Perception of Social and Educational Quality of Life of Minors Diagnosed with Rare Diseases: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
O: To analyse, through a meta-analysis, the indicators of the quality of social and educational life perceived by the patients and their caregivers, in minors diagnosed with rare diseases.
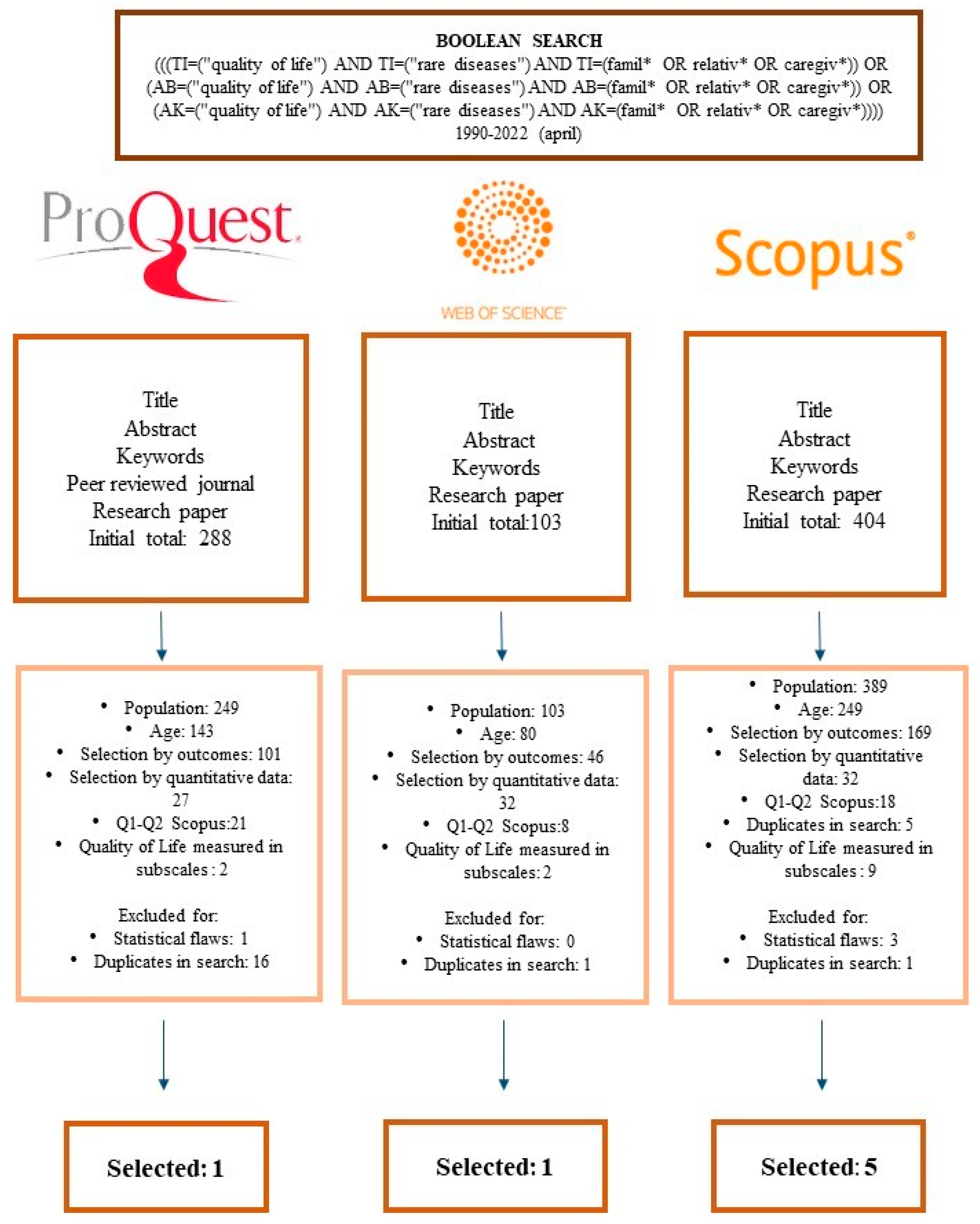
2. Materials and Methods
2.1. Inclusion Criteria
- -
- Sample: Minors diagnosed with RDs aged 0–18 years.Although the paediatric age is up to 14 years, this variable was extended to 18 years to analyse this situation during adolescence and puberty. In this way, the social and educational changes of these stages are contemplated, since they are essential in human development.
- -
- Methodology of the articles: empirical and quantitative. Publication date: From 1990 to 2022 (April).
- -
- Methodological rigour. Studies of recognised prestige, published in Quartile 1 and Quartile 2 journals (Scimago Journal & Country Rank).
- -
- Assessed psychometric instruments present in scientific publications that analyse the quality of life and contain subscales of the quality of social and educational life.
2.2. Exclusion Criteria
- -
- Studies with healthy minors that compare these with RD minors and do not specify the experimental and control groups, or other methodological issues that do not allow extracting accurate data about minors with RDs.
- -
- Studies that include adolescents and young adults aged 18 years or older.
- -
- Specific instruments of quality of life designed for a specific RD.
- -
- -
- ProQuest: “title”, “abstract”, “full text”, “article”, “English”, 1990–2022.
- -
- Web Of Science (WOS): “title,” and type of research “article”, 1990–2022.
- -
- Scopus: “Article title, abstract, keywords”, “English”, 1990–2022.
