Childbearing with Hypermobile Ehlers–Danlos Syndrome and Hypermobility Spectrum Disorders: A Large International Survey of Outcomes and Complications
Abstract
:1. Introduction
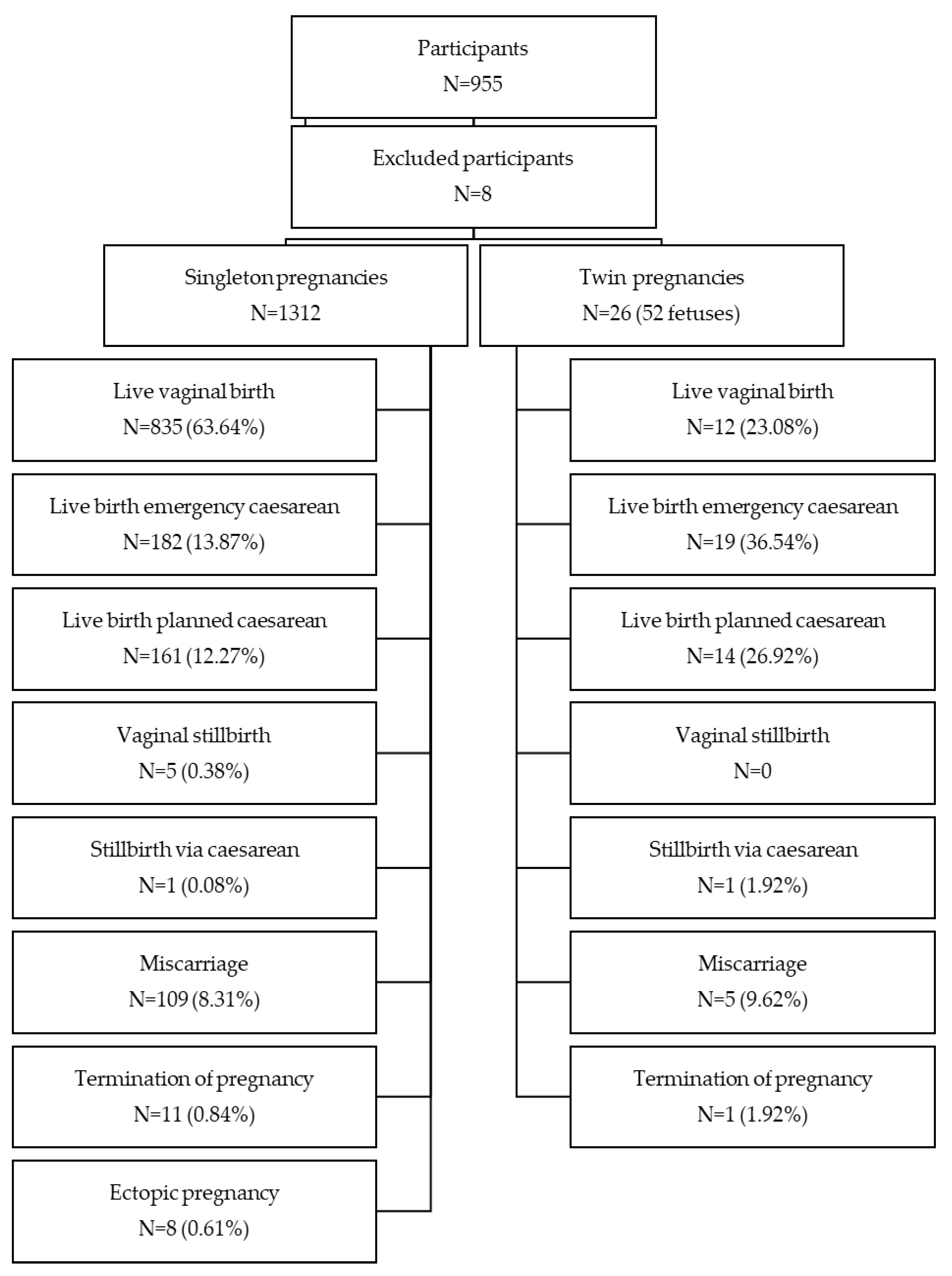
2. Materials and Methods
3. Results
Pregnancy and Birth Outcomes and Complications
| Complications/Outcomes | Pregnancies in Third Trimester (N = 1209) 1 | Percentage of Pregnancies (95% Confidence Intervals) | Published Population Incidence |
|---|---|---|---|
| Hyperemesis Gravidarum | 308 | 25.48% (23.04–28.03) | 3% [46] |
| Antepartum Haemorrhage | 104 | 8.60% (7.08–10.33) | 3–5% [47] |
| Pre-term Rupture of Membranes | 85 | 7.03% (5.65–8.62) | <3% [48] |
| Pre-eclampsia | 115 | 9.51% (7.92–11.31) | 2.4% [49] |
| Eclampsia | 17 | 1.41% (0.82–2.24) | 0.027% [50] |
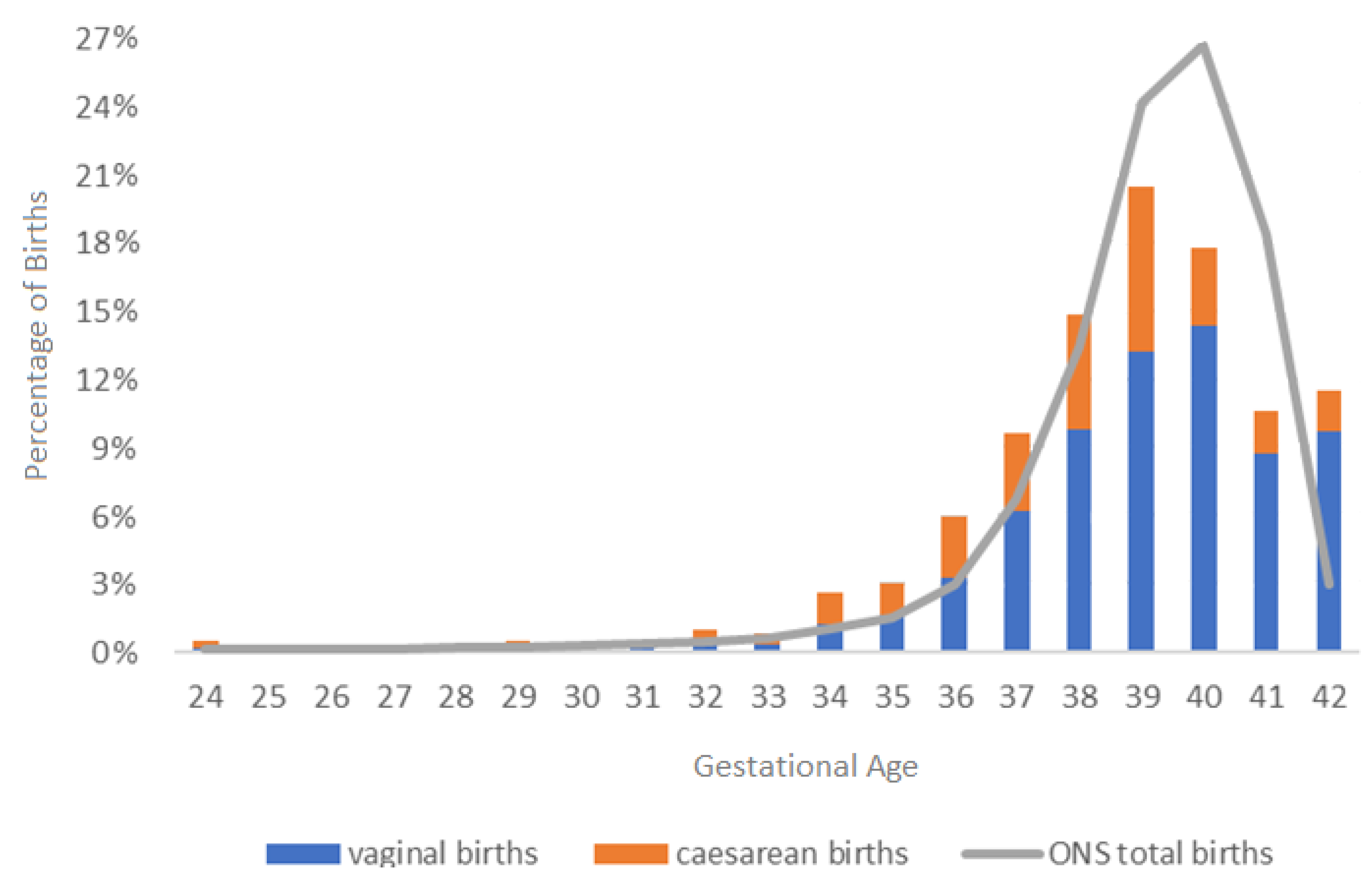
| Preterm birth < 37 weeks (singleton) | 155/1185 | 13.08% (11.21–15.13) | 6.2% [51] |
| Preterm birth < 37 weeks (twin) | 35/46 | 76.09% (61.23–87.41) | 50% [51] |
| Preterm birth < 37 weeks (all) | 190/1230 | 15.45% (13.47–17.59) | 7.8% [51] |
| Stillbirth ≥ 24 weeks (singleton) | 6/1185 | 0.51% (0.19–1.10) | 0.4% [51] |
| Stillbirth ≥ 24 weeks (twin) | 1/46 | 2.1% (0.05–11.5) | 2.3% [51] |
| Stillbirth ≥ 24 weeks (all) | 7/1230 | 0.57% (0.23–1.17) | 0.48% [51] |
| Abnormal foetal presentation | 338 | 27.96% (25.44–30.58) | N/A |
| Precipitate labour (<3 h) | 357/1041 | 34.29% (31.41–37.27) | 0.07–14% [39,40] |
| Born before arrival at intended place of birth | 23 | 1.90% (1.21–2.84) | 0.4% [52] |
| Caesarean section | 363 | 30.02% (27.45–32.70) | 27.6% [53] |
| Shoulder dystocia | 52/847 | 6.14% (4.62–7.97) | 0.18% [54] |
| 1st degree perineal tear | 170/847 | 20.07% (17.42–22.93) | N/A |
| 2nd degree perineal tear | 303/847 | 35.77% (32.54–39.11) | N/A |
| 3rd degree perineal tear | 72/847 | 8.50% (6.71–10.59) | N/A |
| 4th degree perineal tear | 17/847 | 2.01% (1.17–3.19) | N/A |
| Intrapartum haemorrhage | 173 | 14.31% (12.38–16.41) | N/A |
| Postpartum haemorrhage | 249 | 20.60% (18.35–22.99) | 6.38% [55] |
| Ineffective pain relief | 517 | 42.76% (39.95–45.61) | N/A |
| Ineffective epidural | 284 | 23.49% (21.13–25.98) | 8–23% [56] |
