Abstract
Rheumatoid arthritis is potentially connected to ocular disorders, such as corneal inflammation and lacrimal gland destruction. This study aimed to evaluate the risk of dry eye disease (DED) and corneal surface damage among patients with rheumatoid arthritis. In a nationwide cohort study, we utilized Taiwan’s National Health Insurance research database and conducted propensity score matching to compare the risks of DED and corneal surface damage between patients with and without rheumatoid arthritis. Proportional hazards regression analyses were used to calculate the adjusted hazard ratio (aHR) and 95% confidence interval (CI) for the outcomes of interest. The matching procedure generated 33,398 matched pairs with 501,377 person-years of follow-up for analyses. The incidence of DED was 23.14 and 10.25 per 1000 person-years in patients with and without rheumatoid arthritis, respectively. After adjusting for covariates, rheumatoid arthritis was significantly associated with DED (aHR: 2.03, 95% CI: 1.93–2.13, p < 0.0001). The association was generally consistent across the subgroups of age, sex, use of systemic corticosteroids, and different comorbidity levels. In addition, patients with rheumatoid arthritis had a higher risk of corneal surface damage (aHR: 1.36, 95% CI: 1.21–1.51, p < 0.0001) compared to control subjects. Other independent factors for corneal surface damage were age and sleeping disorders. Rheumatoid arthritis was associated with an increased risk of DED and corneal surface damage. Ophthalmological surveillance is required to prevent vision-threatening complications in this susceptible population.
1. Introduction
Dry eye disease (DED) is a growing epidemic worldwide, with an estimated prevalence of 5% to 64% [,]. In Taiwan, epidemiological studies have reported a prevalence rate of DED ranging from 4.6% to 33.7% [,]. DED is potentially associated with ocular surface injury and visual disturbance, which makes it the most common diagnosis in the eye care service []. Additionally, DED adversely affects the quality of life and decreases the workforce productivity, placing a large financial burden on patients and medical care system [,].
The pathogenesis of DED is multifaceted and can be classified into aqueous-deficient and evaporative subtypes []. An unstable tear film, hyperosmolarity, inflammation and damage of ocular surface, and neurosensory abnormalities are deemed as the major contributing factors of DED []. DED might develop into superficial punctate keratitis, filamentary keratitis, and superior limbic keratoconjunctivitis []. Importantly, disruption of the corneal barrier function might result in serious corneal surface pathologies, such as corneal erosion, ulcers, and opacity [].
Rheumatoid arthritis (RA) is one of the most common systemic autoimmune diseases, with an increasing prevalence rate by 7.4% from 1990 to 2017 []. In Taiwan, the annual incidence rate of RA was 15.8 cases per 100,000 population, and the prevalence rate increased steadily from 57.7 in 2000 to 99.6 per 100,000 people in 2007 [,]. RA is characterized by progressive articular damage, potentially leading to functional disability and premature mortality []. RA is also associated with extra-articular manifestations, potentially involving the eyes. Common ocular manifestations of RA include corneal inflammation, lacrimal gland dysfunction, and uveitis, sharing similar pathogenic mechanisms to DED []. Noticeably, studies have shown that DED is more likely to progress into corneal erosion and ulcers among patients with systemic autoimmune diseases [,].
The relationship between RA and DED or corneal surface damages is still not fully clarified due to some methodological limitations of previous studies, including a small sample size (n < 1000) [,,,,,,], insufficient adjustment for confounders [,,,,,], and restriction to specific medical institutions [,,,,,] or populations [,]. Furthermore, relevant risk factors for corneal surface damage among patients with RA remain largely unknown. Accordingly, we conducted a population-based cohort study using Taiwan’s National Health Insurance (NHI) research database to clarify the temporal association between RA and aqueous-deficient DED or corneal surface damage. The objective of this study was to compare the risks of DED and corneal surface injury between patients with and without RA. In addition, we also sought to identify independent factors for corneal diseases associated with RA. Based on the current evidence [,,,,,,], we hypothesized that RA was associated with more aqueous-deficient DED and corneal surface damage in the nationwide autoimmune population.
