Effects of Upper Blepharoplasty Techniques on Headaches, Eyebrow Position, and Electromyographic Outcomes: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
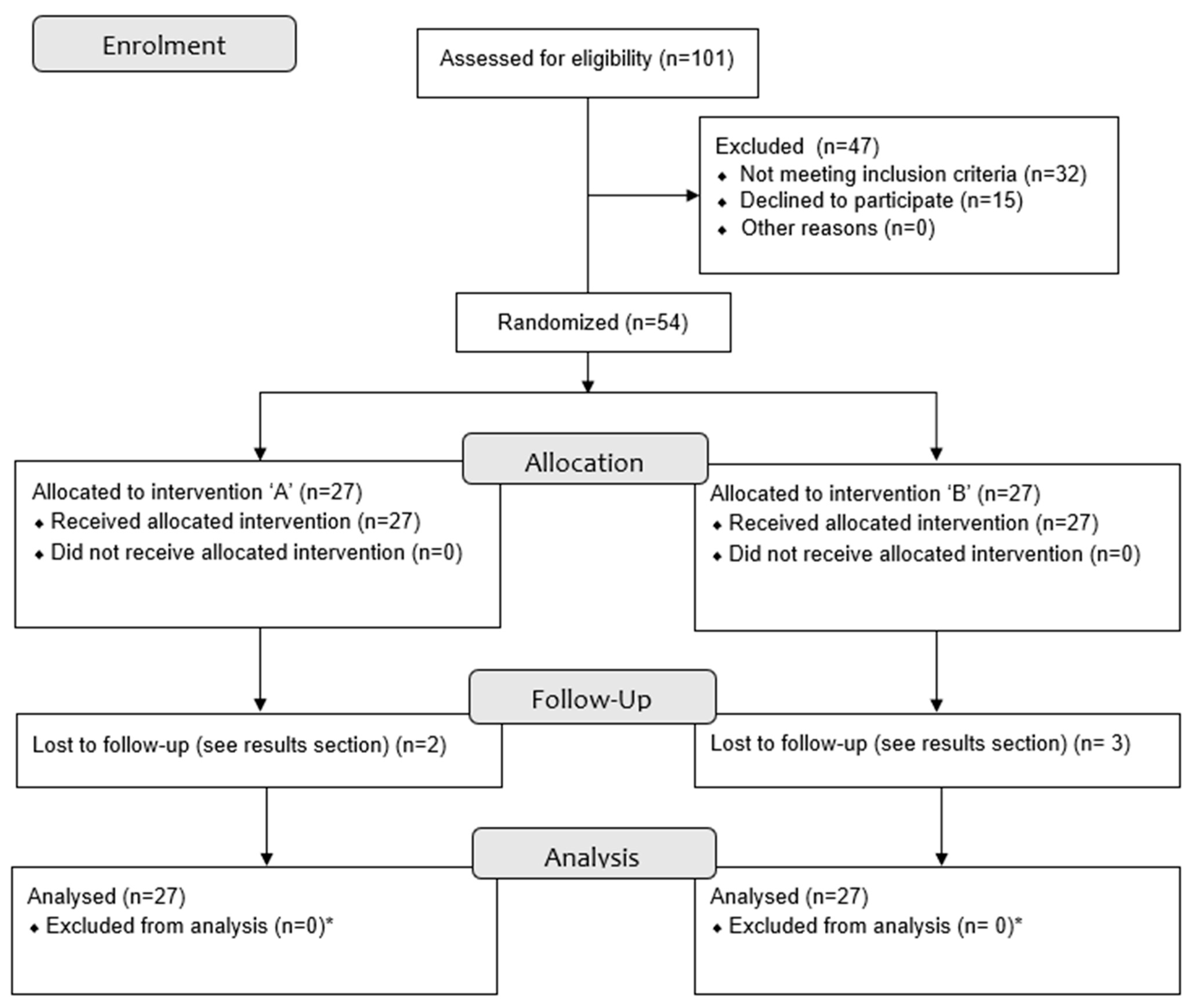
2.3. Blinding and Randomization
2.4. Outcomes
2.4.1. Headache Impact
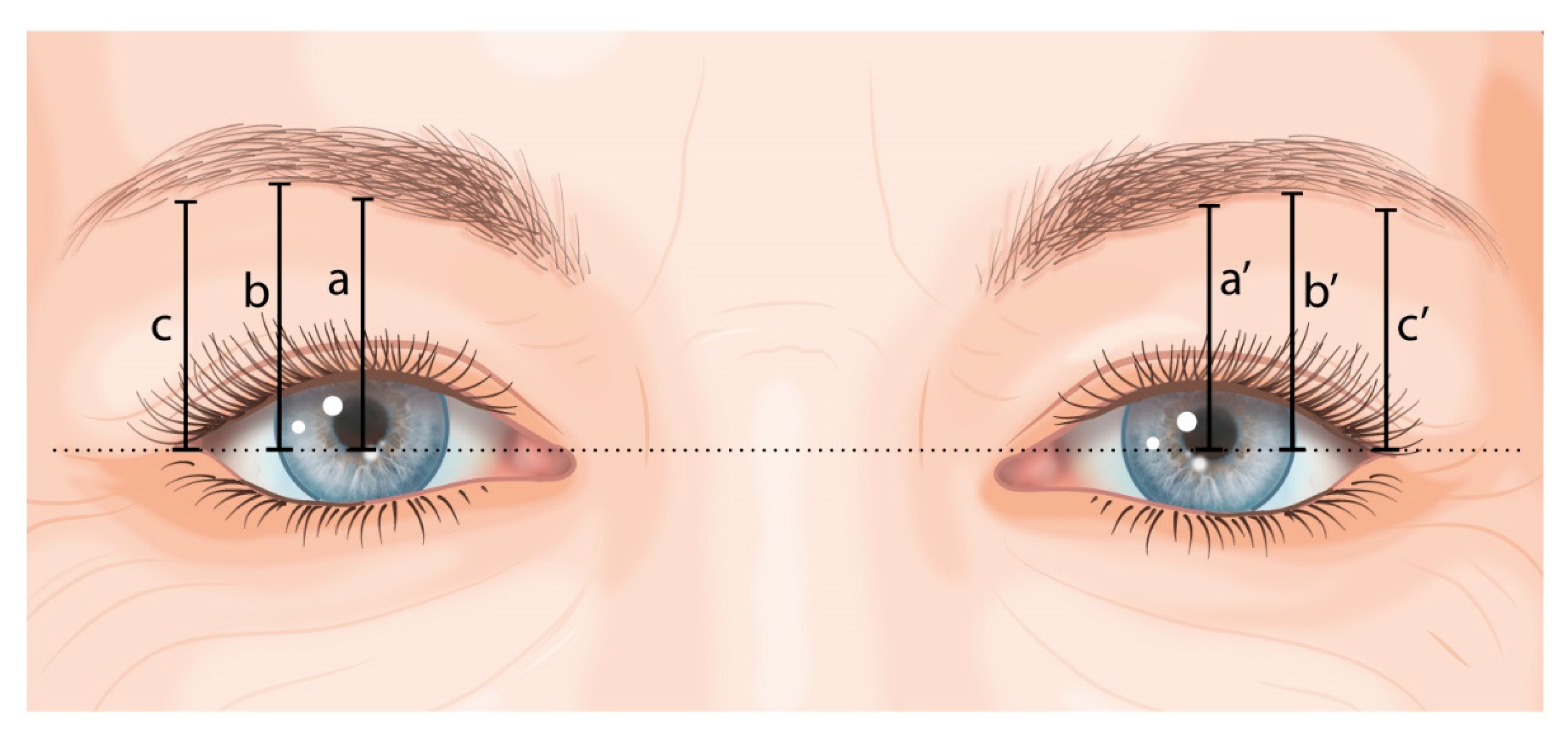
2.4.2. Eyebrow Height
- -
- a and a’: vertical line to the lower boundary of the eyebrow at the pupil’s midline;
- -
- b and b’: vertical line to the lower boundary of the eyebrow at the lateral border of the iris;
- -
- c and c’: vertical line to the lower boundary of the eyebrow at the exocanthion.
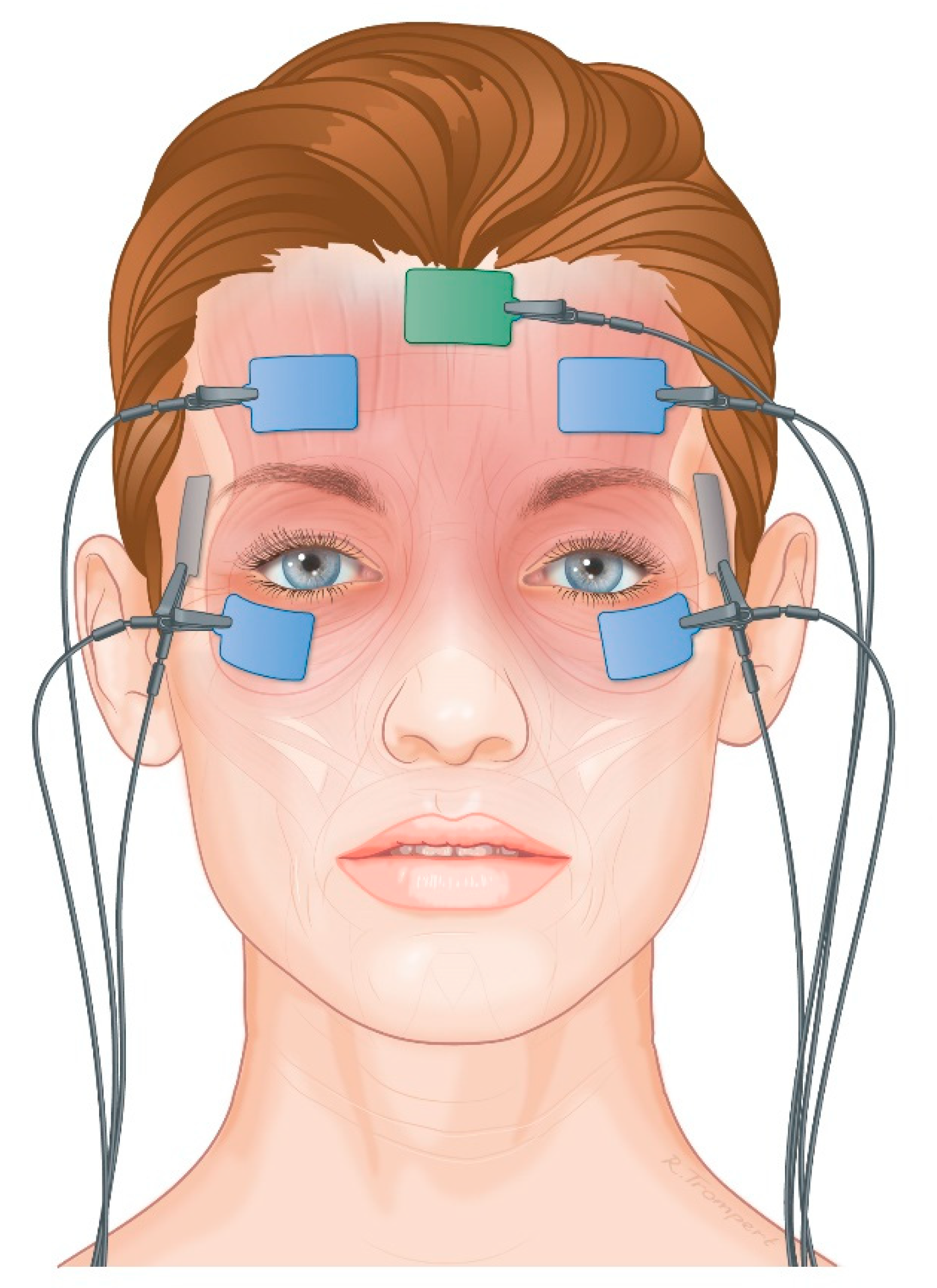
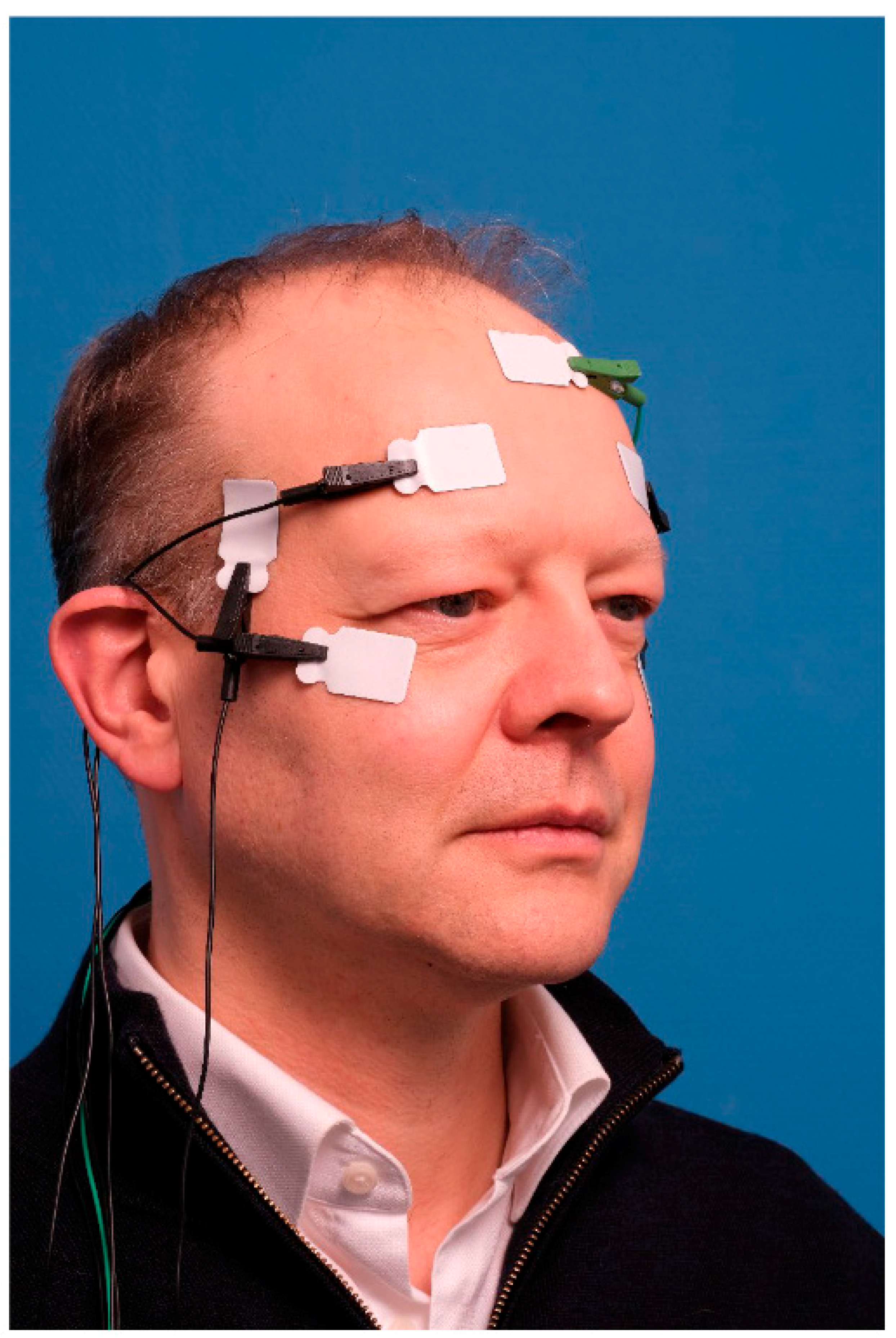
2.4.3. Electromyography
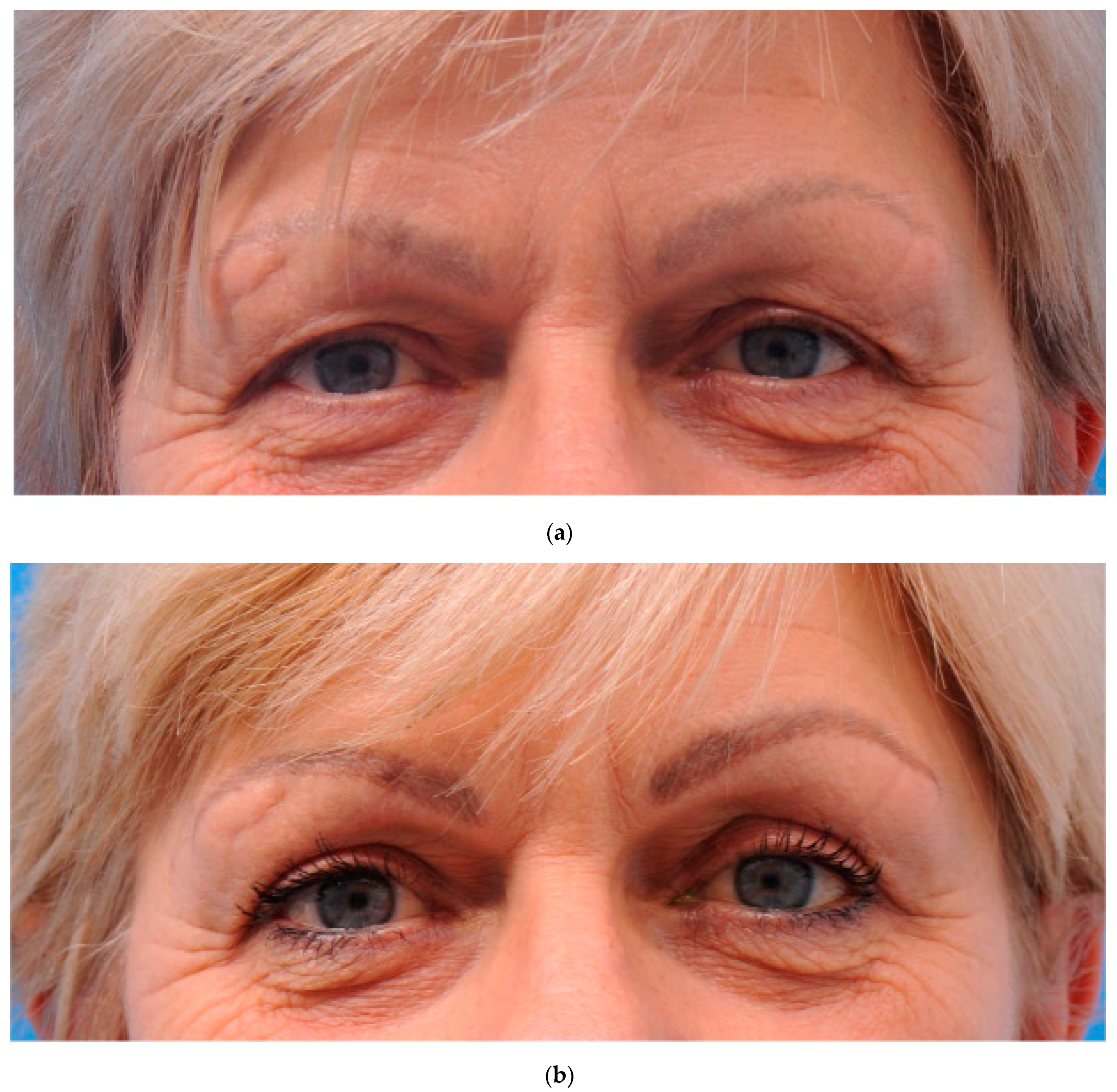
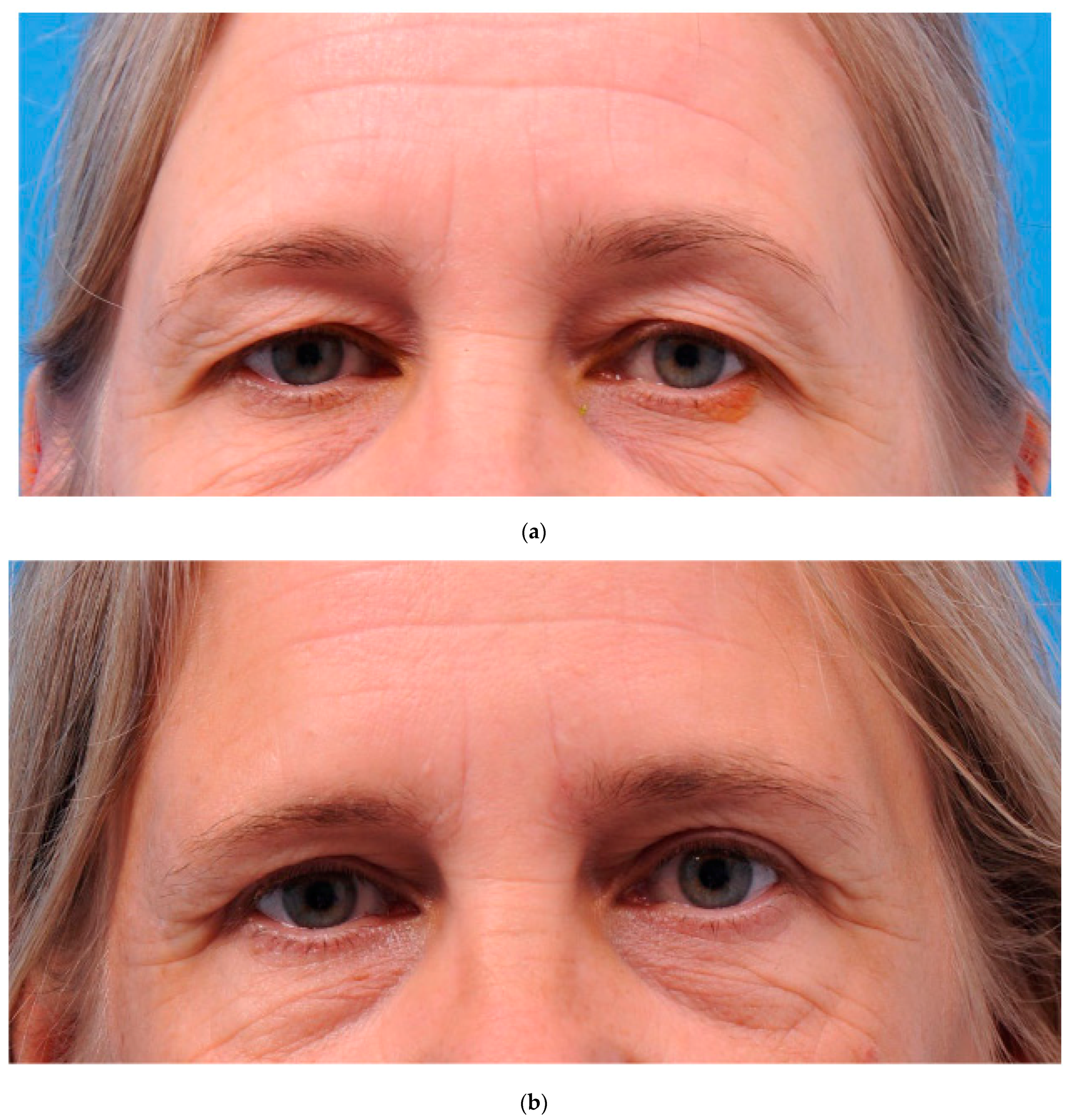
2.5. Surgical Procedure
2.6. Statistical Analysis
3. Results
3.1. HIT-6
3.2. Eyebrow Height
3.3. Electromyography
3.4. Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, D.; Son, D.; Kim, M.; Harijan, A.; Yang, S.; Lee, S. Does upper blepharoplasty affect frontalis tonicity? J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 638–644. [Google Scholar] [CrossRef]
- Jensen, R. Pathophysiological mechanisms of tension-type headache: A review of epidemiological and experimental studies. Cephalalgia 1999, 19, 602–621. [Google Scholar] [CrossRef] [PubMed]
- Wittrock, D.A. The comparison of individuals with tension-type headache and headache-free controls on frontal EMG levels: A meta-analysis. Headache 1997, 37, 424–432. [Google Scholar] [CrossRef]
- Hollander, M.H.J.; Contini, M.; Pott, J.W.; Vissink, A.; Schepers, R.H.; Jansma, J. Functional outcomes of upper eyelid blepharoplasty: A systematic review. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Hollander, M.H.J.; Schortinghuis, J.; Vissink, A.; Jansma, J.; Schepers, R.H. Aesthetic outcomes of upper eyelid blepharoplasty: A systematic review. Int. J. Oral. Maxillofac. Surg. 2020, 49, 750–764. [Google Scholar] [CrossRef]