3. Results
3.1. Quality of Life: Social Subscale
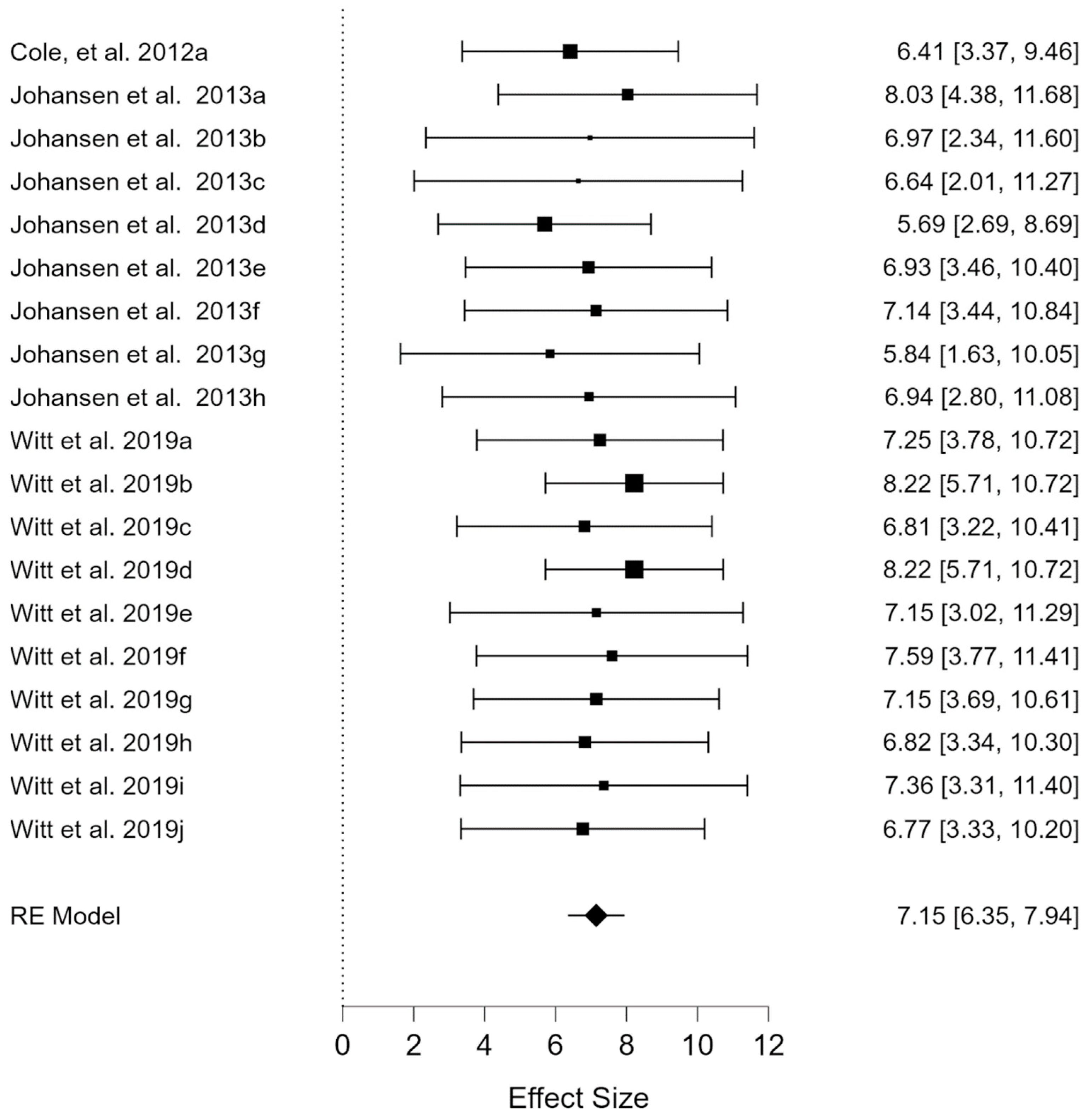
3.2. Quality of Life: Educational L Subscale
4. Discussion
4.1. Perception of the Quality of Social Life
4.2. Perception of the Quality of Life Related to Education
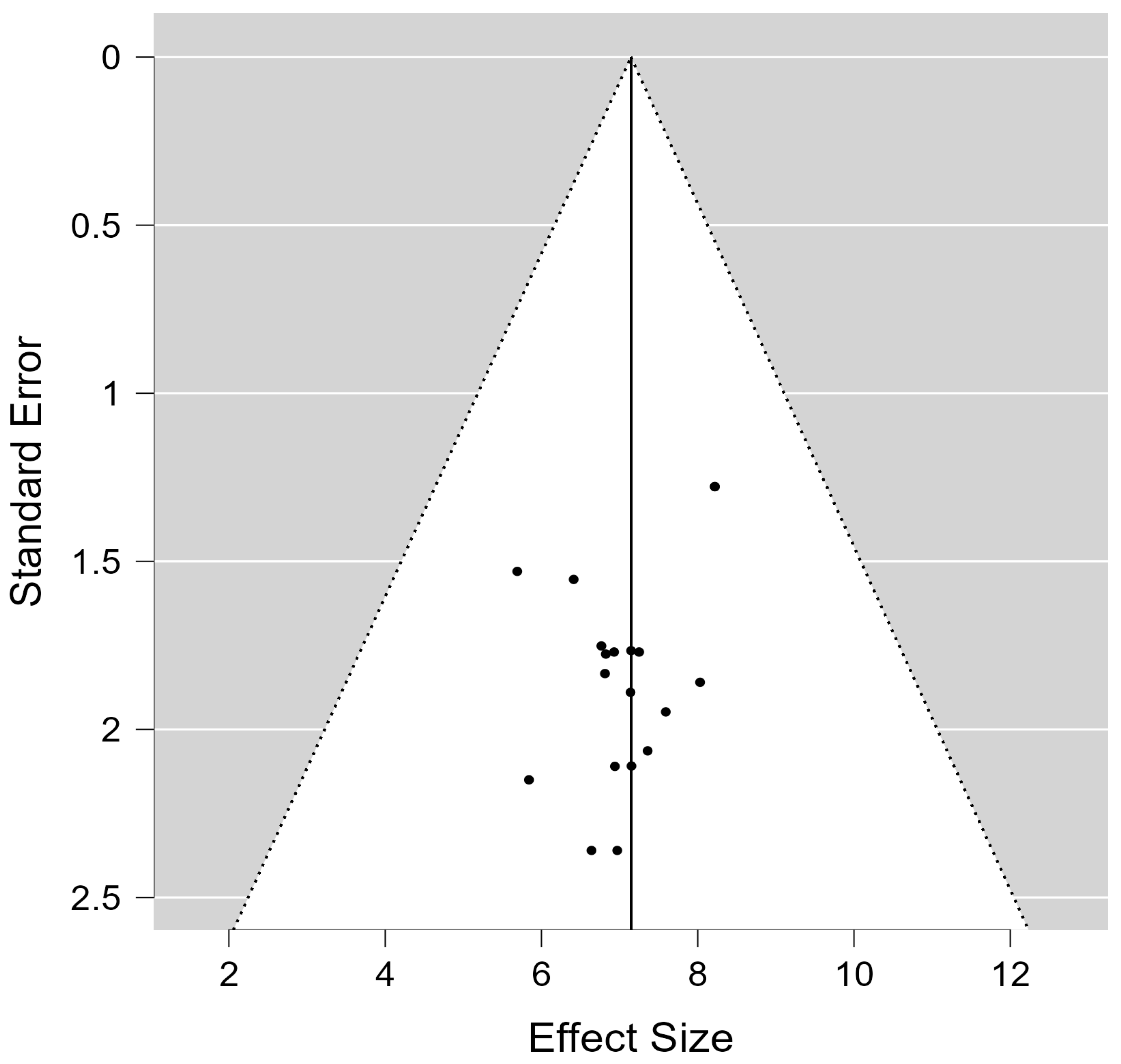
5. Limitations of the Study
6. Conclusions
- The first fundamental conclusion refers to the urgent need of conducting further quality research (quantitative and qualitative) about rare diseases. Currently, the situation of this type of studies is relatively scarce, which hinders the potential of the analysis and the attainment of highly reliable results.
- The instrument to measure the perception of the quality of life, at both the social and educational levels, is relevant. In this sense, the “instrument” model was very significant at the quantitative level.
- The informant is also of great relevance when perceiving the quality of life. These data allow us to warn, for future research, about the need of always indicating who the informant is, as well as her/his gender.
- The perception of the quality of social life is not related, according to our data, to the type of condition. This suggests that many of the people directly or indirectly affected by RDs have similar social needs. Therefore, when making decisions about, e.g., social policies, it would be convenient, in principle, to make such decisions jointly.
- The perception of the quality of life at the educational level is strongly related to the type of disease. This led us to think about the need of training teachers and schools’ directors in general about rare diseases and developing individualized educational plans for individuals with special needs along with providing the needed rehabilitation therapies in school.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Haendel, M.; Vasilevsky, N.; Unni, D.; Bologa, C.; Harris, N.; Rehm, H.; Hamosh, A.; Baynam, G.; Groza, T.; McMurry, J.; et al. How many rare diseases are there? Nat. Rev. Drug Discov. 