SARS-CoV-2 Aerosol and Surface Detections in COVID-19 Testing Centers and Implications for Transmission Risk in Public Facing Workers
Abstract
1. Introduction
2. Materials and Methods
- Probe: 5′/56-FAM/ACACTAAGCC/ZEN/ATCCTTACTGCGCTTCG/3AIBkFG/-3′
- Primer 1: 5′-ATATTGCAGCAGTACGCACACA-3′
- Primer 2: 5′-ACAGGTACGTTAATAGTTAATAGCGT-3′
- 5′-TTCGGAAGAGACAGGTACGTTAATAGTTAATAGCGTACTTCTTTTTCTTGCTTTCGTGGTATTCTTGCTAGTTACACTAGCCATCCTTACTGCGCTTCGATTGTGTGCGTACTGCTGCAATATTGTTAACGTG-3′
- 5.6 µL nuclease free water;
- 12.5 µL Invitrogen 2X Master Mix;
- 0.4 µL MgSO4;
- 1.0 µL Primer/Probe Mix (IDT) × (Primers 10 µM, Probe 5 µM);
- 0.5 µL SuperScript III Platinum Taq;
- 5.0 µL extracted sample RNA, nuclease free water or positive control;
- 25.0 µL Total.
3. Results
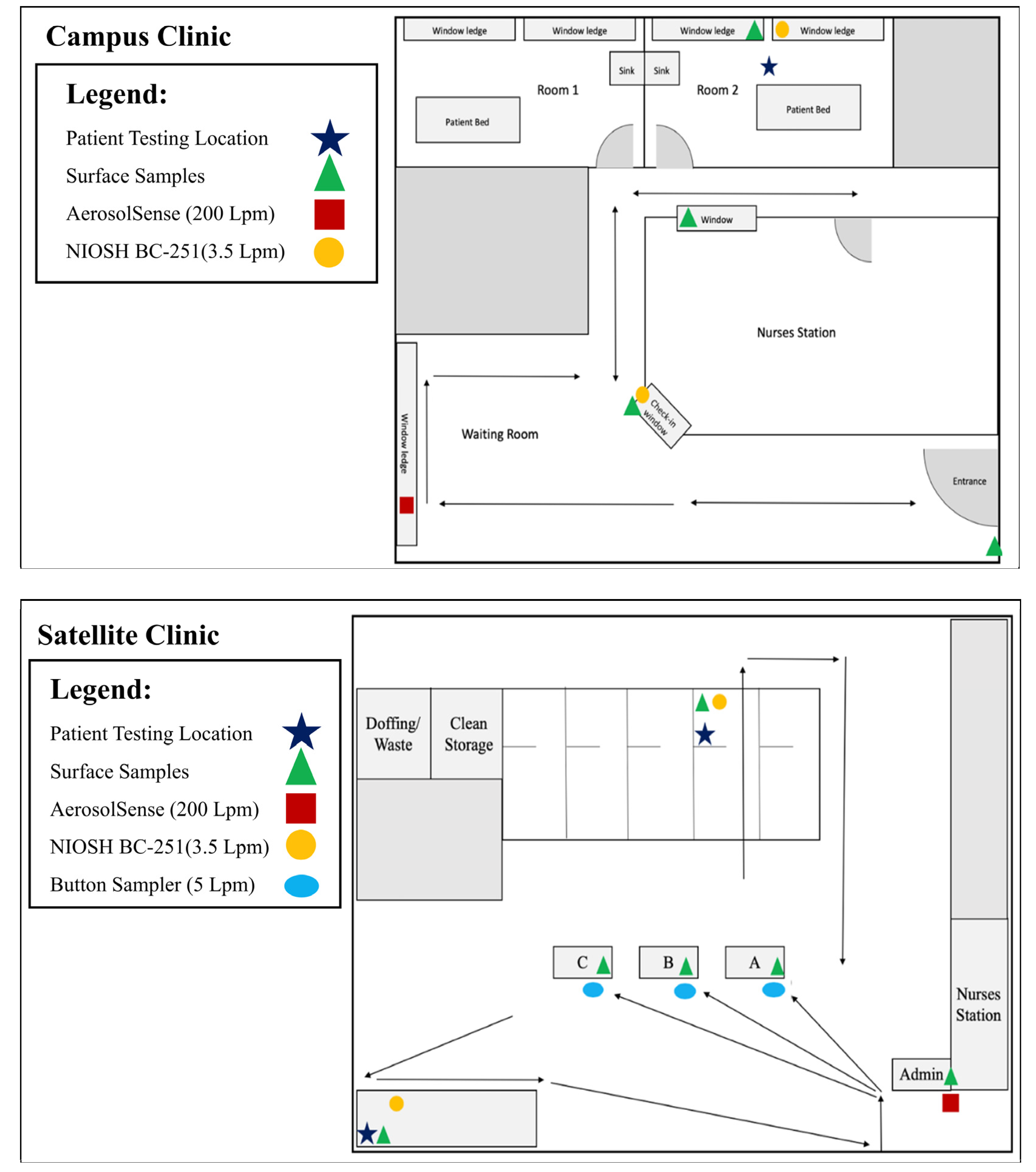
3.1. Satellite Clinic
3.2. Campus Clinic
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 9 February 2022).