2. Material and Methods
2.1. Source of Data
This study was approved by the Institutional Review Board of Taipei Medical University (approval no. TMU-JIRB-N202210011; date of approval on 6 October 2022). Written informed consent was waived by the Institutional Review Board due to the retrospective nature of this study. All methods of this study were performed in accordance with the standards of the Helsinki Declaration-2013 and relevant study guidelines []. Taiwan’s National Health Insurance program was launched in March 1995 and covers more than 99% of Taiwanese and non-Taiwanese working or studying in Taiwan. The NHI research database contains comprehensive registration files and original claims data of the insured beneficiaries, including demographic attributes, medical diagnoses, and prescription drugs. Research articles based on the NHI research database have been published in prominent scientific journals [,,]. The study subjects were included from the three Longitudinal Health Insurance Databases (LHID2000, LHID2005, and LHID2010), which contains original claims data of one million randomly sampled subjects from the original NHI research database in the years 2000, 2005, and 2010, respectively [].
2.2. Inclusion and Exclusion Criteria
Inclusion criteria were patients who had at least two rheumatology clinic visits with the diagnosis of RA from 1 January 2002 to 30 June 2013. The International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes were used to identify the diagnoses of RA, coexisting diseases, and ocular diseases (Supplementary Table S1). The index date was defined as the date of the first RA diagnosis. We excluded patients who had any diagnosis of DED, corneal ulcers, recurrent corneal erosion, corneal opacity, interstitial and deep keratitis, corneal neovascularization, ocular burns, or open globe injury in the ophthalmology service before the index date. We also excluded patients who had been prescribed eye lubricants before the index date. Patients who died during the follow-up period were also excluded.
2.3. Study Outcome
The primary outcome was DED, which was defined as the diagnosis made twice by certified ophthalmologists with prescriptions of cyclosporine ophthalmic emulsion (Restasis®) treatment in the ophthalmology service (Supplementary Table S1). In the reimbursement regulations of Taiwan’s National Health Insurance, Restasis® ophthalmic emulsion is indicated when patients have a Schirmer test score of <5 mm in 5 min [,]. The secondary outcomes were secondary Sjögren’s syndrome (SS) and severe corneal surface damages, which were defined as any diagnosis of corneal ulcers, recurrent corneal erosion, or corneal opacity made twice by certified ophthalmologists.
2.4. Baseline Patient Characteristics
Monthly insurance premium was classified into 0–500, 501–800, and >800 USD. We used the ICD-9-CM codes of physicians’ diagnoses within 24 months before the index date to identify the following coexisting diseases, chosen on the basis of data availability and physiological plausibility: hypertension, diabetes mellitus, ischemic heart disease, chronic obstruction pulmonary disease, chronic liver disease, chronic kidney disease, cerebrovascular disease, thyroid disease, depressive disorder, anxiety disorder, sleeping disorder, and malignancy (Supplementary Table S1) []. The Charlson comorbidity index score was used to assess the comorbidity condition of studied subjects []. The concurrent use of systemic corticosteroids prescribed within 180 days after the index date was also examined in the analysis. We also evaluated the numbers of hospitalizations and emergency visits within 24 months before the index date to examine the level of medical resource utilization of the included subjects.
2.5. Statistical Analysis
Continuous variables were expressed as the mean ± standard deviation or median with interquartile range. Categorical variables were presented as the frequency and percentage. We used a non-parsimonious multivariable logistic regression model to obtain a propensity score for people with or without RA. Each RA patient was matched to a non-RA control using a greedy matching algorithm within a tolerance limit of 0.05 and without replacement to adjust for the distributions of age, sex, and monthly insurance premium between the two groups []. The balance of the baseline covariate distribution between matched pairs was evaluated using absolute standardized mean difference []. Multivariable proportional hazards regressions models were used to calculate the adjusted hazard ratio (aHR) and 95% confidence interval (CI) for the ophthalmological outcome. Factors controlled in the multivariable models were age, sex, monthly insurance premium, coexisting diseases, Charlson comorbidity index score, use of systemic corticosteroids, number of hospitalizations, and number of emergency room visits. We also used Kaplan–Meier curves and log-rank tests to compare the cumulative incidence of study outcomes between the two groups. Subgroup analyses were also conducted by age ≥ or <65 years, male or female, different Charlson comorbidity index scores, and use of systemic corticosteroids or not. A two-sided level of 0.05 was considered statistically significant. All the statistical analyses were conducted using Statistics Analysis System (SAS), Version 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Baseline Patient Characteristics
The propensity score matching analysis generated 33,398 matched pairs with 501,377 person-years of follow-up for comparison (Supplementary Figure S1). Table 1 shows the baseline patient and clinical characteristics of the RA and non-RA subjects. Of note, patients with RA were more likely to have more coexisting diseases, higher Charlson comorbidity index scores, use of systemic corticosteroids, and higher number of hospitalizations and emergence room visits.