| Ineffective local injection | 192 | 15.88% (13.86–18.07) | N/A |
| Caesarean wound infection | 87/363 | 23.97% (19.67–28.70) | 3–15% [57] |
| Vaginal wound infection | 61/847 | 7.20% (5.55–9.16) | N/A |
| Wound dehiscence | 132 | 10.92% (9.22–12.81) | N/A |
| Slow healing | 487 | 40.28% (37.50–43.11) | N/A |
| Pelvic organ prolapse | 149 | 12.32% (10.52–14.31) | N/A |
| Postpartum psychosis | 57 | 4.71% (3.59–6.07) | 0.089–0.26% [58] |
| Post Traumatic Stress Disorder | 227 | 18.78% (16.61–21.09) | 4% [59] |
| Other | 87 | 7.20% (5.80–8.80) | N/A |
| None | 71 | 5.87% (4.61–7.35) | N/A |
- (1)
- Joints moving out of place and pain: “hip dislocation in labour”, “sporadic pelvic displacement, wheelchair from 5 months until 2 weeks after”, “widespread post-partum joint pain and weakness”, “hip subluxation from stirrups (wasn’t aware of hypermobility)”, “severe looseness of shoulders and hips”, “pain, pain, pain”, “pelvic separation leading to bad post-partum pain”, “abdominal divarication, torn ligaments, stretched ligaments, pubis symphysis”; “Pubic Bone Separated at 20 weeks gestation”, “subluxed hips”, “costochondritis as baby moved from under ribs—misdiagnosed as indigestion at the time (EDS not diagnosed at that time)”, “pelvic displacement”, “SI [sacroiliac] joint issues, headaches” and “induced labour caused hip dislocations”.
- (2)
- Issues from epidural anaesthesia: “CSF [cerebral spinal fluid] leak”, “post-dural puncture headache from a ‘technically difficult’ epidural (x3) requiring a blood patch [sic]”, “CSF leak from epidural and have continued pain there since”, “extreme hypotension from epidural”; “hypotension with epidural. Prolonged effects of epidural”, and three people said “broken” or “dislocated coccyx”.
- (3)
- Bruising, tearing and poor wound healing: “very significant bruising”, “bruising on the baby from fast birth”, “site of vaginal tear never fully healed”, “previous Caesarean scar ruptured during this labour”; “polyps in birth canal from tearing”, “torn hip labrum from long labour” and “bilateral femoral nerve damage and bilateral hip labral tears”.
- (4)