2020, 19, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Marwaha, S.; Knowles, J.W.; Ashley, E.A. A guide for the diagnosis of rare and undiagnosed disease: Beyond the exome. Genome Med. 2022, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Millán, J.M.; García-García, G. Genetic Testing for Rare Diseases. Diagnostics 2022, 12, 809. [Google Scholar] [CrossRef] [PubMed]
- Von der Lippe, C.; Diesen, P.S.; Feragen, K.B. Living with a rare disorder: A systematic review of the qualitative literature. Mol. Genet. Genomic Med. 2017, 5, 758–773. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, J.; Denecke, J.; Barkmann, C.; Wiegand-Grefe, S. Quality of Life and Mental Health in Mothers and Fathers Caring for Children and Adolescents with Rare Diseases Requiring Long-Term Mechanical Ventilation. Int. J. Environ. Res. Public Health 2020, 17, 8975. [Google Scholar] [CrossRef] [PubMed]
- Pelentsov, L.J.; Fielder, A.L.; Laws, T.A.; Esterman, A.J. The supportive care needs of parents with a child with a rare disease: Results of an online survey. BMC Fam. Pract. 2016, 17, 88. [Google Scholar] [CrossRef]
- Lenderking, W.R.; Anatchkova, M.; Pokrzywinski, R.; Skalicky, A.; Martin, M.L.; Gelhorn, H. Measuring health-related quality of life in patients with rare disease. J. Patient-Rep. Outcomes 2021, 5, 61. [Google Scholar] [CrossRef]
- Austin, C.P.; Cutillo, C.M.; Lau, L.P.; Jonker, A.H.; Rath, A.; Julkowska, D.; Thomson, D.; Terry, S.F.; de Montleau, B.; Ardigò, D.; et al. Future of Rare Diseases Research 2017–2027: An IRDiRC Perspective. Clin. Transl. Sci. 2018, 11, 21–27. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- PRISMA-Statement Website. PRISMA Translations Policy. Available online: http://www.prisma-statement.org/PRISMAStatement/ (accessed on 22 November 2022).