- Flowers, R.S.; Caputy, G.G.; Flowers, S.S. The biomechanics of brow and frontalis function and its effect on blepharoplasty. Clin. Plast. Surg. 1993, 20, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Rohrich, R.J.; Villanueva, N.L.; Afrooz, P.N. Refinements in upper blepharoplasty: The five-step technique. Plast. Reconstr. Surg. 2018, 141, 1144–1146. [Google Scholar] [CrossRef]
- Jacobs, L.C.; Liu, F.; Bleyen, I.; Gunn, D.; Hofman, A.; Klaver, C.C.W.; Uitterlinden, A.G.; Neumann, H.A.M.; Bataille, V.; Spector, T.D.; et al. Intrinsic and extrinsic risk factors for sagging eyelids. JAMA Dermatol. 2014, 150, 836–843. [Google Scholar] [CrossRef]
- Kosinski, M.; Bayliss, M.; Bjorner, J.B.; Ware, J.E., Jr.; Garber, W.; Batenhorst, A.; Cady, R.; Dahlöf, C.; Dowson, A.; Tepper, S. A six-item short-form survey for measuring headache impact: The HIT-6. Qual. Life Res. 2003, 12, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Blaisdell, B.; Kwong, J.W.; Bjorner, J.B. The short-form headache impact test (HIT-6) was psychometrically equivalent in nine languages. J. Clin. Epidemiol. 2004, 57, 1271–1278. [Google Scholar] [CrossRef]
- Yang, M.; Rendas-Baum, R.; Varon, S.F.; Kosinski, M. Validation of the headache impact test (HIT-6) across episodic and chronic migraine. Cephalalgia 2011, 31, 357–367. [Google Scholar] [CrossRef]
- Bayliss, M.; Batenhorst, A. The HIT-6™ a User’s Guide; QualityMetric Incorporated: Lincoln, RI, USA, 2002. [Google Scholar]
- Rufer, F.; Schroder, A.; Erb, C. White-to-white corneal diameter: Normal values in healthy humans obtained with the orbscan II topography system. Cornea 2005, 24, 259–261. [Google Scholar] [CrossRef]
- Taban, M.; Taban, M.; Perry, J.D. Lower eyelid position after transconjunctival lower blepharoplasty with versus without a skin pinch. Ophthalmic. Plast. Reconstr. Surg. 2008, 24, 7–9. [Google Scholar] [CrossRef]
- Dar, S.A.; Rubinstein, T.J.; Perry, J.D. Eyebrow position following upper blepharoplasty. Orbit 2015, 34, 327–330. [Google Scholar] [CrossRef]