- Blood pressure and syncope issues: “blood pressure dropped severely (PoTS) [Postural (orthostatic) Tachycardia Syndrome]”, “tachycardia”, “syncope several hours following birth”, “low blood pressure” and “high blood pressure after delivery”.
- (5)
- Infections and unexplained allergic reactions: “unexplained anaphylaxis 7 days and 8 days postpartum respectively [sic]”, “chorioamnionitis”, “systemic infection”, “baby born with group B strep”, “infection due to time between waters breaking and giving birth”, “bladder infection”, and four people said “uterine infection”.
4. Discussion
Strengths, Limitations and Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malfait, F.; Francomano, C.; Byers, P.; Belmont, J.; Berglund, B.; Black, J.; Bloom, L.; Bowen, J.M.; Brady, A.F.; Burrows, N.P.; et al. The 2017 international classification of the Ehlers–Danlos syndromes. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, P.R.; Xu, Z.; Tumelty, K.E.; Zhao, R.W.; Monis, W.J.; Harris, K.G.; Gass, J.M.; Cousin, M.A.; Boczek, N.J.; Mitkov, M.V.; et al. Bi-allelic Alterations in AEBP1 Lead to Defective Collagen Assembly and Connective Tissue Structure Resulting in a Variant of Ehlers-Danlos Syndrome. Am. J. Hum. Genet. 2018, 102, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Pezaro, S.; Pearce, G.; Reinhold, E. A clinical update on hypermobile Ehlers-Danlos syndrome during pregnancy, birth and beyond. Br. J. Midwifery 2021, 29, 492–500. [Google Scholar] [CrossRef]
- Tinkle, B.; Castori, M.; Berglund, B.; Cohen, H.; Grahame, R.; Kazkaz, H.; Levy, H. Hypermobile Ehlers–Danlos syndrome (aka Ehlers–Danlos syndrome Type III and Ehlers–Danlos syndrome hypermobility type): Clinical description and natural history. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017, 175, 48–69. [Google Scholar] [CrossRef]
- Demmler, J.C.; Atkinson, M.D.; Reinhold, E.J.; Choy, E.; Lyons, R.A.; Brophy, S.T. Diagnosed prevalence of Ehlers-Danlos syndrome and hypermobility spectrum disorder in Wales, UK: A national electronic cohort study and case–control comparison. BMJ Open 2019, 9, e031365. [Google Scholar] [CrossRef]
- Norris Lab. Ehlers-Danlos Syndrome. Available online: https://www.thenorrislab.com/research/ehlers-danlos-syndrome (accessed on 18 October 2023).
