure in riverine populations of the Madeira river (Amazon basin) near a hydroelectric project. Intern. J. Environ. Res. Public Health 2014, 11, 2437–2455. [Google Scholar] [CrossRef]
- Sirén, A.; Valbo-Jørgensen, J. Quantifying fish catches and fish consumption in the Amazon Basin. Aquat. Ecosyst. Health Manag. 2022, 25, 59–71. [Google Scholar] [CrossRef]
- Santos, E.L.Q.V. A Pesca Comercial de Raias para Consumo Alimentar no Eixo Fluvial Solimões-Amazonas; Dissertação de Mestrado do Programa de Pós Graduação em Ciência Animal e Recursos Pesqueiros; Universidade Federal do Amazonas: Manaus, Brazil, 2022; 47p. [Google Scholar]
- Araújo, M.L. Plano de Monitoramento de Arraias de Água Doce–Rio Negro, Estado do Amazonas; Relatório Final do IBAMA; IBAMA: Manaus, Brazil, 2005; 100p. [Google Scholar]
- Duncan, W.P.; Inomata, S.O.; Fernandes, M.N. Comércio de raias de água doce na Região do Médio Rio Negro, estado do Amazonas, Brasil. Rev. Brasil. Eng. Pesca 2010, 5, XIII–XXII. [Google Scholar]
- Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA). Instrução Normativa; N 204; IBAMA: Manaus, Brazil, 2008.
- Andrade, J.C.; Oliveira, A.T.; Amazonas, M.G.F.M.; Galvan, D.; Tessaro, L.; Conte-Junior, C.A. Fingerprinting based on spectral reflectance and chemometrics—An analytical approach aimed at combating the illegal trade of stingray meat in the Amazon. Food Chem. 2023, 436, 137637. [Google Scholar] [CrossRef] [PubMed]
- Melack, J.H.; Kasper, D.; Amaral, J.H.F.; Barbosa, P.M.; Forsber, B.R. Limnological perspectives on conservation of floodplain lakes in the Amazon basin. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 1041–1055. [Google Scholar] [CrossRef]
- LasPso, C.A.; Rosa, R.S.; Sánchez-Duarte, P.; Morales-Betancourt, M.A.; Agudelo-Córdoba, E.; Lasso, C.A.; Rosa, R.S.; Sánchez-Duarte, P.; Morales-Betancourt, M.A.; Agudelo-Córdoba, E. Rayas de Agua Dulce (Potamotrygonidae) de Suramérica. Parte I. Colombia, Venezuela, Ecuador, Perú, Brasil, Guyana, Surinam y Guayana Francesa: Diversidad, Bioecología, Uso y Conservación; Serie Recursos Hidrobiológicos y Pesqueros Continentales de Colombia, IX; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2013; 368p. [Google Scholar]
- Oliveira, A.T.; Santos, M.Q.C.; Araujo, M.L.G.; Lemos, J.R.G.; Sales, R.S.A.; Pantoja-Lima, J.; Tavares-Dias, M.; Marcon, J.L. Hematological parameters of three freshwater stingray species (Chondrichthyes: Potamotrygonidae) in the middle Rio Negro, Amazonas state. Biochem. Syst. Ecol. 2016, 69, 33–40. [Google Scholar] [CrossRef]
- Oliveira, A.T.; Lemos, J.R.G.; Santos, M.Q.C.; Sales, R.S.A.; Pantoja-Lima, J.; Aride, P.H.R.; Araujo, M.L.G.; Tavares-Dias, M. Morphological, cytochemical and ultrastructural aspects of blood cells in freshwater stingray species in the middle Rio Negro basin of Amazonian Brazil. Sci. Rep. 2021, 11, 15685. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (US-EPA). Guidance for Assessing Chemical Contamination Data for Use in Fish Advisories. In Risk Assessment and Fish Consumption Limits EPA/823-B94-004; United States Environmental Protection Agency: Washington DC, USA, 2000; Volume II. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Evaluations of Certain Contaminants in Food: Seventy-Second Report of the Joint FAO/WHO Expert Committee on Food Additives; Food and Agriculture Organization/WHO: Rome, Italy, 2011; 115p. [Google Scholar]
- Instituto Brasileiro de Geografıa e Estatística (IBGE). Pesquisa de Orçamentos Familiares 2017–2018 em Análise do Consumo Alimentar Pessoal No Brasil; IBGE, Coordenação de Trabalho e Rendimento: Rio de Janeiro, Brazil, 2020; 147p.