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2022. Available online: https://covid19.who.int (accessed on 3 February 2022).
- Wake, R.M.; Morgan, M.; Choi, J.; Winn, S. Reducing nosocomial transmission of COVID-19: Implementation of a COVID-19 triage system. Clin. Med. 2020, 20, e141–e145. [Google Scholar] [CrossRef]
- Kutter, J.S.; Spronken, M.I.; Fraaij, P.L.; Fouchier, R.A.; Herfst, S. Transmission routes of respiratory viruses among humans. Curr. Opin. Virol. 2018, 28, 142–151. [Google Scholar] [CrossRef]
- Stadnytskyi, V.; Bax, C.E.; Bax, A.; Anfinrud, P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc. Natl. Acad. Sci. USA 2020, 117, 11875–11877. [Google Scholar] [CrossRef]
- Meyerowitz, E.A.; Richterman, A.; Gandhi, R.T.; Sax, P.E. Transmission of SARS-CoV-2: A review of viral, host, and environmental factors. Ann. Intern. Med. 2021, 174, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, Y.; Zhang, A.L.; Wang, Y.; Molina, M.J. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc. Natl. Acad. Sci. USA 2020, 117, 14857–14863. [Google Scholar] [CrossRef] [PubMed]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D.; et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 2020, 582, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, J.L.; Rivera, D.N.; Herrera, V.L.; Morwitzer, M.J.; Creager, H.M.; Santarpia, G.W.; Crown, K.K.; Brett-Major, D.M.; Schnaubelt, E.R.; Broadhurst, M.J.; et al. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci. Rep. 2020, 10, 12732. [Google Scholar] [CrossRef]
- Santarsiero, A.; Giustini, M.; Quadrini, F.; D’Alessandro, D.; Fara, G.M. Effectiveness of face masks for the population. Ann. Ig. 2021, 33, 347–359. [Google Scholar]
- Miller, S.L.; Nazaroff, W.W.; Jimenez, J.L.; Boerstra, A.; Buonanno, G.; Dancer, S.J.; Kurnitski, J.; Marr, L.C.; Morawska, L.; Noakes, C. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air 2021, 31, 314–323. [Google Scholar] [CrossRef]
- Buonanno, G.; Morawska, L.; Stabile, L. Quantitative assessment of the risk of airborne transmission of SARS-CoV-2 infection: Prospective and retrospective applications. Environ. Int. 2020, 145, 106112. [Google Scholar] [CrossRef]
- Cai, J.; Sun, W.; Huang, J.; Gamber, M.; Wu, J.; He, G. Indirect virus transmission in cluster of COVID-19 cases, Wenzhou, China, 2020. Emerg. Infect. Dis. 2020, 26, 1343–1345. [Google Scholar] [CrossRef]
- Li, Y.; Qian, H.; Hang, J.; Chen, X.; Cheng, P.; Ling, H.; Wang, S.; Liang, P.; Li, J.; Xiao, S.; et al. Probable airborne transmission of SARS-CoV-2 in a poorly ventilated restaurant. Build. Environ. 2021, 196, 107788. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.W.; Chu, J.T.; Perera, M.R.; Hui, K.P.; Yen, H.L.; Chan, M.C.; Peiris, M.; Poon, L.L. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 2020, 1, e10. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, M.; Hamblin, M.; Rezaei, N. COVID-19: Transmission, prevention, and potential therapeutic opportunities. Clin. Chim. Acta 2020, 508, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Tian, D.; Sun, Y.; Zhou, J.; Ye, Q. The global epidemic of the SARS-CoV-2 delta variant, key spike mutations and immune escape. Front. Immunol. 2021, 12, 751778. [Google Scholar] [CrossRef]
- Tracking SARS-CoV-2 Variants. 2022. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 11 January 2022).