Table 1.
Baseline characteristics of patients with and without rheumatoid arthritis.
3.2. Risk of Dry Eye Disease
In the follow-up period, the incidence of DED was 23.14 and 10.25 per 1000 person-years in the RA and non-RA groups, respectively (Table 2). The duration between enrollment and DED diagnosis was a median of 3.2 (interquartile range: 1.2–6.0) years in the RA patients and 4.3 (1.9–6.9) years in the non-RA controls (p < 0.0001). Table 3 demonstrates the results of univariate and multivariable proportional hazards regression analyses for DED. RA was significantly associated with more DED compared to non-RA controls (aHR: 2.03, 95% CI: 1.93–2.13, p < 0.0001). The cumulative incidence of DED in the two groups is shown in Figure 1A. RA was also associated with increased SS (aHR: 4.11, 95% CI: 3.69–4.58, p < 0.0001). Other independent factors for DED were age (aHR: 1.02), sex (male vs. female, aHR: 0.46), monthly insurance premium (501–800 vs. 0–500 USD, aHR: 0.78; ≥801 vs. 0–500 USD, aHR: 1.02), hypertension (aHR: 0.90), chronic liver disease (aHR: 1.25), thyroid disease (aHR: 1.48), anxiety disorder (aHR: 1.27), sleeping disorder (aHR: 1.13), Charlson comorbidity index (1 vs. 0, aHR: 1.22; 2 vs. 0, aHR: 1.16; ≥3 vs. 0, aHR: 0.68), use of systemic corticosteroids (aHR: 1.36), and number of hospitalizations (1 vs. 0, aHR: 0.88; 2 vs. 0, aHR: 0.94; ≥3 vs. 0, aHR: 0.71). Subgroup analyses showed consistent associations between RA and DED, including different age groups, male or female, different Charlson comorbidity index scores, and use of systemic corticosteroids or not (Table 4).

Table 2.
Risk of dry eye disease and corneal surface damage for patients with and without rheumatoid arthritis.

Table 3.
Univariate and multivariable analyses for dry eye disease.
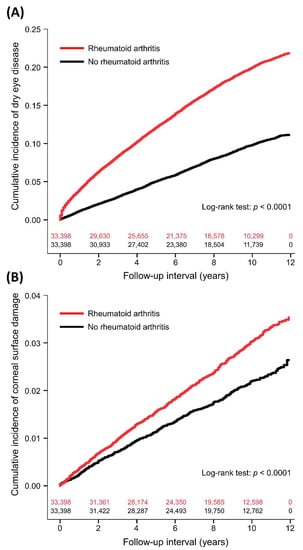
Figure 1.
Cumulative incidence of dry eye disease (A) and corneal surface damage (B) between patients with and without rheumatoid arthritis with number of subjects at risk.

Table 4.
Subgroup analysis of dry eye disease for patients with and without rheumatoid arthritis.