- Tofts, L.J.; Simmonds, J.; Schwartz, S.B.; Richheimer, R.M.; O’connor, C.; Elias, E.; Engelbert, R.; Cleary, K.; Tinkle, B.T.; Kline, A.D.; et al. Pediatric joint hypermobility: A diagnostic framework and narrative review. Orphanet J. Rare Dis. 2023, 18, 104. [Google Scholar] [CrossRef]
- Eccles, J.A.; Thompson, B.; Themelis, K.; Amato, M.L.; Stocks, R.; Pound, A.; Jones, A.-M.; Cipinova, Z.; Shah-Goodwin, L.; Timeyin, J.; et al. Beyond bones: The relevance of variants of connective tissue (hypermobility) to fibromyalgia, ME/CFS and controversies surrounding diagnostic classification: An observational study. Clin. Med. 2021, 21, 53–58. [Google Scholar] [CrossRef]
- Copetti, M.; Morlino, S.; Colombi, M.; Grammatico, P.; Fontana, A.; Castori, M. Severity classes in adults with hypermobile Ehlers-Danlos syndrome/hypermobility spectrum disorders: A pilot study of 105 Italian patients. Rheumatology 2019, 58, 1722–1730. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Selected Heritable Disorders of Connective Tissue and Disability; Wedge, R.A.; Cartaxo, T.; Spicer, C.M. Ehlers-Danlos Syndromes and Hypermobility Spectrum Disorders. In Selected Heritable Disorders of Connective Tissue and Disability; National Academies Press: Washington, DC, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK584966/ (accessed on 18 October 2023).
- Peterson, B.; Coda, A.; Pacey, V.; Hawke, F. Physical and mechanical therapies for lower limb symptoms in children with Hypermobility Spectrum Disorder and Hypermobile Ehlers-Danlos Syndrome: A systematic review. J. Foot Ankle Res. 2018, 11, 59. [Google Scholar] [CrossRef]
- Tinkle, B.T.; Bird, H.A.; Grahame, R.; Lavallee, M.; Levy, H.P.; Sillence, D. The lack of clinical distinction between the hypermobility type of Ehlers-Danlos syndrome and the joint hypermobility syndrome (a.k.a. hypermobility syndrome). Am. J. Med. Genet. A 2009, 149A, 2368–2370. [Google Scholar] [CrossRef]
- Beighton, P.; De Paepe, A.; Danks, D.; Finidori, G.; Gedde-Dahl, T.; Goodman, R.; Hall, J.G.; Hollister, D.W.; Horton, W.; McKusick, V.A.; et al. International nosology of heritable disorders of connective tissue, Berlin, 1986. Am. J. Med. Genet. 1988, 29, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Beighton, P.; De Paepe, A.; Steinmann, B.; Tsipouras, P.; Wenstrup, R.J. Ehlers-Danlos syndromes: Revised nosology, Villefranche, 1997. Am. J. Med. Genet. 1998, 77, 31–37. [Google Scholar] [CrossRef]
- Grahame, R.; Bird, H.A.; Child, A. The revised criteria for the diagnosis of benign joint hypermobility syndrome (BJHS). J. Rheumatol. 2000, 27, 1585. [Google Scholar]
- Powell Davies, J. In Hypermobile Ehlers-Danlos Syndromes and Hypermobile Spectrum Disorders (hEDS/HSD) in Northumberland; NHS North of England Commissioning Support Unit: Durham, UK, 2023; internal report; unpublished (available on request). [Google Scholar]
- Cederlöf, M.; Larsson, H.; Lichtenstein, P.; Almqvist, C.; Serlachius, E.; Ludvigsson, J.F. Nationwide population-based cohort study of psychiatric disorders in individuals with Ehlers-Danlos syndrome or hypermobility syndrome and their siblings. BMC Psychiatry 2016, 16, 207. [Google Scholar] [CrossRef]
- Kole, A.; Faurisson, F. The Voice of 12,000 Patients: Experiences and Expectations of Rare Disease Patients on Diagnosis and Care in Europe. 2009. Available online: https://www.eurordis.org/wp-content/uploads/2009/12/EURORDISCARE_FULLBOOKr.pdf (accessed on 18 October 2023).