- Sanche, S.; Lin, Y.T.; Xu, C.; Romero-Severson, E.; Hengartner, N.; Ke, R. High contagiousness and rapid spread of severe acute respiratory syndrome Coronavirus 2. Emerg. Infect. Dis. 2020, 26, 1470–1477. [Google Scholar] [CrossRef]
- D’Arienzo, M.; Coniglio, A. Assessment of the SARS-CoV-2 basic reproduction number, R (0), based on the early phase of COVID-19 outbreak in Italy. Biosaf. Health 2020, 2, 57–59. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, Q.; Yang, Z.; Liao, J.; Yang, K.; Bai, W.; Lu, X.; Zhang, W. Preliminary prediction of the basic reproduction number of the Wuhan novel coronavirus 2019-nCoV. J. Evid. Based Med. 2020, 13, 3–7. [Google Scholar] [CrossRef]
- Variant Proportions. COVID Data Tracker. 2022. Available online: https://covid.cdc.gov/covid-data-tracker/#variant-proportions (accessed on 11 January 2022).
- What You Need to Know About Variants. Variants of the Virus; About Variants. 2022. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/aboutvarants.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fvariants%2Fvariant.html (accessed on 2 February 2022).
- COVID-19 Genomics and Wastewater Surveillance. 2022. Available online: https://dhhs.ne.gov/Pages/COVID-19-Genomics-and-Wastewater-Surveillance.aspx (accessed on 22 December 2022).
- Lindsley, W.G.; Schmechel, D.; Chen, B. A two-stage cyclone using microcentrifuge tubes for personal bioaerosol sampling. J. Environ. Monit. 2006, 8, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Burton, N.C.; Grinshpun, S.; Reponen, T. Physical collection efficiency of filter materials for bacteria and viruses. Ann. Occup. Hyg. 2007, 51, 143–151. [Google Scholar]
- Leung, N.H.; Zhou, J.; Chu, D.K.; Yu, H.; Lindsley, W.G.; Beezhold, D.H.; Yen, H.L.; Li, Y.; Seto, W.H.; Peiris, J.S.; et al. Quantification of influenza virus RNA in aerosols in patient rooms. PLoS ONE 2016, 11, e0148669. [Google Scholar] [CrossRef]
- Uhrbrand, K.; Koponen, I.K.; Schultz, A.C.; Madsen, A.M. Evaluation of air samplers and filter materials for collection and recovery of airborne norovirus. J. Appl. Microbiol. 2018, 124, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diagnostic Detection of Wuhan Coronavirus 2019 by Real-Time RT-PCR. 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/wuhan-virus-assay-v1991527e5122341d99287a1b17c111902.pdf?sfvrsn=d381fc88_2 (accessed on 11 January 2022).
- U.S. COVID Tracker 2022. Available online: https://covidactnow.org/ (accessed on 22 December 2022).