3.3. Risk of Corneal Surface Damage
The incidence of corneal surface damage was 3.07 and 2.23 per 1000 person-years in the RA and non-RA groups, respectively (Table 2). The time to corneal surface damage was a median of 4.1 years (interquartile range: 1.8–7.1) in the RA patients and 4.0 years (interquartile range: 1.8–6.8) in the non-RA controls (p = 0.7746). After adjusting for covariates, RA was significantly associated with more corneal surface damage compared to non-RA controls (aHR: 1.36, 95% CI: 1.21–1.51, p < 0.0001; Table 5 and Figure 1B). Further analyses showed that RA was significantly associated with increased risks of corneal ulcer (aHR: 1.30, 95% CI: 1.11–1.52, p = 0.0009) and recurrent corneal erosion (aHR: 1.99, 95% CI: 1.55–2.55, p < 0.0001). Other independent factors for corneal surface damage were age (aHR: 1.01) and sleeping disorder (aHR: 0.80).

Table 5.
Univariate and multivariable analyses for corneal surface damage.
4. Discussion
In the present study, we found that RA was significantly associated with increased DED and corneal surface damages in a population-based matched cohort. The association was generally consistent across the subgroups of age, sex, use of systemic corticosteroids, and varying comorbidity levels. We also found some independent factors for corneal diseases associated with RA, providing evidence in early identification and prophylaxis for severe corneal morbidities. Our results highlight the need for regular ophthalmology follow-up for potential corneal complications among patients with RA.
Few studies have compared the incidence of DED and corneal diseases between RA and non-RA subjects. Two studies evaluated the association between RA activity and DED [,]. Three case reports demonstrated the complication of corneal ulcers in patients with RA [,,]. Another study reported that 1.9% patients with RA had inflammatory ocular diseases in an older male population but did not analyze the relative risk []. DED is a highly prevalent disease [,,,], and there is little evidence indicating the risk difference in DED between RA and general population. Our results suggested that patients with RA had an increased risk of DED and corneal surface damages, filling the knowledge gap in the field of eye care for RA. A recent meta-analysis reported that DED is the most common ocular manifestation of RA with an estimated prevalence of 16%, which is slightly lower than our results []. Additionally, our study identified several risk factors for DED and corneal surface damages associated with RA, which were not reported in previous studies [,,,,,,]. These findings might be useful in the risk stratification and early prevention for serious ocular complications.
The biology and pathogenesis of DED and peripheral ulcerative keratitis in RA are still not clearly elucidated. Current evidence suggests that lymphocyte and plasma cell infiltration in cornea and lacrimal gland destruction are possibly responsible for RA-associated DED []. In addition, destructive inflammatory cells in the marginal corneal cause progressive stromal degradation and thinning, corneal perforation, and ulceration [,]. Both T cells and antibodies associated with RA are involved in the corneal inflammation []. Furthermore, cornea-resident antigen-presenting cells, proinflammatory cytokines and chemokines, and the imbalance between matrix metalloproteinases and tissue inhibitors also contribute to corneal epithelial destruction and disease progression in RA [,,].
Our findings highlight the importance of regular ophthalmology examinations for potential corneal manifestations in patients with RA. Early recognition and treatment of DED in RA are essential in preventing progressive corneal injury and irreversible vision impairment []. In an in vivo confocal microscopy study, Villani et al. found that the dendritic cell density of the cornea and levels of interleukin-1α and interleukin-6 of tear fluid were significantly reduced by systemic prednisone and methotrexate in RA patients with secondary SS, but this finding was not observed in those without SS []. These results suggest that the immunopathogenesis of corneal surface inflammation in RA might be different between SS and non-SS patients []. It remains unclear whether standard systemic treatment for RA (e.g., corticosteroids, immunosuppressive agents, and biologic therapy) is effective in preventing the occurrence of DED or peripheral ulcerative keratitis []. Further studies are needed to clarify the molecular process and prophylactic measures of DED and related complications in RA with or without secondary SS.