- Anderson, L.; Lane, K. The diagnostic journey in adults with hypermobile Ehlers–Danlos syndrome and hypermobility spectrum disorders. J. Am. Assoc. Nurse Pract. 2022, 34, 4. [Google Scholar] [CrossRef]
- Grahame, R. Hypermobility: An important but often neglected area within rheumatology. Nat. Clin. Pract. Rheumatol. 2008, 4, 522–524. [Google Scholar] [CrossRef]
- Hakim, A.J.; Grahame, R. Recognizing the scale of joint hypermobility burden: Comment on the article by Mulvey et al. Arthritis Care Res. 2014, 66, 496. [Google Scholar] [CrossRef]
- Mulvey, M.R.; Macfarlane, G.J.; Beasley, M.; Symmons, D.P.M.; Lovell, K.; Keeley, P.; Woby, S.; McBeth, J. Modest Association of Joint Hypermobility with Disabling and Limiting Musculoskeletal Pain: Results from a Large-Scale General Population–Based Survey. Arthritis Care Res. 2013, 65, 1325–1333. [Google Scholar] [CrossRef]
- Pezaro, S.; Pearce, G.; Reinhold, E. Understanding hypermobile Ehlers-Danlos syndrome and Hypermobility Spectrum Disorders in the context of childbearing: An international qualitative study. Midwifery 2020, 88, 102749. [Google Scholar] [CrossRef] [PubMed]
- Gazit, Y.; Nahir, A.M.; Grahame, R.; Jacob, G. Dysautonomia in the joint hypermobility syndrome. Am. J. Med. 2003, 115, 33–40. [Google Scholar] [CrossRef]
- Knight, I. The role of narrative medicine in the management of joint hypermobility syndrome/Ehlers–Danlos syndrome, hypermobility type. Am. J. Med. Genet. C Semin Med. Genet. 2015, 169, 123–129. [Google Scholar] [CrossRef]
- Bennett, S.E.; Walsh, N.; Moss, T.; Palmer, S. Understanding the psychosocial impact of joint hypermobility syndrome and Ehlers–Danlos syndrome hypermobility type: A qualitative interview study. Disabil. Rehabil. 2021, 43, 795–804. [Google Scholar] [CrossRef]
- Bell, L.; Pearce, G. Parents’ experiences of children’s health care for hypermobile Ehlers–Danlos syndrome and hypermobility spectrum disorders. Child. Health Care 2022, 51, 37–61. [Google Scholar] [CrossRef]
- Atwell, K.; Michael, W.; Dubey, J.; James, S.; Martonffy, A.; Anderson, S.; Rudin, N.; Schrager, S. Diagnosis and Management of Hypermobility Spectrum Disorders in Primary Care. J. Am. Board Fam. Med. 2021, 34, 838–848. [Google Scholar] [CrossRef]
- World Health Organization. WHO Recommended Interventions for Improving Maternal and Newborn Health: Integrated Management of Pregnancy and Childbirth; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- World Health Organization. WHO Recommendations on Intrapartum Care for a Positive Childbirth Experience; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- National Institute for Health and Care Excellence. Intrapartum Care for Healthy Women and Babies Clinical Guideline [CG190]; National Institute for Health and Care Excellence: London, UK, 2022. [Google Scholar]
- Spiegel, E.; Nicholls-Dempsey, L.; Czuzoj-Shulman, N.; Abenhaim, H.A. Pregnancy outcomes in women with Ehlers-Danlos Syndrome. J. Matern. Neonatal Med. 2022, 35, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Hanif, M.; Mirza, E.; Jaleel, S. Ehlers-Danlos Syndrome in Pregnancy: A Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 255, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Hugon-Rodin, J.; Lebègue, G.; Becourt, S.; Hamonet, C.; Gompel, A. Gynecologic symptoms and the influence on reproductive life in 386 women with hypermobility type Ehlers-Danlos syndrome: A cohort study. Orphanet J. Rare Dis. 2016, 11, 1–6. [Google Scholar] [CrossRef]
- Hurst, B.S.; Lange, S.S.; Kullstam, S.M.B.; Usadi, R.S.; Matthews, M.L.; Marshburn, P.B.; Templin, M.A.; Merriam, K.S.B. Obstetric and gynecologic challenges in women with Ehlers-Danlos syndrome. Obstet. Gynecol. 2014, 123, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Lind, J.; Wallenburg, H.S. Pregnancy and the Ehlers-Danlos syndrome: A retrospective study in a Dutch population. Acta Obstet. Gynecol. Scand. 2002, 81, 293–300. [Google Scholar] [CrossRef]
- Sundelin, H.E.K.; Stephansson, O.; Johansson, K.; Ludvigsson, J.F. Pregnancy outcome in joint hypermobility syndrome and Ehlers–Danlos syndrome. Acta Obstet. Gynecol. Scand. 2017, 96, 114–119. [Google Scholar] [CrossRef]
- Castori, M.; Morlino, S.; Dordoni, C.; Celletti, C.; Camerota, F.; Ritelli, M.; Morrone, A.; Venturini, M.; Grammatico, P.; Colombi, M. Gynecologic and obstetric implications of the joint hypermobility syndrome (a.k.a. Ehlers–Danlos syndrome hypermobility type) in 82 Italian patients. Am. J. Med. Genet. A 2012, 158A, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S. Clinical significance of precipitous labor. J. Clin. Med. Res. 2015, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Sheiner, E.; Levy, A.; Mazor, M. Precipitate labor: Higher rates of maternal complications. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 116, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Pearce, G.; Bell, L.; Magee, P.; Pezaro, S. Co-created solutions for perinatal professionals and childbearing needs for people with hypermobile Ehlers-Danlos syndrome and Hypermobility Spectrum Disorders. Int. J. Environ. Res. Public Health 2023, accepted. [Google Scholar]
- Gov.UK. Prove Your Knowledge of English for Citizenship and Settling. Available online: https://www.gov.uk/english-language#:~:text=You%20might%20need%20to%20prove,taught%20or%20researched%20in%20English (accessed on 18 October 2023).