- Botella, J.; Sánchez, J. Meta-Análisis en Ciencias Sociales y de La Salud; Síntesis: Madrid, Spain, 2015. [Google Scholar]
- Moreau, D.; Gamble, B. Conducting a meta-analysis in the age of open science: Tools, tips, and practical recommendations. Psychol. Method 2020, 7, 426–432. [Google Scholar] [CrossRef]
- Hunter, J.E.; Schmidt, F.L. Methods of Meta-Analysis: Correcting Error and Bias in Research Findings; Sage: Newbury Park, CA, USA, 2004. [Google Scholar] [CrossRef]
- Friese, M.; Frankenbach, J. p-Hacking and publication bias interact to distort meta-analytic effect size estimates. Psychol. Method 2020, 25, 456–471. [Google Scholar] [CrossRef]
- Botella, J.; Gambara, H. Qué es el Meta-Análisis; Biblioteca Nueva: Santander, Cantabria, 2022. [Google Scholar]
- Behan, L.; Driessens, C.; Carr, S.; Dell, S.; Harris, A.; Knibb, R.; Lucas, J. A Parent Reported Quality of Life Measure for Young Children with Primary Ciliary Dyskinesia: QOL-PCDPR. Eur. Resp. J. 2021, 58, OA1592. [Google Scholar] [CrossRef]
- Bosch, A.M.; Burlina, A.; Cunningham, A.; Bettiol, E.; Moreau-Stucker, F.; Koledova, E.; Benmedjahed, K.; Regnault, A. Assessment of the impact of phenylketonuria and its treatment on quality of life of patients and parents from seven European countries. Orphanet. J. Rare Dis. 2015, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Caliandro, P.; Grugni, G.; Padua, L.; Kodra, Y.; Tonali, P.; Gargantini, L.; Ragusa, L.; Crinò, A.; Taruscio, D. Quality of life assessment in a sample of patients affected by Prader–Willi syndrome. J. Paediatr. Child. Health 2007, 43, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.; McKendrick, F.; Titman, P.; Cant, A.J.; Pearce, M.S.; Cale, C.M.; Goldblatt, D.; Gennery, A.R. Health related quality of life and emotional health in children with chronic granulomatous disease: A comparison of those managed conservatively with those that have undergone haematopoietic stem cell transplant. J. Clin. Immunol. 2013, 33, 8–13. [Google Scholar] [CrossRef]
- Johansen, H.; Dammann, B.; Andresen, I.L.; Fagerland, M.W. Health-related quality of life for children with rare diagnoses, their parents’ satisfaction with life and the association between the two. Health Qual. Life Outcomes 2013, 11, 152. [Google Scholar] [CrossRef]
- Qi, X.; Xu, J.; Shan, L.; Li, Y.; Cui, Y.; Liu, H.; Wang, K.; Gao, L.; Kang, Z.; Wu, Q. Economic burden and health related quality of life of ultra-rare Gaucher disease in China. Orphanet. J. Rare Dis. 2021, 16, 358. [Google Scholar] [CrossRef]
- Witt, S.; Bloemeke, J.; Bullinger, M.; Dingemann, J.; Dellenmark-Blom, M.; Quitmann, J. Agreement between mothers’, fathers’, and children’s’ ratings on health-related quality of life in children born with esophageal atresia—A German cross-sectional study. BMC Pediatr. 2019, 19, 330. [Google Scholar] [CrossRef]
- Der Simonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials. 2015, 45, 139–145. [Google Scholar] [CrossRef]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Bonett, D.G.; Price Jr, R.M. Varying coefficient meta-analysis methods for odds ratios and risk ratios. Psych. Method 2015, 20, 394–406. [Google Scholar] [CrossRef]
- Martin-Andrés, A.; Luna del Castillo, J. Bioestadística Para Las Ciencias de la Salud; Ediciones Norma—Capitel: Madrid, Spain, 2004. [Google Scholar]
- Eggers, J. Nonlinear dynamics and breakup of free-surface flows. Rev. Mod. Phys. 1997, 69, 865. [Google Scholar] [CrossRef]
- Jak, S.; Cheung, M.W.L. Meta-analytic structural equation modeling with moderating effects on SEM Parameters. Psychol. Method 2020, 25, 430–455. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, J.; Boettcher, M.; Wiegand-Grefe, S.; Zapf, H. Being the Pillar for Children with Rare Diseases—A Systematic Review on Parental Quality of Life. Int. J. Environ. Res. Public Health 2021, 18, 4993. [Google Scholar] [CrossRef]