Our study had several strengths to detect the putative relationship between RA and corneal pathologies. First, a large-scale nationwide cohort was used to increase the statistical power and generalizability of study results. The subjects were followed up for up to 12 years to evaluate the temporal association of RA with corneal diseases. Second, our analyses considered a comprehensive list of patient and clinical factors to better clarify the independent association between RA and ocular complications. However, there were some limitations to our study. Firstly, our data did not contain information about objective physical measures (e.g., ocular test results), biochemical profiles (e.g., autoimmunity and inflammation markers), and clinical data on RA management (e.g., types and doses of disease-modifying antirheumatic drugs and biologic agents) that were not available in the NHI research database. Secondly, the activity and severity of RA could not be evaluated because of data unavailability. Therefore, whether RA activity is linked to development and severity of DED or corneal damages remains unclear. However, our multivariable analyses showed that use of systemic corticosteroids after diagnosis of RA was linked to a higher risk of DED. This finding might partly reflect a positive association between RA severity and DED risk. Thirdly, the study cohort did not include patients with subclinical DED or corneal inflammation who did not seek conventional eye care service. Fourthly, the prescriptions of cyclosporine ophthalmic emulsion as a part of primary outcome ascertainment might have underestimated the incidence of DED in this study. Fifthly, we did not analyze the economic impact of RA-associated DED and corneal surface injury due to a lack of accurate data on the costs of related treatments in the NHI research database. Lastly, our cohort was only followed up until 31 December 2013, due to the regulations of the NHI research database.
5. Conclusions
Patients with RA had an increased risk of DED and corneal surface damages compared to non-RA controls in the 12 year population-based cohort. Our study identified several risk factors for corneal surface injury, providing an implication for early identification and prevention for serious corneal sequela in patients with RA. Regular ophthalmology surveillance might be needed to mitigate the adverse effect of corneal inflammation on this susceptible population. Future studies are warranted to clarify the biological mechanism and to evaluate the effective prophylactic and therapeutic strategies for corneal complications in RA.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph20021584/s1: Table S1. ICD-9-CM codes of exposure factors, coexisting diseases, and study outcomes; Figure S1. Flow diagram for patient selection.
Author Contributions
Conceptualization, S.-C.L. and Y.-H.T.; methodology, Y.-H.T.; software, Y.-H.T.; validation, C.-W.W., Y.-M.W. and H.-L.W.; formal analysis, Y.-H.T.; investigation, S.-C.L. and C.-W.W.; resources, S.-C.L. and Y.-G.C.; data curation, T.-J.C. and Y.-X.D.; writing—original draft preparation, S.-C.L.; writing—review and editing, C.-W.W., Y.-M.W., Y.-X.D., T.-J.C., H.-L.W., Y.-G.C. and Y.-H.T.; project administration, Y.-H.T.; funding acquisition, Y.-H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Taipei Medical University, Taiwan, grant number TMU110-AE1-B11. The APC was funded by Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Taipei Medical University (TMU-JIRB-N202210011; date of approval on 6 October 2022).
Informed Consent Statement