- Hsieh, H.-F.; Shannon, S.E. Three approaches to qualitative content analysis. Qual. Health Res. 2005, 15, 1277–1288. [Google Scholar] [CrossRef]
- Vaismoradi, M.; Turunen, H.; Bondas, T. Content analysis and thematic analysis: Implications for conducting a qualitative descriptive study. Nurs. Health Sci. 2013, 15, 398–405. [Google Scholar] [CrossRef]
- Office for National Statistics. Birth Characteristics in England and Wales. Available online: www.ons.gov.uk (accessed on 18 October 2023).
- McParlin, C.; O’Donnell, A.; Robson, S.C.; Beyer, F.; Moloney, E.; Bryant, A.; Bradley, J.; Muirhead, C.R.; Nelson-Piercy, C.; Newbury-Birch, D.; et al. Treatments for Hyperemesis Gravidarum and Nausea and Vomiting in Pregnancy: A Systematic Review. JAMA 2016, 316, 1392–1401. [Google Scholar] [CrossRef]
- Royal College of Obstetricians and Gynaecologists. Antepartum Haemorrhage Green-Top Guideline No. 63; Royal College of Obstetricians and Gynaecologists: London, UK, 2011. [Google Scholar]
- Thomson, A.J.; Royal College of Obstetricians and Gynaecologists. Care of Women Presenting with Suspected Preterm Prelabour Rupture of Membranes from 24(+0) Weeks of Gestation: Green-top Guideline No. 73. BJOG 2019, 126, e152–e166. [Google Scholar] [CrossRef]
- Leon, L.J.; McCarthy, F.P.; Direk, K.; Gonzalez-Izquierdo, A.; Prieto-Merino, D.; Casas, J.P.; Chappell, L. Preeclampsia and Cardiovascular Disease in a Large UK Pregnancy Cohort of Linked Electronic Health Records. Circulation 2019, 140, 1050–1060. [Google Scholar] [CrossRef] [PubMed]
- Knight, M. Eclampsia in the United Kingdom 2005. BJOG 2007, 114, 1072–1078. [Google Scholar] [CrossRef]
- Tingleff, T.; Räisänen, S.; Vikanes, Å.; Sandvik, L.; Sugulle, M.; Murzakanova, G.; Laine, K. Different pathways for preterm birth between singleton and twin pregnancies: A population-based registry study of 481 176 nulliparous women. BJOG 2023, 130, 387–395. [Google Scholar] [CrossRef] [PubMed]
- McLelland, G.; McKenna, L.; Morgans, A.; Smith, K. Epidemiology of unplanned out-of-hospital births attended by paramedics. BMC Pregnancy Childbirth 2018, 18, 15. [Google Scholar] [CrossRef]
- OECD. Health at a Glance 2015: OECD Indicators; OECD Publishing: Paris, France, 2015. [Google Scholar]
- Heinonen, K.; Saisto, T.; Gissler, M.; Kaijomaa, M.; Sarvilinna, N. Rising trends in the incidence of shoulder dystocia and development of a novel shoulder dystocia risk score tool: A nationwide population-based study of 800 484 Finnish deliveries. AOGS 2021, 100, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Carroli, G.; Cuesta, C.; Abalos, E.; Gulmezoglu, A.M. Epidemiology of postpartum haemorrhage: A systematic review. Best Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 999–1012. [Google Scholar] [CrossRef]
- Ismail, S.; Raza, A.; Munshi, K.; Tabassum, R. Failure rate of labor epidural: An observational study among different levels of trainee anesthesiologists in a university hospital of a developing country. J. Anaesthesiol. Clin. Pharmacol. 2021, 37, 210–215. [Google Scholar] [CrossRef]
- Kvalvik, S.A.; Rasmussen, S.; Thornhill, H.F.; Baghestan, E. Risk factors for surgical site infection following cesarean delivery: A hospital-based case–control study. Acta Obstet. Gynecol. Scand. 2021, 100, 2167–2175. [Google Scholar] [CrossRef] [PubMed]
- VanderKruik, R.; on behalf of the Maternal Morbidity Working Group; Barreix, M.; Chou, D.; Allen, T.; Say, L.; Cohen, L.S. The global prevalence of postpartum psychosis: A systematic review. BMC Psychiatry 2017, 17, 272. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, P.D.; Ayers, S.; Phillips, L. The prevalence of posttraumatic stress disorder in pregnancy and after birth: A systematic review and meta-analysis. J. Affect. Disord. 2017, 208, 634–645. [Google Scholar] [CrossRef]
- Wilcox, A.J.; Weinberg, C.R.; O’Connor, J.F.; Baird, D.D.; Schlatterer, J.P.; Canfield, R.E.; Armstrong, E.G.; Nisula, B.C. Incidence of early loss of pregnancy. N. Engl. J. Med. 1988, 319, 189–194. [Google Scholar] [CrossRef]
- Tikkanen, M. Placental abruption: Epidemiology, risk factors and consequences. Acta Obstet. Gynecol. Scand. 2011, 90, 140–149. [Google Scholar] [CrossRef]
- Mikuscheva, A.; Strassding, F.; MacKenzie, E. Three Cases of Severe Placental Abruption as a First Symptom of Preeclampsia. Case Rep. Obstet. Gynecol. 2021, 2021, 3863607. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Hypertension in Pregnancy: Diagnosis and Management NICE Guideline. 2019. Available online: https://www.nice.org.uk/guidance/ng133 (accessed on 18 October 2023).
