Implementation of A Year-Long Antimicrobial Stewardship Program in A 227-Bed Community Hospital in Southern Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
2.2. Program Development
2.2.1. Antimicrobial Oversight
“Front End” Strategy
“Back End” Strategy
2.2.2. Development and Application of Hospital Recommendations and Guidelines
2.2.3. Development and Use of Clinical and Decision Support Algorithms
2.2.4. Training and Updating
2.3. Program Outcomes
2.3.1. Clinical Outcomes
- − Hospital length of stay;
- − Total deaths and deaths from infections;
- − Intensive care transfers for infectious complications;
- − Unscheduled re-admission to hospital within 30 days of discharge.
2.3.2. Microbiologic Outcomes
- − Laboratory diagnosis of Clostridium Difficile infection.
2.3.3. Economic Outcomes
- − Quantitative consumption of antibiotics;
- − Antibiotic cost.
2.4. Statistical Analysis
3. Results
3.1. Clinical Outcomes
3.2. Economic Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magill, S.S.; O’Leary, E.; Janelle, S.J.; Thompson, D.L.; Dumyati, G.; Nadle, J.; Wilson, L.E.; Kainer, M.A.; Lynfield, R.; Greissman, S.; et al. Emerging Infections Program Hospital Prevalence Survey Team. Changes in Prevalence of Health Care-Associated Infections in U.S. Hospitals. N. Engl. J. Med. 2018, 379, 1732–1744. [Google Scholar] [CrossRef] [PubMed]
- Suetens, C.; Latour, K.; Kärki, T.; Ricchizzi, E.; Kinross, P.; Moro, M.L.; Jans, B.; Hopkins, S.; Hansen, S.; Lyytikäinen, O.; et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: Results from two European point prevalence surveys, 2016 to 2017. Euro Surveill. 2018, 23, 1800516. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Report on Infection Prevention and Control. Executive Summary. Available online: https://cdn.who.int/media/docs/default-source/integrated-health-services-(ihs)/ipc/ipc-global-report/who_ipc_global-report_executive-summary.pdf (accessed on 28 September 2022).
- Bell, B.G.; Shellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis on the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 13. [Google Scholar]
- Bakhit, M.; Hoffmann, T.; Scott, A.M.; Beller, E.; Rathbone, J.; Del Mar, C. Resistance decay in individuals after antibiotic exposure in primary care: A systematic review and meta-analysis. BMC Med. 2018, 16, 126. [Google Scholar] [CrossRef] [PubMed]
- Fridkin, S.K.; Steward, C.D.; Edwards, J.R.; Pryor, E.R.; McGowan Jr, J.E.; Archibald, L.K.; Gaynes, R.P.; Tenover, F.C.; Project Intensive Care Antimicrobial Resistance Epidemiology (ICARE) Hospitals. Surveillance of antimicrobial use and antimicrobial resistance in United States hospitals: Project ICARE phase 2. Project Intensive Care Antimicrobial Resistance Epidemiology (ICARE) hospitals. Clin. Infect. Dis. 1999, 29, 245–252. [Google Scholar] [CrossRef][Green Version]
- Weinstein, R.; Gaynes, R.; Edwards, J.R. Overview of nosocomial infections caused by gram-negative bacilli. Clin. Infect. Dis. 2005, 41, 848–854. [Google Scholar] [CrossRef]
- Weiner-Lastinger, L.M.; Abner, S.; Edwards, J.R.; Kallen, A.J.; Karlsson, M.; Magill, S.S.; Polloc, D.; See, I.; Soe, M.M.; Walters, M.S.; et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect. Control Hosp. Epidemiol. 2020, 41, 1–18. [Google Scholar] [CrossRef]
- Cosgrove, S.E. The relationship between antimicrobial resistance and patient outcomes: Mortality, length of hospital stay, and health care costs. Clin. Infect. Dis. 2006, 42, S82–S89. [Google Scholar] [CrossRef]
- Medina, E.; Pieper, D.H. Tackling Threats and Future Problems of Multidrug-Resistant Bacteria. Curr. Top. Microbiol. Immunol. 2016, 398, 3–33. [Google Scholar]
- Friedman, N.D.; Temkin, E.; Carmeli, Y. The negative impact of antibiotic resistance. Clin. Microbiol. Infect. 2016, 22, 416–422. [Google Scholar] [CrossRef]
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention. The Core Elements of Hospital Antibiotic Stewardship Programs: 2019. Available online: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf (accessed on 28 September 2022).