- Santarpia, J.L.; Herrera, V.L.; Rivera, D.N.; Ratnesar-Shumate, S.; Reid, S.P.; Ackerman, D.N.; Denton, P.W.; Martens, J.W.; Fang, Y.; Conoan, N.; et al. The size and culturability of patient-generated SARS-CoV-2 aerosol. J. Expo. Sci. Environ. Epidemiol. 2021, 32, 706–711. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Flamholz, A.; Phillips, R.; Milo, R. SARS-CoV-2 (COVID-19) by the numbers. Elife 2020, 9, e57309. [Google Scholar] [CrossRef] [PubMed]
- Aboubakr, H.A.; Sharafeldin, T.; Goyal, S. Stability of SARS-CoV-2 and other coronaviruses in the environment and on common touch surfaces and the influence of climatic conditions: A review. Transbound. Emerg. Dis. 2021, 68, 296–312. [Google Scholar] [CrossRef]
- Kampf, G.; Pfaender, S.; Goldman, E.; Steinmann, E. SARS-CoV-2 detection rates from surface samples do not implicate public surfaces as relevant sources for transmission. Hygiene 2021, 1, 24–40. [Google Scholar] [CrossRef]
- Ma, J.; Qi, X.; Chen, H.; Li, X.; Zhang, Z.; Wang, H.; Sun, L.; Zhang, L.; Guo, J.; Morawska, L.; et al. Coronavirus disease 2019 patients in earlier stages exhaled millions of severe acute respiratory syndrome Coronavirus 2 per hour. Clin. Infect. Dis. 2021, 72, e652–e654. [Google Scholar] [CrossRef]
- Santarpia, J.L.; Markin, N.W.; Herrera, V.L.; Ackerman, D.N.; Rivera, D.N.; Lucero, G.A.; Lisco, S.J. Infectious aerosol capture mask as environmental control to reduce spread of respiratory viral particles. Viruses 2022, 14, 1275. [Google Scholar] [CrossRef]
| Date | Surface | Air | Symptomatic Patients | Total Patients | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hand Sanitizer | Check-In | Testing Room | Check-In Station | Testing/Collection Room | AerosolSense | |||||||||||||
| <1 µm | 1–4 µm | >4.1 µm | ||||||||||||||||
| Ct | Copies/cm2 | Ct | Copies/cm2 | Ct | Copies/cm2 | Ct | Copies/L of Air | Ct | Copies/L of Air | Ct | Copies/L of Air | Ct | Copies/L of Air | Ct | Copies/L of Air | |||
| 29 June 2021 | ND | 37.78 ND ND | 194.94 | ND | 35.84 ND ND | 0.22 | ND | ND | ND | ND | 6 | 52 | ||||||
| 30 June 2021 | ND | ND | ND | ND | ND | ND | ND | ND | 8 | 50 | ||||||||
| 1 July 2021 | ND | ND | ND | ND | ND | ND | ND | ND | 8 | 16 | ||||||||
| 6 July 2021 | ND | ND | ND | ND | ND | ND | ND | ND | None | 38 | ||||||||
| 7 July 2021 | ND | ND | ND | ND | ND | ND | ND | ND | 6 | 53 | ||||||||
| 8 July 2021 | ND | ND | ND | ND | ND | ND | ND | ND | 18 | 34 | ||||||||
| 13 July 2021 | ND | ND | 38.08 ND ND | 165.27 | ND | ND | ND | ND | ND | 13 | 42 | |||||||
| 14 July 2021 | ND | 37.71 37.87 ND | 388.33 | ND | ND | ND | ND | ND | ND | 5 | 43 | |||||||
| 15 July 2021 | ND | ND | ND | ND | ND | ND | ND | ND | 10 | 23 | ||||||||
| 27 July 2021 | ND | ND | ND | ND | ND | ND | ND | ND | 17 | 35 | ||||||||
| Date | Surface | Air | Clinic Positive Tests | Percentage of Positive Tests | Percent Community Positivity | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hand Sanitizer | Check-In | Door Handle | Exam Room | Check-In | Exam Room | AerosolSense | Positive COVID-19 Tests | Total COVID-19 Tests | Percent | Douglas County | Sarpy County | Washington County | Douglas County | Sarpy County | Washington County | Omaha Metro | ||||||||||||||||
| <1 µm | 1–4 µm | >4.1 µm | <1 µm | 1–4 µm | >4.1 µm | |||||||||||||||||||||||||||
| Ct | Copies/cm2 | Ct | Copies/cm2 | Ct | Copies/cm2 | Ct | Copies/cm2 | Ct | Copies/L of Air | Ct | Copies/L of Air | Ct | Copies/L of Air | Ct | Copies/L of Air | Ct | Copies/L of Air | Ct | Copies/L of Air | Ct | Copies/L of Air | |||||||||||
| 7 September 2021 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | N/A | 5 | 46 | 10.87% | NC | NC | NC | NC | NC | NC | NC | |||||||||||