- Rajyalakshmi, R.; Prakash, W.D.; Ali, M.J.; Naik, M.N. Periorbital biometric measurements using ImageJ software: Standardisation of technique and assessment of intra- and interobserver variability. J. Cutan. Aesthet. Surg. 2017, 10, 130–135. [Google Scholar] [PubMed]
- Pennock, J.D.; Johnson, P.C.; Manders, E.K.; VanSwearingen, J.M. Relationship between muscle activity of the frontalis and the associated brow displacement. Plast. Reconstr. Surg. 1999, 104, 1789–1797; discussion 1798–1799. [Google Scholar] [CrossRef] [PubMed]
- Tarata, M.T. Mechanomyography versus electromyography, in monitoring the muscular fatigue. Biomed. Eng. Online 2003, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- van Boxtel, A. Optimal signal bandwidth for the recording of surface EMG activity of facial, jaw, oral, and neck muscles. Psychophysiology 2001, 38, 22–34. [Google Scholar] [CrossRef]
- Bahceci Simsek, I. Association of upper eyelid ptosis repair and blepharoplasty with headache-related quality of life. JAMA Facial. Plast. Surg. 2017, 19, 293–297. [Google Scholar] [CrossRef]
- Castien, R.F.; Blankenstein, A.H.; Windt Daniëlle AWM van der Dekker, J. Minimal clinically important change on the headache impact test-6 questionnaire in patients with chronic tension-type headache. Cephalalgia 2012, 32, 710–714. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Evans, J.D. Straightforward Statistics for the Behavioral Sciences; Thomson Brooks/Cole Publishing, Co.: Pacific Grove, CA, USA, 1996. [Google Scholar]
- Sinha, K.R.; Al Shaker, S.; Yeganeh, A.; Moreno, T.; Rootman, D.B. The relationship between eyebrow and eyelid position in patients with ptosis, dermatochalasis and controls. Ophthalmic. Plast. Reconstr. Surg. 2019, 35, 85–90. [Google Scholar] [CrossRef]
- Prado, R.B.; Silva-Junior, D.E.; Padovani, C.R.; Schellini, S.A. Assessment of eyebrow position before and after upper eyelid blepharoplasty. Orbit 2012, 31, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Frankel, A.S.; Kamer, F.M. The effect of blepharoplasty on eyebrow position. Arch. Otolaryngol. Head Neck Surg. 1997, 123, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Starck, W.J.; Griffin, J.E.; Jr Epker, B.N. Objective evaluation of the eyelids and eyebrows after blepharoplasty. J. Oral. Maxillofac. Surg. 1996, 54, 297–302; discussion 302–303. [Google Scholar] [CrossRef]