- British Society of Antimicrobial Chemotherapy. Antimicrobial Stewardship: From Principles to Practise; British Society of Antimicrobial Chemotherapy: Birmingham, UK, 2018. [Google Scholar]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardships Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef]
- Pollack, L.; Srinivasan, A. Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin. Infect. Dis. 2014, 59 (Suppl. 3), S97–S100. [Google Scholar] [CrossRef] [PubMed]
- Elligsen, M.; Walker, S.; Simor, A.; Daneman, N. Prospective audit and feedback of antimicrobial stewardship in critical care: Program implementation, experience, and challenges. Can. J. Hosp. Pharm. 2012, 65, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Elligsen, M.; Walker, S.; Pinto, R.; Simor, A.; Mubareka, S.; Rachlis, A.; Allen, V.; Daneman, N. Audit and feedback to reduce broad-spectrum antibiotic use among intensive care unit patients: A controlled interrupted time series analysis. Infect. Control Hosp. Epidemiol. 2012, 33, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Albano, G.D.; Bertozzi, G.; Maglietta, F.; Montana, A.; Di Mizio, G.; Esposito, M.; Mazzeo, P.; D’Errico, S.; Salerno, M. Medical records quality as prevention tool for healthcare-associated infections (HAIs) related litigation: A case series. Curr. Pharm. Biotechnol. 2018, 20, 653–657. [Google Scholar] [CrossRef]
- Levy Hara, G. Antimicrobial stewardship in hospitals: Does it work and can we do it? J. Glob. Antimicrob. Resist. 2014, 2, 1–6. [Google Scholar] [CrossRef]
- Mendelson, M.; Morris, A.M.; Thursky, K.; Pulcini, C. How to start an antimicrobial stewardship programme in a hospital. Clin. Microbiol. Infect. 2019, 26, 447–453. [Google Scholar] [CrossRef]
- Spellberg, B.; Bartlett, J.G.; Gilbert, D.N. How to Pitch an Antibiotic Stewardship Program to the Hospital C-Suite. Open Forum Infect. Dis. 2016, 3, ofw210. [Google Scholar] [CrossRef]
- WHO Access, Watch, Reserve (AWaRe) Classification of Antibiotics for Evaluation and Monitoring of Use. 2021. Available online: https://www.who.int/publications-detail-redirect/2021-aware-classification (accessed on 28 September 2022).
- Sharland, M.; Gandra, S.; Huttner, B.; Moja, L.; Pulcini, C.; Zeng, M.; Mendelson, M.; Cappello, B.; Cooke, G.; Magrini, N.; et al. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use—The new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect. Dis. 2019, 19, 1278–1280. [Google Scholar] [CrossRef]
- Dik, J.W.; Hendrix, R.; Poelman, R.; Niesters, H.G.; Postma, M.J.; Sinha, B.; Friedrich, A.W. Measuring the impact of antimicrobial stewardship programs. Expert Rev. Anti Infect. Ther. 2016, 14, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Pollack, L.A.; Diamantis, P.; Gruhler, H.; Ronda, S.C.; Monnet, D.L.; Weber, T. Transatlantic Taskforce on Antimicrobial Resistance, Summary the Modified Delphi Process for Common Structure and Process Indicators for Hospital Antimicrobial Stewardship Programs. Available online: https://www.cdc.gov/drugresistance/pdf/summary_of_tatfar_recommendation_1.pdf (accessed on 28 September 2022).