- Collins, H. Magnesium and Ehlers-Danlos Syndrome. 2013. Available online: https://tcapp.org/wp-content/uploads/2017/09/Magnesium-and-Ehlers-Danlos-Syndrome.pdf (accessed on 18 October 2023).
- Wei, X.; Yang, X. The central role of natural killer cells in preeclampsia. Front. Immunol. 2023, 14, 1009867. [Google Scholar] [CrossRef]
- Fukui, A.; Yokota, M.; Funamizu, A.; Nakamua, R.; Fukuhara, R.; Yamada, K.; Kimura, H.; Fukuyama, A.; Kamoi, M.; Tanaka, K.; et al. Changes of NK cells in preeclampsia. Am. J. Reprod. Immunol. 2012, 67, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Pezaro, S.; Pearce, G.; Reinhold, E. Hypermobile Ehlers-Danlos Syndrome during pregnancy, birth and beyond. BJM 2018, 26, 217–223. [Google Scholar] [CrossRef]
- Schubart, J.R.; Schaefer, E.; Janicki, P.; Adhikary, S.D.; Schilling, A.; Hakim, A.J.; Bascom, R.; Francomano, C.A.; Raj, S.R. Resistance to local anesthesia in people with the Ehlers-Danlos Syndromes presenting for dental surgery. J. Dent. Anesth. Pain Med. 2019, 19, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Ängeby, K.; Wilde-Larsson, B.; Hildingsson, I.; Sandin-Bojö, A.-K. Prevalence of Prolonged Latent Phase and Labor Outcomes: Review of Birth Records in a Swedish Population. J. Midwifery Women’s Health 2018, 63, 33–44. [Google Scholar] [CrossRef]
- Memon, H.U.; Handa, V.L. Vaginal childbirth and pelvic floor disorders. Womens Health 2013, 9, 265–277; quiz 276–277. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, I.; Barber, M.D.; Burgio, K.L.; Kenton, K.; Meikle, S.; Schaffer, J.; Spino, C.; Whitehead, W.E.; Wu, J.; Brody, D.J.; et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA 2008, 300, 1311–1316. [Google Scholar] [CrossRef]
- Wiesmann, T.; Castori, M.; Malfait, F.; Wulf, H. Recommendations for anesthesia and perioperative management in patients with Ehlers-Danlos syndrome(s). Orphanet J. Rare Dis. 2014, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.; Hefele, J.G.; Ritter, G.; Darden, J.; Firneno, C.; Hendrich, A. Population-based risk factors for shoulder dystocia. J. Obstet. Gynecol. Neonatal Nurs. 2018, 47, 32–42. [Google Scholar] [CrossRef]
- Royal College of Obstetricians & Gynaecologists. Shoulder Dystocia: Green–Top Guideline No. 42, 2nd ed.; Royal College of Obstetricians & Gynaecologists: London, UK, 2012; Available online: https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/shoulder-dystocia-green-top-guideline-no-42/ (accessed on 18 October 2023).
- Bothou, A.; Apostolidi, D.-M.; Tsikouras, P.; Iatrakis, G.; Sarella, A.; Iatrakis, D.; Peitsidis, P.; Gerente, A.; Anthoulaki, X.; Nikolettos, N.; et al. Overview of techniques to manage shoulder dystocia during vaginal birth. Eur. J. Midwifery 2021, 5, 48. [Google Scholar] [CrossRef]
- Keriakos, R.; Bhatta, S.R.C.; Morris, F.; Mason, S.; Buckley, S. Pelvic girdle pain during pregnancy and puerperium. J. Obstet. Gynaecol. 2011, 31, 572–580. [Google Scholar] [CrossRef]
- Zheng, L.; Li, H.; Zhang, H. Cohort study of use of the hands-and knees-position as the first approach to resolving shoulder dystocia and preventing neonatal birth trauma. Gynecol. Obstet. Clin. Med. 2021, 1, 160–163. [Google Scholar] [CrossRef]
- Monaco, A.; Choi, D.; Uzun, S.; Maitland, A.; Riley, B. Association of mast-cell-related conditions with hypermobile syndromes: A review of the literature. Immunol. Res. 2022, 70, 419–431. [Google Scholar] [CrossRef]
- Wong, S.; Hasan, S.; Parducci, C.; Riley, B.A. The gastrointestinal effects amongst Ehlers-Danlos syndrome, mast cell activation syndrome and postural orthostatic tachycardia syndrome. AIMS Allergy Immunol. 2022, 6, 19–24. [Google Scholar] [CrossRef]
- Wang, E.; Ganti, T.; Vaou, E.; Hohler, A. The relationship between mast cell activation syndrome, postural tachycardia syndrome, and Ehlers-Danlos syndrome. Allergy Asthma Proc. 2021, 42, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Seidel, H.; Oldenburg, J.; Meis, K.; Kolck, U.W.; Homann, J.; Hertfelder, H.-J.; Molderings, G.J. Bleeding diathesis in patients with mast cell activation disease. Thromb. Haemost. 2017, 106, 987–989. [Google Scholar] [CrossRef]