- Michel, G.; Bisegger, C.; Fuhr, D.C.; Abel, T.; The KIDSCREEN Group. Age and gender differences in health-related quality of life of children and adolescents in Europe: A multilevel analysis. Qual. Life Res. 2009, 18, 1147. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, T.M.; Aldao, A. Gender differences in emotion expression in children: A meta-analytic review. Psychol. Bull. 2013, 139, 735–765. [Google Scholar] [CrossRef] [PubMed]
- Christov-Moore, L.; Simpson, E.A.; Coudé, G.; Grigaityte, K.; Iacoboni, M.; Ferrari, P.F. Empathy: Gender effects in brain and behavior. Neurosci. Biobehav. Rev. 2014, 46, 604–627. [Google Scholar] [CrossRef] [PubMed]
- Briegel, W.; Greuel, J.; Stroth, S.; Heinrichs, N. Parents’ Perception of Their 2-10-Year-Old Children’s Contribution to The Dyadic Parent-Child Relationship in Terms of Positive and Negative Behaviors. Int. J. Environ. Res. Public Health 2019, 16, 1123. [Google Scholar] [CrossRef]
- Quittner, A.L.; Nicolais, C.J.; Saez-Flores, E. Integrating Patient-Reported Outcomes Into Research and Clinical Practice. In Kendig’s Disorders of the Respiratory Tract in Children; Wilmott, R., Bush, A., Deterding, R., Ratjen, F., Sly, P., Zar, H., Li, A.P., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 231–240. [Google Scholar] [CrossRef]
- Varni, J.W.; Seid, M.; Kurtin, P.S. PedsQL™ 4.0: Reliability and validity of the Pediatric Quality of Life Inventory™ Version 4.0 Generic Core Scales in healthy and patient populations. Med. Care 2001, 1, 800–812. [Google Scholar] [CrossRef]
- Witt, S.; Kolb, B.; Bloemeke, J.; Mohnike, K.; Bullinger, M.; Quitmann, J. Quality of life of children with achondroplasia and their parents-a German cross-sectional study. Orphanet. J. Rare Dis. 2019, 14, 194. [Google Scholar] [CrossRef]
- Thomasgard, M.; Metz, W.P. The vulnerable child syndrome revisited. J. Dev. Behav. Pediatr. 1995, 16, 47–53. [Google Scholar] [CrossRef]
- Schweid, R. The Caring Class. Home Health Aides in Crisis; Cornell University Press: New York, NY, USA, 2021. [Google Scholar]
- Calzada, E.J.; Eyberg, S.M.; Rich, B.; Querido, J.G. Parenting disruptive preschoolers: Experiences of mothers and fathers. J. Abnorm. Child. Psychol. 2004, 32, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.Y.; Chin-Chen, W.; Chun-Ying, W. Gender Differences in Caring for Children with Genetic or Rare Diseases: A Mixed-Methods Study. Child 2022, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Al-Janabi, H.; Mallett, A.; Quinlan, C.; Scheffer, I.E.; Howell, K.B.; Goranitis, I. Parental health spillover effects of paediatric rare genetic conditions. Qual. Life Res. 2020, 29, 2445–2454. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Xu, H.; Wu, B. Gender differences in quality of life among community-dwelling older adults in low- and middle-income countries: Results from the Study on global AGEing and adult health (SAGE). BMC Public Health 2020, 20, 114. [Google Scholar] [CrossRef] [PubMed]
- Uhlenbusch, N.; Löwe, B.; Depping, M.K. Perceived burden in dealing with different rare diseases: A qualitative focus group study. BMJ Open 2019, 9, e033353. [Google Scholar] [CrossRef]
- Uhlenbusch, N.; Löwe, B.; Härter, M.; Schramm, C.; Weiler-Normann, C.; Depping, M.K. Depression and anxiety in patients with different rare chronic diseases: A cross-sectional study. PLoS ONE 2019, 14, e0211343. [Google Scholar] [CrossRef]
- Drukker, M.; Kaplan, C.; Feron, F.; van Os, J. Children’s health-related quality of life, neighbourhood socio-economic deprivation and social capital. A contextual analysis. Soc. Sci. Med. 2003, 57, 825–841. [Google Scholar] [CrossRef]
- Eurordis. European Organization for Rare Disorders. Rare Diseases: Understanding this Public Health Priority; European Organization for Rare Disorders: Paris, France, 2005; Available online: https://www.eurordis.org/wp-content/uploads/2009/12/princeps_document-EN.pdf (accessed on 16 November 2022).