- Targino, F.J.; Ribeiro, J.D.d.N.; Simões, J.S.; Carneiro, C.S.; Lazzarini, S.M.; Souza, A.R.; Ferreira, M.d.S.; Mano, S.B.; Mársico, E.T. Total Mercury Content in the Tissues of Freshwater Chelonium (Podocnemis expansa) and a Human Health Risk Assessment for the Amazon Population in Brazil. Int. J. Environ. Res. Public Health 2023, 20, 6489. [Google Scholar] [CrossRef]
- Kasper, D.; Forsberg, B.R.; Amaral, J.H.F.; Py-Daniel, S.S.; Bastos, W.R.; Malm, O. Methylmercury Modulation in Amazon Rivers Linked to Basin Characteristics and Seasonal Flood-Pulse. Environ. Sci. Technol. 2017, 51, 14182–14191. [Google Scholar] [CrossRef]
- Azevedo-Silva, C.E.; Almeida, R.; Carvalho, D.P.; Ometto, J.P.H.B.; Camargo, P.B.; Dorneles, P.R.; Azeredo, A.; Bastos, W.R.; Malm, O.; Torres, J.P.M. Mercury biomagnification and the trophic structure of the ichthyofauna from a remote lake in the Brazilian Amazon. Environ. Res. 2016, 151, 286–296. [Google Scholar] [CrossRef]
- Shibuya, A. A review of the ecological role of the Neotropical freshwater stingrays (Chondrichthyes: Potamotrygoninae). Food Webs 2022, 32, e00244. [Google Scholar] [CrossRef]
- Liebl, A.R.S.; Cao, M.A.; Nascimento, M.S.; Castro, P.D.S.; Duncan, W.L.P.; Pantoja-Lima, J.; Aride, P.H.R.; Bussons, M.R.F.M.; Furuya, W.M.; Faggio, C.; et al. Dietary lysine requirements of Colossoma macropomum (Cuvier 1818) based on growth performance, hepatic and intestinal morphophysiology and hematology. Vet. Res. Commun. 2022, 46, 1–17. [Google Scholar] [CrossRef]
- Amazonas, M.A.; Olentino, D.; Furtado, C.L.C.; Yamamoto, K.C.; Duncan, W.L.P. Length-weight relationship for Potamotrygon wallacei (Carvalho, Rosa and Araújo, 2016) caught in the middle Negro River, Barcelos, Brazilian Amazon. Braz. J. Biol. 2022, 84, e253497. [Google Scholar] [CrossRef] [PubMed]
- Taffer, A.K.; Staudinger, M.D.; Taylor, D.L.; Juanes, F. Trophic influences on mercury accumulation in top pelagic predators from offshore New England waters of the northwest Atlantic Ocean. Mar. Environ. Res. 2014, 101, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Bastos, W.R.; Dórea, J.G.; Bernardi, J.V.E.; Lauthartte, L.C.; Mussy, M.H.; Lacerda, L.D.; Malm, O. Mercury in fish of the Madeira river (temporal and spatial assessment), Brazilian Amazon. Environ. Res. 2015, 140, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Hedeholm, R.B.; Heinemeier, J.; Bushnell, P.G.; Christiansen, J.S.; Olsen, J.; Ramsey, C.B.; Brill, R.W.; Simon, M.; Steffensen, K.F.; et al. Eye lens radiocarbon reveals centuries of longevity in the Greenland shark (Somniosus microcephalus). Science 2016, 353, 702–704. [Google Scholar] [CrossRef]
- Graham, R.T.; Witt, M.J.; Castellanos, D.W.; Remolina, F.; Maxwell, S.; Godley, B.J.; Hawkes, L.A. Satellite tracking of manta rays highlights challenges to their conservation. PLoS ONE 2012, 7, e36834. [Google Scholar] [CrossRef]
- Alves, L.M.F.; Marco, F.L.; Lemos, H.C.; Sara, C.N. Elasmobranchs as bioindicators of pollution in the marine environment. Mar. Pollut. Bull. 2022, 176, 113418. [Google Scholar] [CrossRef]
- Achá, D.; Pabón, C.A.; Hintelmann, H. Mercury methylation and hydrogen sulfide production among unexpected strains isolated from periphyton of two macrophytes of the Amazon. FEMS Microb. Ecol. 2012, 80, 637–645. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária (ANVISA), Ministério da Saúde. RDC N° 42, 29 de Agosto de 2013. In Dispõe sobre o Regulamento Técnico Mercosul sobre Limites Máximos de Contaminantes Inorgânicos em Alimentos; Diário Oficial da República Federativa do Brasil: Brasılia, DF, Brazil.