- Huijing, M.A.; van der Palen, J.; van der Lei, B. The effect of upper eyelid blepharoplasty on eyebrow position. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Pool, S.M.; van der Lei, B. Asymmetry in upper blepharoplasty: A retrospective evaluation study of 365 bilateral upper blepharoplasties conducted between january 2004 and december 2013. J. Plast. Reconstr. Aesthet. Surg. 2015, 68, 464–468. [Google Scholar] [CrossRef]
- Baker, M.S.; Shams, P.N.; Allen, R.C. The quantitated internal suture browpexy: Comparison of two brow-lifting techniques in patients undergoing upper blepharoplasty. Ophthal. Plast. Reconstr. Surg. 2016, 32, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, S.E.; Khajouei Kermani, H. Brow ptosis after upper blepharoplasty: Findings in 70 patients. World J. Plast. Surg. 2016, 5, 58–61. [Google Scholar]
- Prantl, L.; Heidekrueger, P.I.; Broer, P.N.; Knoll, S.; Thiha, A.; Grundl, M. Female eye attractiveness—Where beauty meets science. J. Craniomaxillofac. Surg. 2019, 47, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences; Houghton Mifflin College Division: Boston, MA, USA, 2003; Volume 663. [Google Scholar]
- Mokhtarzadeh, A.; McClelland, C.; Lee, M.S.; Smith, S.; Harrison, A.R. The bleph and the brain: The effect of upper eyelid surgery on chronic headaches. Ophthal. Plast. Reconstr. Surg. 2017, 33, 178–181. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.J. Myoelectrical manifestations of localized muscular fatigue in humans. Crit. Rev. Biomed. Eng. 1984, 11, 251–279. [Google Scholar]
- Chen, C.H.C.; Chung, H. The review of applications and measurements in facial electromyography. J. Med. Biol. Eng. 2004, 25, 15–20. [Google Scholar]