Patient consent was waived by the Institutional Review Board.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dana, R.; Bradley, J.L.; Guerin, A.; Pivneva, I.; Stillman, I.Ö.; Evans, A.M.; Schaumberg, D.A. Estimated Prevalence and Incidence of Dry Eye Disease Based on Coding Analysis of a Large, All-age United States Health Care System. Am. J. Ophthalmol. 2019, 202, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Shanti, Y.; Shehada, R.; Bakkar, M.M.; Qaddumi, J. Prevalence and associated risk factors of dry eye disease in 16 northern West bank towns in Palestine: A cross-sectional study. BMC Ophthalmol. 2020, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Cheng, C.Y.; Hsu, W.M.; Tsai, S.Y.; Lin, M.W.; Liu, J.H.; Chou, P. Association between symptoms and signs of dry eye among an elderly Chinese population in Taiwan: The Shihpai Eye Study. Invest. Ophthalmol. Vis. Sci. 2005, 46, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.K.; Lin, I.C.; Chien, L.N.; Lin, T.Y.; How, Y.T.; Chen, K.H.; Dusting, G.J.; Tseng, C.L. Dry Eye Disease: A Review of Epidemiology in Taiwan, and its Clinical Treatment and Merits. J. Clin. Med. 2019, 8, 1227. [Google Scholar] [CrossRef]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Yang, W.; Luo, Y.; Wu, S.; Niu, X.; Yan, Y.; Qiao, C.; Ming, W.; Zhang, Y.; Wang, H.; Chen, D.; et al. Estimated Annual Economic Burden of Dry Eye Disease Based on a Multi-Center Analysis in China: A Retrospective Study. Front. Med. 2021, 8, 771352. [Google Scholar] [CrossRef]
- Chan, C.; Ziai, S.; Myageri, V.; Burns, J.G.; Prokopich, C.L. Economic burden and loss of quality of life from dry eye disease in Canada. BMJ Open Ophthalmol. 2021, 6, e000709. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; de Paiva, C.S. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology 2017, 124, S4–S13. [Google Scholar] [CrossRef]
- Nelson, J.D.; Craig, J.P.; Akpek, E.K.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Clayton, J.A.; Dogru, M.; Dua, H.S.; Foulks, G.N.; et al. TFOS DEWS II Introduction. Ocul. Surf. 2017, 15, 269–275. [Google Scholar] [CrossRef]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Safiri, S.; Kolahi, A.A.; Hoy, D.; Smith, E.; Bettampadi, D.; Mansournia, M.A.; Almasi-Hashiani, A.; Ashrafi-Asgarabad, A.; Moradi-Lakeh, M.; Qorbani, M.; et al. Global, regional and national burden of rheumatoid arthritis 1990–2017: A systematic analysis of the Global Burden of Disease study 2017. Ann. Rheum. Dis. 2019, 78, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.F.; Luo, S.F.; See, L.C.; Chou, I.J.; Chang, H.C.; Yu, K.H. Rheumatoid arthritis prevalence, incidence, and mortality rates: A nationwide population study in Taiwan. Rheumatol. Int. 2013, 33, 355–360. [Google Scholar] [CrossRef]
- Lai, C.H.; Lai, M.S.; Lai, K.L.; Chen, H.H.; Chiu, Y.M. Nationwide population-based epidemiologic study of rheumatoid arthritis in Taiwan. Clin. Exp. Rheumatol. 2012, 30, 358–363. [Google Scholar] [PubMed]
- Finckh, A.; Gilbert, B.; Hodkinson, B.; Bae, S.C.; Thomas, R.; Deane, K.D.; Alpizar-Rodriguez, D.; Lauper, K. Global epidemiology of rheumatoid arthritis. Nat. Rev. Rheumatol. 2022, 18, 591–602. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, W.; Wu, J.; Zhang, H.; Zhou, H. Peripheral Ulcerative Keratitis Associated with Autoimmune Disease: Pathogenesis and Treatment. J. Ophthalmol. 2017, 2017, 7298026. [Google Scholar] [CrossRef]
- Sura, A.A.; McCallum, R.M. Peripheral ulcerative keratitis due to systemic diseases. Curr. Opin Ophthalmol. 2022, 33, 543–550. [Google Scholar] [CrossRef]
- Fujita, M.; Igarashi, T.; Kurai, T.; Sakane, M.; Yoshino, S.; Takahashi, H. Correlation between dry eye and rheumatoid arthritis activity. Am. J. Ophthalmol. 2005, 140, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Karampatakis, V.; Konidaris, V.; Michailidou, M.; Gerofotis, A.; Daniilidis, M. Peripheral corneal ulceration associated with rheumatoid arthritis. Am. J. Case Rep. 2013, 14, 318–321. [Google Scholar]
- Singh, G.; Salvador, V.B.; Bagchi, A.; Tushabe, R.; Abrudescu, A. Rheumatoid arthritis-associated corneal ulceration with superimposed infection by methicillin-resistant Staphylococcus aureus: A complicated type of corneal melt. Am. J. Case Rep. 2014, 15, 523–525. [Google Scholar]