- Razzaque, M.S. Implementation of antimicrobial stewardship to reduce antimicrobial drug resistance. Expert Rev. Anti Infect. Ther. 2021, 19, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.A.A.; Singh, K.; Hilaire, M.G.; Rahman, S.; Sa, B.; Haque, M. Tackling antimicrobial resistance by promoting antimicrobial stewardship in medical and allied health professional curricula. Expert Rev. Anti Infect. Ther. 2020, 18, 1245–1258. [Google Scholar] [CrossRef]
- Cangini, A.; Fortinguerra, F.; Di Filippo, A.; Pierantozzi, A.; Da Cas, R.; Villa, F.; Trotta, F.; Moro, M.L.; Gagliotti, C. Monitoring the community use of antibiotics in Italy within the National Action Plan on antimicrobial resistance. Br. J. Clin. Pharmacol 2021, 87, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Olesen, S.W.; Barnett, M.L.; MacFadden, D.R.; Brownstein, J.S.; Hernández-Díaz, S.; Lipsitch, M.; Grad, Y.H. The distribution of antibiotic use and its association with antibiotic resistance. Elife 2018, 7, e39435. [Google Scholar] [CrossRef] [PubMed]
- Olesen, S.W. Uses of mathematical modeling to estimate the impact of mass drug administration of antibiotics on antimicrobial resistance within and between communities. Infect. Dis. Poverty 2022, 11, 75. [Google Scholar] [CrossRef]
- Machowska, A.; Lundborg, C.S. Drivers of irrational use of antibiotics in Europe. Int. J. Environ. Res. Public Health 2019, 16, 27. [Google Scholar] [CrossRef]
- Zarauz, J.M.; Zafrilla, P.; Ballester, P.; Cerda, B. Study of the Drivers of Inappropriate Use of Antibiotics in Community Pharmacy: Request for Antibiotics Without a Prescription, Degree of Adherence to Treatment and Correct Recycling of Leftover Treatment. Infect. Drug Resist. 2022, 1, 6773–6783. [Google Scholar] [CrossRef]
- Crespo-Rivas, J.C.; Guisado-Gil, A.B.; Peñalva, G.; Rodríguez-Villodres, Á.; Martín-Gandul, C.; Pachón-Ibáñez, M.E.; Cisneros, J.M. Are antimicrobial stewardship interventions effective and safe in long-term care facilities? A systematic review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 1431–1438. [Google Scholar] [CrossRef]
- Fitzpatrick, L.P.; Levkovich, B.; McGloughlin, S.; Litton, E.; Cheng, A.C.; Bailey, M.; Pilcher, D. Infection management processes in intensive care and their association with mortality. J. Antimicrob. Chemother. 2021, 76, 1920–1927. [Google Scholar] [CrossRef]
- Schuts, E.C.; Hulscher, M.E.; Mouton, J.W.; Verduin, C.M.; Stuart, J.W.C.; Overdiek, H.W.; Prins, J.M. Current evidence on hospital antimicrobial stewardship objectives: A systematic review and meta-analysis. Lancet Infect. Dis. 2016, 16, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Abad, C.L.R.; Safdar, N. A review of clostridioides difficile infection and antibiotic-associated diarrhea. Gastroenterol. Clin. 2021, 50, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, J.G. Narrative review: The new epidemic of Clostridium difficile-associated enteric disease. Ann. Intern. Med. 2006, 145, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, O.U.; Ab Rahman, N.S.; Zin, C.S. A narrative review of antimicrobial stewardship interventions within in-patient settings and resultant patient outcomes. J. Pharm. Bioallied. Sci. 2020, 12, 369. [Google Scholar] [PubMed]