- Halaki, M.; Ginn, K. Normalization of EMG signals: To normalize or not to normalize and what to normalize to. In Computational Intelligence in Electromyography Analysis—A Perspective on Current Applications and Future Challenges; Intech: Rang-Du-Fliers, France, 2012; pp. 175–194. [Google Scholar] [CrossRef]
- Jensen, R.; Fuglsang-Frederiksen, A.; Olesen, J. Quantitative surface EMG of pericranial muscles. reproducibility and variability. Electroencephalogr. Clin. Neurophysiol./Evoked Potentials Sect. 1993, 89, 1–9. [Google Scholar] [CrossRef] [PubMed]
| Treatment A n = 27 | Treatment B n = 27 | p Value | ||||
|---|---|---|---|---|---|---|
| Gender (number and % female) | 21 (78%) | 23 (85%) | 0.484 | |||
| Age (years; mean ± SD [range]) | 58 ± 8.6 [43–70] | 55 ± 9.1 [39–70] | 0.241 | |||
| Dermatochalasis severity score (number of patients) | Right eye Normal: 0 Mild: 11 Moderate: 15 Severe: 1 | Left eye Normal: 0 Mild: 10 Moderate: 16 Severe: 1 | Right eye Normal: 0 Mild: 12 Moderate: 13 Severe: 2 | Left eye Normal: 0 Mild: 13 Moderate: 12 Severe: 2 | Right eye p = 0.771 | Left eye p = 0.523 |
| Removed skin (g; mean ± SD [range]) | Right eye | Left eye | Right eye | Left eye | Right eye | Left eye |
| 0.30 ± 0.08 [0.18–0.42] | 0.32 ± 0.08 [0.21–0.51] | 0.32 ± 0.11 [0.18–0.61] | 0.34 ± 0.12 [0.14–0.65] | p = 0.563 | p = 0.703 | |
| Removed muscle (g; mean ± SD [range]) | - | - | Right eye | Left eye | - | |
| 0.11 ± 0.07 [0.05–0.40] | 0.11 ± 0.07 [0.05–0.40] | |||||
| Medial fat removal (no. of patients) | 2 * | 0 | p = 0.552 | |||
| Group A Median [Q1;Q3] (p-Value *) | Group B Median [Q1;Q3] (p-Value *) | Adjusted Difference between Groups A and B ** (95% CI) and p-Value | ||
|---|---|---|---|---|
| Preoperatively | 46 [40;55] | 42 [40;58] | n.a. | |
| 6 months postoperatively | 40 [36;44] | 38 [36;45] | −2 (−7–3) | p = 0.383 |
| (p = 0.126) | (p = 0.052) | |||
| 12 months postoperatively | 37 [36;42] | 38 [36;41] | 3 (−3–9) | p = 0.301 |
| (p = 0.003) | (p = 0.029) | |||
| Preoperatively | 6 Months Postoperatively | 12 Months Postoperatively | ||