| 20 September 2021 | 37.20 ND ND | 269.11 | ND | ND | ND | ND | ND | ND | ND | ND | ND | N/A | 3 | 36 | 8.33% | NC | NC | NC | NC | NC | NC | NC | ||||||||||
| 20–22 September 2021 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 35.08 35.27 36.11 | 0.047 | 11 | 145 | 7.59% | 11.80% | 13.30% | 19.10% | 0.19% | 0.28% | 0.10% | 0.23% | ||||||||||
| 27 September 2021 | ND | ND | ND | ND | 38.33 ND ND | 17.14 | ND | ND | ND | ND | ND | N/A | 4 | 91 | 4.40% | NC | NC | NC | NC | NC | NC | NC | ||||||||||
| 28 September 2021 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | N/A | 4 | 82 | 4.88% | NC | NC | NC | NC | NC | NC | NC | |||||||||||
| 29 September 2021 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | N/A | 7 | 85 | 8.24% | NC | NC | NC | NC | NC | NC | NC | |||||||||||
| 27–29 September 2021 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 35.39 35.92 37.49 | 0.033 | 18 | 307 | 5.86% | 11.70% | 12.70% | 20.80% | 0.17% | 0.21% | 0.22% | 0.19% | ||||||||||
| 6 October 2021 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | N/A | 4 | 94 | 4.26% | NC | NC | NC | NC | NC | NC | NC | |||||||||||
| 2021/10/7 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | N/A | 8 | 97 | 8.25% | NC | NC | NC | NC | NC | NC | NC | |||||||||||
| 6–7 October 2021 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 37.62 ND ND | 0.009 | 12 | 191 | 6.28% | 11.90% | 12.00% | 11.10% | 0.17% | 0.21% | 0.23% | 0.19% | ||||||||||
| 21 October 2021 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | N/A | 6 | 45 | 13.33% | NC | NC | NC | NC | NC | NC | NC | |||||||||||
| 22 October 2021 | ND | 36.98 ND ND | 304 | ND | ND | ND | ND | ND | ND | ND | ND | N/A | 5 | 57 | 8.77% | NC | NC | NC | NC | NC | NC | NC | ||||||||||
| 20–25 October 2021 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 37.72 ND ND | 0.002 | 30 | 387 | 7.75% | 11.40% | 11.40% | 12.80% | 0.15% | 0.19% | 0.18% | 0.15% | ||||||||||
| 25–29 October 2021 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 35.67 37.39 ND | 0.009 | 32 | 422 | 7.58% | 13.20% | 12.40% | 13.70% | 0.16% | 0.15% | 0.17% | 0.20% | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stein, S.J.; Ravnholdt, A.R.; Herrera, V.L.; Rivera, D.N.; Williams, P.T.; Santarpia, J.L. SARS-CoV-2 Aerosol and Surface Detections in COVID-19 Testing Centers and Implications for Transmission Risk in Public Facing Workers. Int. J. Environ. Res. Public Health 2023, 20, 976. https://doi.org/10.3390/ijerph20020976
Stein SJ, Ravnholdt AR, Herrera VL, Rivera DN, Williams PT, Santarpia JL. SARS-CoV-2 Aerosol and Surface Detections in COVID-19 Testing Centers and Implications for Transmission Risk in Public Facing Workers. International Journal of Environmental Research and Public Health. 2023; 20(2):976. https://doi.org/10.3390/ijerph20020976
Chicago/Turabian StyleStein, Sarah J., Ashley R. Ravnholdt, Vicki L. Herrera, Danielle N. Rivera, Paul T. Williams, and Joshua L. Santarpia. 2023. "SARS-CoV-2 Aerosol and Surface Detections in COVID-19 Testing Centers and Implications for Transmission Risk in Public Facing Workers" International Journal of Environmental Research and Public Health 20, no. 2: 976. https://doi.org/10.3390/ijerph20020976
APA StyleStein, S. J., Ravnholdt, A. R., Herrera, V. L., Rivera, D. N., Williams, P. T., & Santarpia, J. L. (2023). SARS-CoV-2 Aerosol and Surface Detections in COVID-19 Testing Centers and Implications for Transmission Risk in Public Facing Workers. International Journal of Environmental Research and Public Health, 20(2), 976. https://doi.org/10.3390/ijerph20020976