- Lindsay, P.J.; Rohailla, S.; Taggart, L.R.; Lightfoot, D.; Havey, T.; Daneman, N.; Muller, M.P. Antimicrobial stewardship and intensive care unit mortality: A systematic review. Clin. Infect. Dis. 2019, 68, 748–756. [Google Scholar] [CrossRef]
- Albano, G.D.; Rifiorito, A.; Malta, G.; Sorrentino, E.S.; Falco, V.; Firenze, A.; Argo, A.; Zerbo, S. The Impact on Healthcare Workers of Italian Law n. 24/2017 “Gelli–Bianco” on Patient Safety and Medical Liability: A National Survey. Int. J. Environ. Res. Public Health 2022, 19, 8448. [Google Scholar] [CrossRef]
- Lanza, G.L.M.; Gaglio, V.; Marotta, C.; Ippolito, M.; Mulè, G.; Albano, G.D.; Gialdino, A.C.; Mescolo, F.; Presti, T.L.; Di Natale, K.; et al. Knowledge and viewpoints on the effects of corruption on healthcare: A survey conducted among students of palermo university medical school, Italy. Euromediterranean Biomed. J. 2018, 13, 31–36. [Google Scholar]
- Costantino, C.; Cannizzaro, E.; Verso, M.G.; Tramuto, F.; Maida, C.M.; Lacca, G.; Alba, D.; Cimino, L.; Conforto, A.; Cirrincione, L.; et al. SARS-CoV-2 infection in healthcare professionals and general population during “first wave” of COVID-19 pandemic: A cross-sectional study conducted in Sicily, Italy. Front. Public Health 2021, 286, 644008. [Google Scholar] [CrossRef]
- Zerbo, S.; Malta, G.; Argo, A. Guidelines and current assessment of health care responsibility in Italy. Risk Manag. Healthc. Policy 2020, 13, 183–189. [Google Scholar] [CrossRef]
| Organization | Our Institution Developed an AMS Program |
|---|---|
| Education | Promotion of education campaign for all healthcare personnel about HAI prevention and control and AMS. |
| Good Clinical Practices and Guidelines | Sharing good clinical practices and evidence-based guidelines in infection control and AMS among healthcare personnel. |
| Antimicrobial Stewardship Program | Antibiotic prescription restriction and supervision; clinical choice support for antimicrobic therapy; training and updating; higher standard of care in managing CAIs and HAIs; availability of AMS team in the hospital. |
| Audit and Feedback | Dissemination of practice of audit and feedback about prescription and authorization of therapeutic plans. |
| Surveillance | Medical records and pharmacy activity in-depth evaluation regarding antibiotic prescription and use. |
| Results Monitoring | Comparison of results after one year of AMS program. |
| Carbapenems | Imipenem-Cilastatin, Meropenem, Ertapenem, Doripenem |
|---|---|
| Carbapenems + β-lactamase inhibitor combination | Meropenem/Vaborbactam, Imipenem/Relebactam |
| Oxazolidinones | Linezolid, Tedizolid |
| Lypo(Glico)peptides | Daptomicin, Dalbavancin, Oritavancin |
| Streptogramin | Fosfomycin |