|---|---|---|---|---|
| Group A Median [Q1;Q3] | Group b Median [q1;q3] | Adjusted Difference * between Groups a and b (95% ci) and p-Value | Adjusted Difference * between Groups A and B (95% CI) and p-Value | |
| Landmark a and a’ | 15.8 [13.6;19.3] | 16.5 [14.6;19.1] | −0.3 [−1.0;0.5] p = 0.502 | 0.1 [−0.9–1.1] p = 0.897 |
| Landmark b and b’ | 16.7 [13.5;19.8] | 17.5 [15.4;20.5] | −0.8 [−1.6;0.1] p = 0.082 | −0.3 [−1.3;0.7} p = 0.575 |
| Landmark c and c’ | 16.7 [13.2;18.6] | 16.8 [15.2;19.7] | −0.7 [−1.8;0.3] p = 0.169 | −0.4 [−1.6;0.7] p = 0.474 |
| Preoperatively | 6 Months Postoperatively | 12 Months Postoperatively | |||
|---|---|---|---|---|---|
| Median [Q1;Q3] | Median [Q1;Q3] | Difference Compared to Baseline (p-Value) | Median [Q1;Q3] | Difference Compared to Baseline (p-Value) | |
| Group A | |||||
| Landmark a and a’ | 15.8 | 13.2 | −2.6 | 13.6 | −2.2 |
| [13.6;19.3] | [11.7;16.2] | (p < 0.001) | [11.4;16.9] | (p < 0.001) | |
| Landmark b and b’ | 16.7 | 14.0 | −2.7 | 13.1 | −3.6 |
| [13.5;19.8] | [11.7;16.6] | (p < 0.001) | [11.7;16.7] | (p < 0.001) | |
| Landmark c and c’ | 16.7 | 13.7 | −3.0 | 12.4 | −4.3 |
| [13.2;18.6] | [11.4;16.2] | (p < 0.001) | [11.1;16.4] | (p < 0.001) | |
| Group B | |||||
| Landmark a and a’ | 16.5 | 14.4 | −2.1 | 15.1 | −1.4 |
| [14.6;19.1] | [13.0;16.4] | (p < 0.001) | [13.1;17.3] | (p < 0.001) | |
| Landmark b and b’ | 17.5 | 14.6 | −2.9 | 15.5 | −2.0 |
| [15.4;20.5] | [12.8;16.7] | (p < 0.001) | [12.9;18.1] | (p < 0.001) | |
| Landmark c and c’ | 16.8 | 14.3 | −2.5 | 14.5 | −2.3 |
| [15.2;19.7] | [11.9;16.8] | (p < 0.001) | [12.7;16.9] | (p < 0.001) | |
| Preoperatively | 2 Months Postoperatively | 6 Months Postoperatively | 12 Months Postoperatively | |||||
|---|---|---|---|---|---|---|---|---|
| Median [Q1;Q3] | MEDIAN [Q1;Q3] (p-Value *) | Adjusted Difference between Groups A and B ** (95% CI) and p-Value | Median [Q1;Q3] (p-Value *) | Adjusted Difference between Groups A and B ** (95% CI) and p-Value | Median [Q1;Q3] (p-Value *) | Adjusted Difference between Groups A and B ** (95% CI) and p-Value | Group | |