- Passos, C.J.; Mergler, D. Human mercury exposure and adverse health effects in the Amazon: A review. Cad. Saúde Pública 2008, 24, s503–s520. [Google Scholar] [CrossRef]
- Souza-Araujo, J.; Giarrizzo, T.; Lima, M.O.; Souza, M.B.G. Mercury and methyl mercury in fishes from Bacajá River (Brazilian Amazon): Evidence for bioaccumulation and biomagnification. J. Fish Biol. 2016, 89, 249–263. [Google Scholar] [CrossRef]
- Lino, A.S.; Kasper, D.; Guida, Y.S.; Thomaz, J.R.; Malm, O. Mercury and selenium in fishes from the Tapajós River in the Brazilian Amazon: An evaluation of human exposure. J. Trace Elem. Med. Biol. 2018, 48, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, A.C.S.D.; Hallwass, G.; Bezerra, J.G.; Aciole, A.N.S.; Meneses, H.N.D.M.; Lima, M.D.O.; Jesus, I.M.D.; Hacon, S.D.S.; Basta, P.C. Health Risk Assessment of Mercury Exposure from Fish Consumption in Munduruku Indigenous Communities in the Brazilian Amazon. Int. J. Environ. Res. Public Health 2021, 18, 7940. [Google Scholar] [CrossRef]
- Vasconcellos, A.C.S.; Ferreira, S.R.B.; De Sousa, C.C.; De Oliveira, M.W.; Oliveira Lima, M.; Basta, P.C. Health Risk Assessment Attributed to Consumption of Fish Contaminated with Mercury in the Rio Branco Basin, Roraima, Amazon, Brazil. Toxics 2022, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Barai, A.A.; Souza, A.F.L.; Viana, A.P.; Inhamuns, A.J. Seasonal influence on centesimal composition and yield of Amazonian fish. Ciência Tecnol. Aliment. 2021, 42, 1–5. [Google Scholar] [CrossRef]
- Tiktak, G.P.; Butcherb, D.; Lawrence, P.J.; Norreya, J.; Bradley, L.; Shawa, K.; Preziosi, R.; Megsona, D. Are concentrations of pollutants in sharks, rays, and skates (Elasmobranchii) a cause for concern? A systematic review. Mar. Pollut. Bull. 2020, 160, 111701. [Google Scholar] [CrossRef]
- Alcala-Orozco, M.; Caballero-Gallardo, K.; Olivero-Verbel, J. Biomonitoring of Mercury, Cadmium, and Selenium in Fish and the Population of Puerto Nariño, at the Southern Corner of the Colombian Amazon. Arch. Environ. Contam. Toxicol. 2020, 79, 354–370. [Google Scholar] [CrossRef]
- Olivero-Verbel, J.; Carranza-Lopez, L.; Caballero-Gallardo, K.; Ripoll-Arboleda, A.; Muñoz-Sosa, D. Human exposure and risk assessment associated with mercury pollution in the Caqueta River, Colombian Amazon. Environ. Sci. Pollut. Res. 2016, 23, 1–11. [Google Scholar] [CrossRef]
- Silva, M.S.; Ríos-Villamizar, E.A.; Miranda, S.A.F.; Ferreira, S.J.F.; Bringel, S.R.B.; Gomes, N.A.; Silva, L.M.; Pascoaloto, D.; Santana, G.P.; Cunha, H.B. Contribution to the hydrochemistry and water typology of the Amazon river and its tributaries. Geogr. Path 2019, 20, 360–374. [Google Scholar] [CrossRef]

| Developmental Stage | Disc Width (DW, cm) | Weight (g) |
|---|---|---|
| Neonates | 12.50 ± 0.91 | 115.00 ± 12.91 |
| Young | 22.42 ± 6.23 | 673.85 ± 565.67 |
| Species | EMI (mg·kg−1 Month) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Children | Young | Adult | ||||||||||