| Polymyxin | Colistin |
| β-lactam/β-lactamase combination | Ceftazidime-Avibactam, Ceftolozane-Tazobactam |
| Interventions |
|---|
| Authorization for restricted antibiotics |
| Conversion of intravenous to oral for high-bioavailability antibiotics |
| Switch from broad spectrum to narrow spectrum |
| Descalation therapy based on microbiologic data |
| Stop antibiotics if no infection is diagnosed |
| Stop antibiotics if criteria to define healing are present |
| Step-down therapy |
| OPAT |
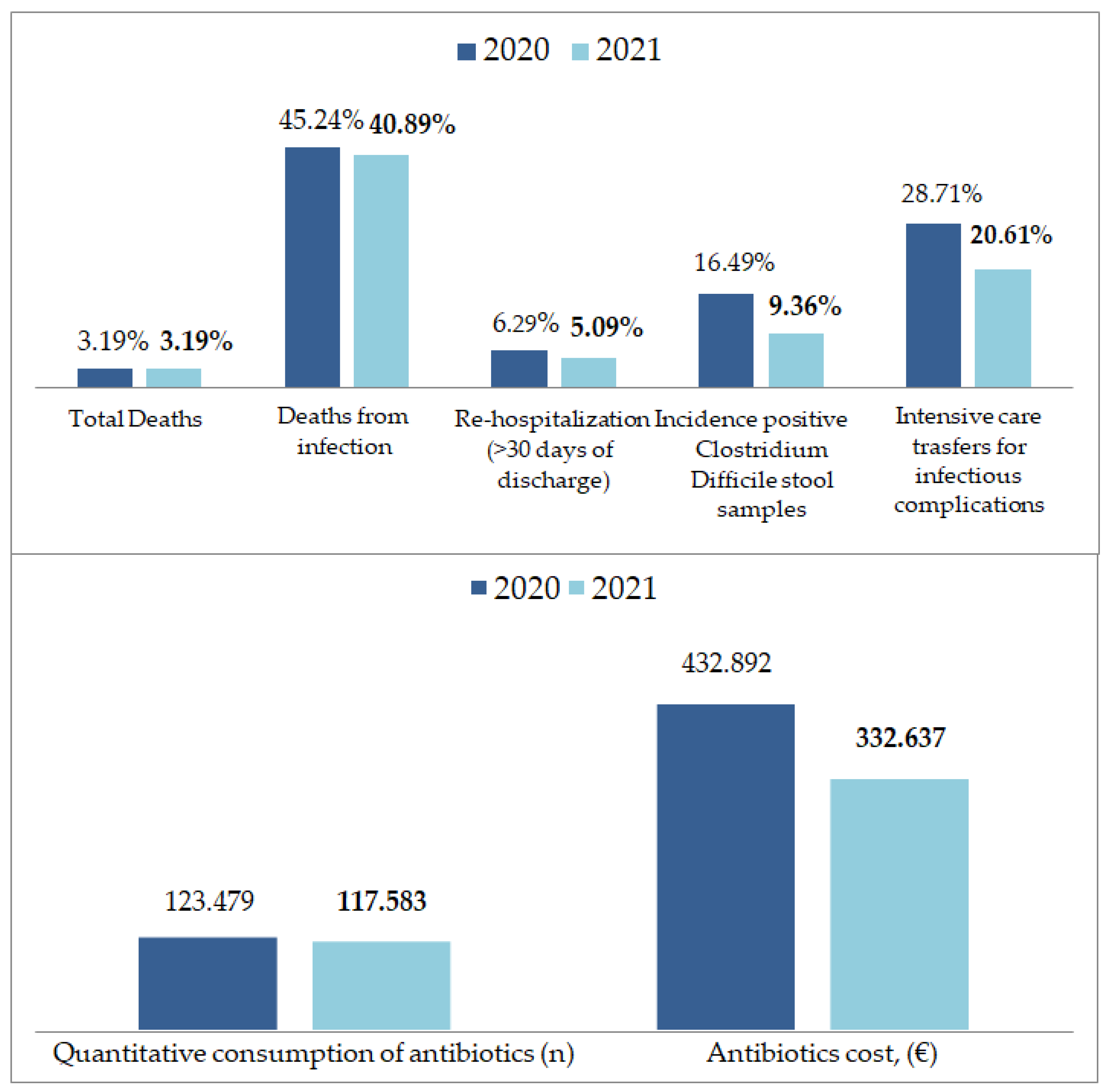
| 2020 | 2021 | 2020 vs. 2021 | p-Value | |
|---|---|---|---|---|
| Admissions/year, n (%) | 6582 (31.72%) | 7053 (33.99%) | 0.0714 | <0.001 |
| Total deaths, n (%) | 210 (3.19%) | 225 (3.19%) | −0.01% | 0.2073 |
| Deaths from infection, n (%) | 95 (45.24%) | 92 (40.89%) | −9.62% | 0.3599 |
| Hospital stay, mean (SD) | 10.8 (20.5) | 10.3 (18.9) | −4.06% | 0.0324 |
| Re-hospitalization (>30 days of discharge), n (%) | 414 (6.29%) | 359 (5.09%) | −19.08% | 0.0025 |
| Quantitative consumption of antibiotics, (n) | 123.479 | 117.583 | −4.77% | <0.001 |
| Incidence positive Clostridium Difficile stoolsamples, n (%) | 16 (16.49%) | 16 (9.36%) | −43.24% | 0.0833 |
| Intensive care transfers, n (%) | 209 (3.18%) | 228 (3.23%) | 1.57% | 0.1958 |
| Intensive care transfers for infectious complications, n (%) | 60 (28.71%) | 47 (20.61%) | −28.21% | 0.0493 |
| Antibioticscost, (EUR) | 432.892 | 332.637 | −23.16% | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albano, G.D.; Midiri, M.; Zerbo, S.; Matteini, E.; Passavanti, G.; Curcio, R.; Curreri, L.; Albano, S.; Argo, A.; Cadelo, M. Implementation of A Year-Long Antimicrobial Stewardship Program in A 227-Bed Community Hospital in Southern Italy. Int. J. Environ. Res. Public Health 2023, 20, 996. https://doi.org/10.3390/ijerph20020996
Albano GD, Midiri M, Zerbo S, Matteini E, Passavanti G, Curcio R, Curreri L, Albano S, Argo A, Cadelo M. Implementation of A Year-Long Antimicrobial Stewardship Program in A 227-Bed Community Hospital in Southern Italy. International Journal of Environmental Research and Public Health. 2023; 20(2):996. https://doi.org/10.3390/ijerph20020996
Chicago/Turabian StyleAlbano, Giuseppe Davide, Mauro Midiri, Stefania Zerbo, Emanuele Matteini, Giulia Passavanti, Rosario Curcio, Lidia Curreri, Salvatore Albano, Antonina Argo, and Marcello Cadelo. 2023. "Implementation of A Year-Long Antimicrobial Stewardship Program in A 227-Bed Community Hospital in Southern Italy" International Journal of Environmental Research and Public Health 20, no. 2: 996. https://doi.org/10.3390/ijerph20020996
APA StyleAlbano, G. D., Midiri, M., Zerbo, S., Matteini, E., Passavanti, G., Curcio, R., Curreri, L., Albano, S., Argo, A., & Cadelo, M. (2023). Implementation of A Year-Long Antimicrobial Stewardship Program in A 227-Bed Community Hospital in Southern Italy. International Journal of Environmental Research and Public Health, 20(2), 996. https://doi.org/10.3390/ijerph20020996