- Verger, S.; Negre, F.; Roselló, M.R.; Paz-Lourido, B. Inclusion and equity in educational services for children with rare diseases: Challenges and opportunities. Child. Youth Serv. Rev. 2020, 119, 10551. [Google Scholar] [CrossRef]
- Paz-Lourido, B.; Negre, F.; de la Iglesia, B.; Verger, S. Influence of schooling on the health-related quality of life of children with rare diseases. Health Qual. Life Outcomes 2020, 18, 109. [Google Scholar] [CrossRef]
- Jaeger, G.; Röjvik, A.; Berglund, B. Participation in society for people with a rare diagnosis. Disabil. Health J. 2015, 8, 44–50. [Google Scholar] [CrossRef]
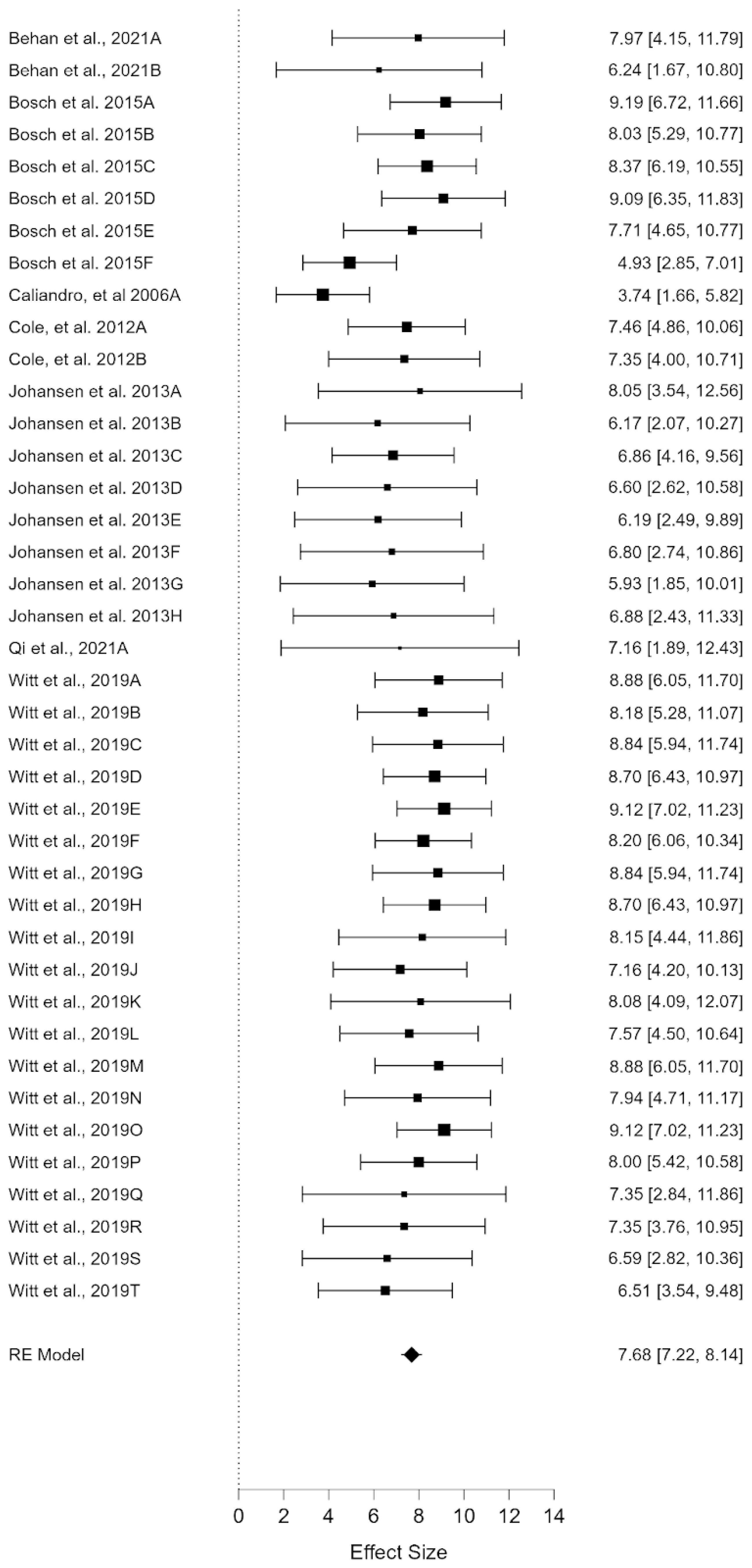

| Studies | N | Mean Age | Nationality | Geographic Area | Instrument | Rare Disease |
|---|---|---|---|---|---|---|
| Behan et al., 2021A [16] | 71 | 9.5 | United Kingdom, United States of America, Ireland and Canada | Mixed | QOL-PCD | Primary ciliary diskinesia |
| Behan et al., 2021B [16] | 85 | 15.4 | United Kingdom, United States of America, Ireland and Canada | Mixed | QOL-PCD | Primary ciliary diskinesia |
| Bosch et al., (2015)A [17] | 92 | 9.8 | France, Germany, Italy, Holland, Spain, Turkey, United Kingdom | Europe | PedsQL | Phenylketonuria (PKU) |
| Bosch et al., (2015)B [17] | 92 | 9.8 | France, Germany, Italy, Holland, Spain, Turkey, United Kingdom | Europe | PedsQL | Phenylketonuria (PKU) |
| Bosch et al., (2015)C [17] | 92 | 9.8 | France, Germany, Italy, Holland, Spain, Turkey, United Kingdom | Europe | PedsQL | Phenylketonuria (PKU) |
| Bosch et al., (2015)D [17] | 110 | 14.5 | France, Germany, Italy, Holland, Spain, Turkey, United Kingdom | Europe | PedsQL | Phenylketonuria (PKU) |
| Bosch et al., (2015)E [17] | 110 | 14.5 | France, Germany, Italy, Holland, Spain, Turkey, United Kingdom | Europe | PedsQL | Phenylketonuria (PKU) |
| Bosch et al., (2015)F [17] | 253 | null | France, Germany, Italy, Holland, Spain, Turkey, United Kingdom | Europe | CHQ-PF28 | Phenylketonuria (PKU) |
| Caliandro et al., (2006)A [18] | 9 | 11.67 | Italy | Europe | CHQ-PF56 | Prader Willi |
| Cole et al., (2012)A [19] | 17 | 9 | UK, Ireland | Europe | PedsQL | Chronic Granulomatous Disease |
| Cole et al., (2012)B [19] | 17 | 9 | UK, Ireland | Europe | PedsQL | Chronic Granulomatous Disease |
| Johansen et al., (2013)A [20] | 67 | 11 | Norway | Europe | PedsQL | Chronic Granulomatous Disease |
| Johansen et al., (2013)B [20] | 17 | 12 | Norway | Europe | PedsQL | Arthrogryposis multiplex congenita (AMC) |
| Johansen et al., (2013)C [20] | 11 | 14 | Norway | Europe | PedsQL | Marfan’s syndrome (MRF) |
| Johansen et al., (2013)D [20] | 21 | 13 | Norway | Europe | PedsQL | Ehlers–Danlos syndrome (EDS) |
| Johansen et al., (2013)E [20] | 28 | 10 | Norway | Europe | PedsQL | Short stature due to skeletal dysplasia (StSh) |
| Johansen et al., (2013)F [20] | 23 | 12 | Norway | Europe | PedsQL | Osteogenesis imperfect (OI) |
| Johansen et al., (2013)G [20] | 42 | 11 | Norway | Europe | PedsQL | Spina bifida/myelomeningocele (MMC). |
| Johansen et al., (2013)H [20] | 209 | 14 | Norway | Europe | PedsQL | CLD + AMC + MRF + EDS + StSh + OI + MMC |
| Qi et al., (2021)A [21] | 49 | 7.5 | China | Asia | SF-36 | Gaucher disease |
| Witt et al., (2019)A [22] | 16 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)B [22] | 16 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)C [22] | 16 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)D [22] | 16 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)E [22] | 16 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)F [22] | 16 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)G [22] | 16 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)H [22] | 16 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)I [22] | 23 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)J [22] | 23 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)K [22] | 23 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)L [22] | 23 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)M [22] | 17 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)N [22] | 17 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)O [22] | 17 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)P [22] | 17 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)Q [22] | 47 | 9.75 | German | Europe | PedsQL | Achondroplasia |