| RMS during maximal contraction (mV) | ||||||||
| Frontalis muscle | 80 [22;125] | 73 [42;157] p = 0.255 | −0.225 (−0.441–−0.008) p = 0.042 | 49 [29;97] p = 0.525 | −0.131 (−0.338–0.076) p = 0.215 | 39 [18;107] p = 0.026 | 0.090 (−0.134–0.314) p = 0.430 | Group A |
| Frontalis muscle | 46 [32;73] | 62 [23;113] p = 0.253 | 76 [43;123] p = 0.253 | 59 [27;119] p = 0.253 | Group B | |||
| Orbicularis oculi muscle | 51 [27;96] | 44 [31;100] p = 0.145 | −0.020 (−0.196–0.157) p = 0.826 | 51 [30;104] p = 0.145 | −0.117 (−0.301–0.067) p = 0.211 | 40 [22;70] p = 0.145 | 0.282 (0.045–0.520) p = 0.020 | Group A |
| Orbicularis oculi muscle | 61 [22;100] | 50 [28;96] p = 0.801 | 66 [34;118] p = 0.801 | 56 [36;111] p = 0.801 | Group B | |||
| Index (RMS/maximal amplitude) during maximal contraction (normalised value) | ||||||||
| Frontalis muscle | 0.21 [0.13;0.30] | 0.21 [0.16;0.25] p = 0.392 | −0.02 (−0.07–0.03) p = 0.386 | 0.18 [0.15;0.21] p = 0.392 | −0.04 (−0.09–0.02) p = 0.225 | 0.19 [0.15;0.22] p = 0.392 | −0.03 (−0.09–0.04) p = 0.459 | Group A |
| Frontalis muscle | 0.20 [0.12;0.27] | 0.19 [0.14;0.22] p = 0.840 | 0.19 [0.13;0.22] p = 0.840 | 0.19 [0.16;0.24] p = 0.840 | Group B | |||
| Orbicularis oculi muscle | 0.21 [0.16;0.24] | 0.20 [0.18;0.26] p = 0.187 | −0.001 (−0.04–0.04) p = 0.958 | 0.17 [0.12;0.20] p = 0.187 | 0.03 (−0.01–0.07) p = 0.102 | 0.19 [0.16;0.22] p = 0.187 | 0.01 (−0.02–0.04) p = 0.666 | Group A |
| Orbicularis oculi muscle | 0.18 [0.16;0.21] | 0.19 [0.17;0.23] p = 0.535 | 0.18 [0.13;0.22] p = 1.000 | 0.18 [0.14;0.22] p = 1.000 | Group B | |||
| Median frequency during maximal contraction (Hz) | ||||||||
| Frontalis muscle | 71 [62;85] | 77 [60;100] p = 0.102 | −0.066 (−0.134–0.001) p = 0.054 | 65 [59;74] p = 0.102 | −0.037 (−0.090–0.016) p = 0.169 | 65 [59;77] p = 0.102 | 0.004 (−0.050–0.058) p = 0.889 | Group A |
| Frontalis muscle | 64 [56;72] | 64 [60;74] p = 0.172 | 64 [57;69] p = 0.172 | 66 [58;100] p = 0.172 | Group B | |||