- Levitt, A.E.; McManus, K.T.; McClellan, A.L.; Davis, J.L.; Goldhardt, R.; Galor, A. Ocular Inflammation in the Setting of Concomitant Systemic Autoimmune Conditions in an Older Male Population. Cornea 2015, 34, 762–767. [Google Scholar] [CrossRef]
- Abd-Allah, N.M.; Hassan, A.A.; Omar, G.; Hamdy, M.; Abdelaziz, S.T.A.; Abd El Hamid, W.M.; Moussa, R.A. Dry eye in rheumatoid arthritis: Relation to disease activity. Immunol. Med. 2020, 43, 92–97. [Google Scholar] [CrossRef]
- Benchérifa, S.; Amine, B.; El Binoune, I.; Rostom, S.; Bahiri, R. Two cases of perforated corneal ulcers complicating rheumatoid arthritis treated successfully by biological therapy. BMC Rheumatol. 2020, 4, 6. [Google Scholar] [CrossRef]
- Ma, W.; Wang, G.; Li, X.; Wu, H.; Liu, Z.; Dong, N.; Li, C. Study of Factors Influencing Dry Eye in Rheumatoid Arthritis. J. Ophthalmol. 2020, 2020, 5768679. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Ting, H.C.; Ma, S.H.; Tai, Y.H.; Dai, Y.X.; Chang, Y.T.; Chen, T.J.; Chen, M.H. Association between alopecia areata and retinal diseases: A nationwide population-based cohort study. J. Am. Acad. Dermatol. 2022, 87, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.J.; Tai, Y.H.; Dai, Y.X.; Lee, D.D.; Chang, Y.T.; Chen, T.J.; Chen, M.H. Obsessive-compulsive disorder and the associated risk of autoimmune skin diseases: A nationwide population-based cohort study. CNS Spectr. 2022, 1–7. [Google Scholar] [CrossRef]
- Dai, Y.X.; Tai, Y.H.; Chang, Y.T.; Chen, T.J.; Chen, M.H. Bidirectional association between psoriasis and atopic dermatitis: A nationwide population-based cohort study. Dermatology 2021, 237, 521–527. [Google Scholar] [CrossRef]
- National Health Insurance Research Database. Data Subsets. Available online: https://nhird.nhri.org.tw/en/Data_Subsets.html (accessed on 28 October 2022).
- Hung, N.; Kang, E.Y.; Lee, T.W.; Chen, T.H.; Shyu, Y.C.; Sun, C.C. The Risks of Corneal Surface Damage in Aqueous-Deficient Dry Eye Disease: A 17-Year Population-Based Study in Taiwan. Am. J. Ophthalmol. 2021, 227, 231–239. [Google Scholar] [CrossRef]
- Li, B.; Evans, D.; Faris, P.; Dean, S.; Quan, H. Risk adjustment performance of Charlson and Elixhauser comorbidities in ICD-9 and ICD-10 administrative databases. BMC Health Serv. Res. 2008, 8, 12. [Google Scholar] [CrossRef]
- Austin, P.C. A comparison of 12 algorithms for matching on the propensity score. Stat. Med. 2014, 33, 1057–1069. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef] [PubMed]
- Turk, M.A.; Hayworth, J.L.; Nevskaya, T.; Pope, J.E. Ocular Manifestations in Rheumatoid Arthritis, Connective Tissue Disease, and Vasculitis: A Systematic Review and Metaanalysis. J. Rheumatol. 2021, 48, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Lamba, N.; Lee, S.; Chaudhry, H.; Foster, S.C. A review of the ocular manifestations of rheumatoid arthritis. Cogent. Med. 2016, 3, 1. [Google Scholar] [CrossRef]
- Galor, A.; Thorne, J.E. Scleritis and peripheral ulcerative keratitis. Rheum. Dis. Clin. North Am. 2007, 33, 835–854. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Thumboo, J.; Tan, Y.K.; Wong, T.Y.; Albani, S. The eye: A window of opportunity in rheumatoid arthritis? Nat. Rev. Rheumatol. 2014, 10, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Villani, E.; Galimberti, D.; Del Papa, N.; Nucci, P.; Ratiglia, R. Inflammation in dry eye associated with rheumatoid arthritis: Cytokine and in vivo confocal microscopy study. Innate. Immun. 2013, 19, 420–427. [Google Scholar] [CrossRef]
- Verjee, M.A.; Brissette, A.R.; Starr, C.E. Dry Eye Disease: Early Recognition with Guidance on Management and Treatment for Primary Care Family Physicians. Ophthalmol. Ther. 2020, 9, 877–888. [Google Scholar] [CrossRef]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2021, 73, 1108–1123. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).