| Witt et al., (2019)R [22] | 47 | 9.75 | German | Europe | PedsQL | Achondroplasia |
| Witt et al., (2019)S [22] | 73 | 9.75 | German | Europe | PedsQL | Achondroplasia |
| Witt et al., (2019)T [22] | 73 | 9.75 | German | Europe | PedsQL | Achondroplasia |
| Model Name | TauSq | R² | Q | Df | p-Value |
|---|---|---|---|---|---|
| Model 1—Simple | 1.31 | 0.00 | 607.44 | 20 | <0.00 |
| Model 2—Masculine gender | 1.31 | 0.00 | 607.44 | 20 | <0.00 |
| Model 6—Nationality | 2.69 | 0.00 | 2210.12 | 39 | <0.00 |
| Model 7—Measurement instrument | 2.69 | 0.69 | 2210.12 | 39 | <0.00 |
| Model 8—Informant | 2.69 | 0.61 | 2210.12 | 39 | <0.00 |
| Covariate | Coefficient | Standard Error | 95% Lower | 95% Upper | Z-Value | p-Value | VIF |
|---|---|---|---|---|---|---|---|
| Intercept | 4.93 | 0.91 | 3.13 | 6.72 | 5.4 | 0 | |
| PedsQL | 2.39 | 0.93 | 0.55 | 4.24 | 2.55 | 0.01 | Q = 37.30. df = 5. p = 0.0000 |
| CHQ-PF56 | −1.19 | 1.33 | −3.81 | 1.43 | −0.89 | 0.37 | |
| Pv PEdsQOL | 3.47 | 0.94 | 1.62 | 5.32 | 3.67 | 0.00 | |
| QOL-PCD | 2.17 | 1.13 | −0.03 | 4.39 | 1.93 | 0.05 | |
| SF-36 | 2.23 | 1.34 | −0.40 | 4.86 | 1.66 | 0.09 |
| Covariate | Coefficient | Standard Error | 95% Lower | 95% Upper | Z-Value | p-Value | VIF |
|---|---|---|---|---|---|---|---|
| Intercept | 8.36 | 0.43 | 7.5054 | 9.21 | 19.17 | 0 | Q = 23.89. df = 4. p = 0.0001 |
| Mother | −0.16 | 0.62 | −1.384 | 1.05 | −0.27 | 0.78 | |
| No report | −2.18 | 0.76 | −3.6782 | −0.68 | −2.86 | 0.00 | |
| Patients | −0.15 | 0.53 | −1.1998 | 0.88 | −0.3 | 0.76 | |
| Parents | −1.69 | 0.52 | −2.7328 | −0.66 | −3.22 | 0.00 |
| Covariate | Coefficient | Standard Error | 95% Lower | 95% Upper | Z-Value | p-Value | VIF |
|---|---|---|---|---|---|---|---|
| Intercept | 6.94 | 0.87 | 5.23 | 8.65 | 7.96 | 0 | |
| Arthrogryposis multiplex congenita (AMC) | −0.77 | 1.99 | −4.6 | 3.14 | −0.39 | 0.69 | Q = 12.18. df = 14. p = 0.59 |
| CLD + AMC + MRF + EDS + StSh + OI + MMC | −0.06 | 1.93 | −3.86 | 3.73 | −0.03 | 0.97 | |
| Congenital limb deficiency (CLD) | 1.10 | 1.95 | −2.72 | 4.93 | 0.56 | 0.57 | |
| Ehlers-Danlos syndrome (EDS) | −0.34 | 1.98 | −4.23 | 3.54 | −0.18 | 0.86 | |
| Chronic Granulomatous Disease | 0.46 | 1.52 | −2.52 | 3.44 | 0.3 | 0.76 | |
| Oesophageal atresia | 1.45 | 0.97 | −0.46 | 3.36 | 1.49 | 0.13 | |
| Marfan’s syndrome (MRF) | −0.08 | 1.97 | −3.96 | 3.78 | −0.04 | 0.96 | |
| Osteogenesis imperfect (OI) | −0.14 | 1.98 | −4.03 | 3.73 | −0.07 | 0.94 | |
| Phenylketonuria (PKU) | 0.93 | 1.12 | −1.26 | 3.13 | 0.84 | 0.40 | |
| Prader Willi | −3.20 | 1.96 | −7.05 | 0.64 | −1.63 | 0.10 | |
| Primary ciliary diskinesia | 0.15 | 1.50 | −2.80 | 3.11 | 0.1 | 0.91 | |
| Short stature due to skeletal dysplasia (StSh) | −0.75 | 1.96 | −4.61 | 3.09 | −0.39 | 0.70 | |
| Spina bifida/myelomeningocele (MMC). | −1.01 | 1.96 | −4.85 | 2.82 | −0.52 | 0.60 | |
| Gaucher disease | 0.21 | 1.97 | −3.65 | 4.07 | 0.11 | 0.91 |
| Studies | N | Mean Age | Nationality | Geographic Area | Instrument | Rare Disease |
|---|---|---|---|---|---|---|
| Cole et al., (2012)a [19] | 17 | 9 | UK, Ireland | Europe | PedsQL | Chronic Granulomatous Disease |
| Johansen et al., (2013)a [20] | 67 | 11 | Norway | Europe | PedsQL | Congenital limb deficiency (CLD) |
| Johansen et al., (2013)b [20] | 17 | 12 | Norway | Europe | PedsQL | Arthrogryposis multiplex congenita (AMC) |
| Johansen et al., (2013)c [20] | 11 | 14 | Norway | Europe | PedsQL | Marfan’s syndrome (MRF) |