- Dorff, S.R.; Afrin, L.B. Mast cell activation syndrome in pregnancy, delivery, postpartum and lactation: A narrative review. J. Obstet. Gynaecol. 2020, 40, 889–901. [Google Scholar] [CrossRef]
- Molderings, G.J.; Haenisch, B.; Bogdanow, M.; Fimmers, R.; Nöthen, M.M. Familial Occurrence of Systemic Mast Cell Activation Disease. PLoS ONE 2013, 8, e76241. [Google Scholar] [CrossRef] [PubMed]
- Gensemer, C.; Burks, R.; Kautz, S.; Judge, D.P.; Lavallee, M.; Norris, R.A. Hypermobile Ehlers-Danlos syndromes: Complex phenotypes, challenging diagnoses, and poorly understood causes. Dev. Dyn. 2021, 250, 318–344. [Google Scholar] [CrossRef]
- Morgan, K.; Smith, A.; Blitshteyn, S. POTS and Pregnancy: A Review of Literature and Recommendations for Evaluation and Treatment. Int. J. Womens Health 2022, 14, 1831–1847. [Google Scholar] [CrossRef]
- National Organization for Rare Disorders (NORD). Hyperemesis Gravidarum; NORD: Quincy, MA, USA, 2020. [Google Scholar]
- Halverson, C.M.E.; Penwell, H.L.; Francomano, C.A. Clinician-associated traumatization from difficult medical encounters: Results from a qualitative interview study on the Ehlers-Danlos Syndromes. SSM Qual. Res. Health 2023, 3, 100237. [Google Scholar] [CrossRef] [PubMed]
- Whiffen, T.; Akbari, A.; Paget, T.; Lowe, S.; Lyons, R. How effective are population health surveys for estimating prevalence of chronic conditions compared to anonymised clinical data? Int. J. Popul. Data Sci. 2020, 5, 1151. [Google Scholar] [CrossRef]
- Delnord, M.; Mortensen, L.; Hindori-Mohangoo, A.D.; Blondel, B.; Gissler, M.; Richards, J.L.; Deb-Rinker, P.; Rouleau, J.; Morisaki, N.; Nassar, N.; et al. International variations in the gestational age distribution of births: An ecological study in 34 high-income countries. Eur. J. Public Health 2018, 28, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A. ABC of subfertility: Extent of the problem. BMJ 2003, 327, 434–436. [Google Scholar] [CrossRef] [PubMed]
- Bearak, J.; Popinchalk, A.; Alkema, L.; Sedgh, G. Global, regional, and subregional trends in unintended pregnancy and its outcomes from 1990 to 2014: Estimates from a Bayesian hierarchical model. Lancet Glob. 2018, 6, e380–e389. [Google Scholar] [CrossRef] [PubMed]
- Pezaro, S.; Crowther, R.; Pearce, G.; Jowett, A.; Godfrey-Isaacs, L.; Samuels, I.; Valentine, V. Perinatal Care for Trans and Nonbinary People Birthing in Heteronormative “Maternity” Services: Experiences and Educational Needs of Professionals. Gend. Soc. 2023, 37, 124–151. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Venkat-Raman, N. Hypermobile Ehlers-Danlos syndrome and pregnancy. Obstet. Med. 2018, 11, 104–109. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pearce, G.; Bell, L.; Pezaro, S.; Reinhold, E. Childbearing with Hypermobile Ehlers–Danlos Syndrome and Hypermobility Spectrum Disorders: A Large International Survey of Outcomes and Complications. Int. J. Environ. Res. Public Health 2023, 20, 6957. https://doi.org/10.3390/ijerph20206957
Pearce G, Bell L, Pezaro S, Reinhold E. Childbearing with Hypermobile Ehlers–Danlos Syndrome and Hypermobility Spectrum Disorders: A Large International Survey of Outcomes and Complications. International Journal of Environmental Research and Public Health. 2023; 20(20):6957. https://doi.org/10.3390/ijerph20206957
Chicago/Turabian StylePearce, Gemma, Lauren Bell, Sally Pezaro, and Emma Reinhold. 2023. "Childbearing with Hypermobile Ehlers–Danlos Syndrome and Hypermobility Spectrum Disorders: A Large International Survey of Outcomes and Complications" International Journal of Environmental Research and Public Health 20, no. 20: 6957. https://doi.org/10.3390/ijerph20206957
APA StylePearce, G., Bell, L., Pezaro, S., & Reinhold, E. (2023). Childbearing with Hypermobile Ehlers–Danlos Syndrome and Hypermobility Spectrum Disorders: A Large International Survey of Outcomes and Complications. International Journal of Environmental Research and Public Health, 20(20), 6957. https://doi.org/10.3390/ijerph20206957