| Male | Female | Male | Female | Male | Female | |||||||
| U | R | U | R | U | R | U | R | U | R | U | R | |
| Potamotrygon motoro | 0.0221 | 0.0777 | 0.0201 | 0.0710 | 0.0129 | 0.0453 | 0.0157 | 0.0553 | 0.0119 | 0.0419 | 0.0140 | 0.0495 |
| Species | IRmm (kg·month−1) | THg (mg·kg−1 w·w) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Children | Young | Adult | |||||||||||
| Male | Female | Male | Female | Male | Female | ||||||||
| U | R | U | R | U | R | U | R | U | R | U | R | ||
| Potamotrygon motoro | 2.63 | 2.88 | 4.51 | 3.69 | 4.88 | 4.13 | 0.272 ± 0.054 | ||||||
| Species | HQ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Children | Young | Adults | ||||||||||
| Male | Female | Male | Female | Male | Female | |||||||
| U | R | U | R | U | R | U | R | U | R | U | R | |
| Potamotrygon motoro | 55.1 | 194.3 | 50.3 | 177.4 | 32.2 | 113.4 | 39.2 | 138.3 | 29.7 | 104.6 | 35.1 | 123.7 |
| Variable | Value |
|---|---|
| Dissolved oxygen (mg/L) | 3.90 ± 0.72 |
| pH | 6.29 ± 0.15 |
| Temperature (°C) | 30.58 ± 1.86 |
| Transparency (m) | 1.80 ± 0.15 |
| Alkalinity (mg/L) | 47.50 ± 9.57 |
| Hardness (mg/L) | 35.0 ± 5.77 |
| Nitrite (mg/L) | 0.01 ± 0.01 |
| Nitrate (mg/L) | 0.10 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, A.T.d.; Rodrigues, P.d.A.; Ramos Filho, A.M.; Gomes, M.F.d.S.; Liebl, A.R.d.S.; de Pinho, J.V.; Aride, P.H.R.; Conte-Junior, C.A. Levels of Total Mercury and Health Risk Assessment of Consuming Freshwater Stingrays (Chondrichthyes: Potamotrygoninae) of the Brazilian Amazon. Int. J. Environ. Res. Public Health 2023, 20, 6990. https://doi.org/10.3390/ijerph20216990
Oliveira ATd, Rodrigues PdA, Ramos Filho AM, Gomes MFdS, Liebl ARdS, de Pinho JV, Aride PHR, Conte-Junior CA. Levels of Total Mercury and Health Risk Assessment of Consuming Freshwater Stingrays (Chondrichthyes: Potamotrygoninae) of the Brazilian Amazon. International Journal of Environmental Research and Public Health. 2023; 20(21):6990. https://doi.org/10.3390/ijerph20216990
Chicago/Turabian StyleOliveira, Adriano Teixeira de, Paloma de Almeida Rodrigues, Alexandre Mendes Ramos Filho, Maria Fernanda da Silva Gomes, Ariany Rabello da Silva Liebl, Júlia Vianna de Pinho, Paulo Henrique Rocha Aride, and Carlos Adam Conte-Junior. 2023. "Levels of Total Mercury and Health Risk Assessment of Consuming Freshwater Stingrays (Chondrichthyes: Potamotrygoninae) of the Brazilian Amazon" International Journal of Environmental Research and Public Health 20, no. 21: 6990. https://doi.org/10.3390/ijerph20216990
APA StyleOliveira, A. T. d., Rodrigues, P. d. A., Ramos Filho, A. M., Gomes, M. F. d. S., Liebl, A. R. d. S., de Pinho, J. V., Aride, P. H. R., & Conte-Junior, C. A. (2023). Levels of Total Mercury and Health Risk Assessment of Consuming Freshwater Stingrays (Chondrichthyes: Potamotrygoninae) of the Brazilian Amazon. International Journal of Environmental Research and Public Health, 20(21), 6990. https://doi.org/10.3390/ijerph20216990