| Johansen et al., (2013)d [20] | 21 | 13 | Norway | Europe | PedsQL | Ehlers-Danlos syndrome (EDS) |
| Johansen et al., (2013)e [20] | 28 | 10 | Norway | Europe | PedsQL | Short stature due to skeletal dysplasia (StSh) |
| Johansen et al., (2013)f [20] | 23 | 12 | Norway | Europe | PedsQL | Osteogenesis imperfect (OI) |
| Johansen et al., (2013)g [20] | 42 | 11 | Norway | Europe | PedsQL | Spina bifida/myelomeningocele (MMC). |
| Johansen et al., (2013)h [20] | 209 | 15 | Norway | Europe | PedsQL | CLD + AMC + MRF + EDS + StSh + OI + MMC |
| Witt et al., (2019)a [22] | 16 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)b [22] | 16 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)c [22] | 16 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)d [22] | 16 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)e [22] | 23 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)f [22] | 23 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)g [22] | 17 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)h [22] | 17 | 8.03 | France | Europe | Pv-PEdsQOL | Oesophageal atresia |
| Witt et al., (2019)i [22] | 47 | 9.75 | German | Europe | PedsQL | Achondroplasia |
| Witt et al., (2019)j [22] | 73 | 9.75 | German | Europe | PedsQL | Achondroplasia |
| Model Name | TauSq | R² | Q | df | p-Value |
|---|---|---|---|---|---|
| Model 1 simple (intercept) | 0.61 | 0.00 | 50.75 | 9 | <0.00 |
| Model 5 measurement instrument | 0.61 | 0.16 | 84.56 | 18 | <0.00 |
| Model 6 type of rare disease | 0.61 | 0.51 | 84.56 | 18 | <0.00 |
| Covariate | Coefficient | Standard Error | 95% Lower | 95% Upper | Z-Value | p-Value | VIF |
|---|---|---|---|---|---|---|---|
| Intercept | 7.03 | 0.35 | 6.34 | 7.72 | 19.94 | 0 | Q = 20.82. df = 10. p = 0.02 |
| Arthrogryposis multiplex congenita (AMC) | −0.06 | 0.79 | −1.62 | 1.49 | −0.08 | 0.93 | |
| CLD + AMC + MRF + EDS + StSh + OI + MMC | −0.09 | 0.57 | −1.22 | 1.03 | −0.16 | 0.86 | |
| Congenital limb deficiency (CLD) | 0.99 | 0.60 | −0.18 | 2.17 | 1.66 | 0.09 | |
| Ehlers-Danlos syndrome (EDS) | −1.34 | 0.64 | −2.61 | −0.07 | −2.07 | 0.03 | |
| Chronic Granulomatous Disease | −0.62 | 0.67 | −1.93 | 0.69 | −0.93 | 0.32 | |
| Oesophageal atresia | 0.42 | 0.40 | −0.37 | 1.23 | 1.05 | 0.29 | |
| Marfan’s syndrome (MRF) | −0.39 | 0.90 | −2.16 | 1.37 | −0.44 | 0.66 | |
| Osteogenesis imperfect (OI) | 0.10 | 0.68 | −1.23 | 1.44 | 0.15 | 0.87 | |
| Short stature due to skeletal dysplasia (StSh) | −0.10 | 0.64 | −1.37 | 1.16 | −0.16 | 0.87 | |
| Spina bifida/myelomeningocele (MMC). | −1.19 | 0.64 | −2.46 | 0.07 | −1.84 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coca, J.R.; Gómez-Redondo, S.; Soto-Sánchez, A.; Lozano-Blasco, R.; Romero-Gonzalez, B. Perception of Social and Educational Quality of Life of Minors Diagnosed with Rare Diseases: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 933. https://doi.org/10.3390/ijerph20020933
Coca JR, Gómez-Redondo S, Soto-Sánchez A, Lozano-Blasco R, Romero-Gonzalez B. Perception of Social and Educational Quality of Life of Minors Diagnosed with Rare Diseases: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2023; 20(2):933. https://doi.org/10.3390/ijerph20020933
Chicago/Turabian StyleCoca, Juan R., Susana Gómez-Redondo, Alberto Soto-Sánchez, Raquel Lozano-Blasco, and Borja Romero-Gonzalez. 2023. "Perception of Social and Educational Quality of Life of Minors Diagnosed with Rare Diseases: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 20, no. 2: 933. https://doi.org/10.3390/ijerph20020933
APA StyleCoca, J. R., Gómez-Redondo, S., Soto-Sánchez, A., Lozano-Blasco, R., & Romero-Gonzalez, B. (2023). Perception of Social and Educational Quality of Life of Minors Diagnosed with Rare Diseases: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 20(2), 933. https://doi.org/10.3390/ijerph20020933