| Orbicularis oculi muscle | 100 [95;122] | 110 [100;123] p = 0.724 | −0.035 (−0.106–0.036) p = 0.337 | 106 [95;122] p = 0.724 | −0.033 (−0.085–0.020) p = 0.222 | 108 [100;118] p = 0.724 | 0.044 (−0.009–0.096) p = 0.101 | Group A |
| Orbicularis oculi muscle | 98 [73;116] | 100 [80;114] p = 0.086 | 97 [71;106] p = 0.086 | 110 [100;124] p = 0.086 | Group B | |||
| Group A | Frontalis Muscle | Orbicularis Oculi Muscle | ||||||
| Median Frequency (Hz) | Median Frequency (Hz) | Difference (%) | p-Value * | Median Frequency (Hz) | Median Frequency (Hz) | Difference (%) | p-Value * | |
| Start | End | Start | End | |||||
| Preoperatively | 71 | 67 | −5.6 % | - | 112 | 101 | −9.8% | - |
| [62;79] | [58;77] | [91;133] | [70;121] | |||||
| 2 months postoperatively | 75 | 69 | −8.0% | 0.381 | 124 | 105 | −15.3% | 0.345 |
| [61;126] | [58;102] | [103;146] | [80;133] | |||||
| 6 months postoperatively | 69 | 66 | −4.3% | 0.381 | 106 | 99 | −6.6% | 0.345 |
| [60;75] | [57;77] | [84;125] | [64;121] | |||||
| 12 months postoperatively | 66 | 69 | +4.5% | 0.381 | 111 | 97 | −12.6% | 0.345 |
| [57;83] | [59;85] | [96;130] | [52;117] | |||||
| Group B | Frontalis muscle | Orbicularis oculi muscle | ||||||
| Median frequency (Hz) | Median frequency (Hz) | Difference (%) | p-value * | Median frequency (Hz) | Median frequency (Hz) | Difference (%) | p-value * | |
| Start | End | Start | End | |||||
| Preoperatively | 65 | 56 | −13.8% | - | 95 | 67 | −29.5% | - |
| [54;71] | [51;71] | [67;113] | [55;98] | |||||
| 2 months postoperatively | 65 | 58 | −10.8% | 0.331 | 110 | 85 | −22.7% | 0.417 |
| [57;76] | [53;73] | [90;125] | [54;106] | |||||
| 6 months postoperatively | 62 | 60 | −3.2% | 0.331 | 97 | 91 | −6.2% | 0.417 |
| [55;73] | [53;71] | [63;107] | [58;106] | |||||
| 12 months postoperatively | 63 | 65 | +4.8% | 0.331 | 114 | 105 | −7.9% | 0.417 |
| [57;85] | [57;96] | [98;135] | [77;128] | |||||
| Change in Headache (rs and p-Value) | Change in Eyebrow Height (rs and p-Value) | Change in EMG Frontalis Muscle (rs and p-Value) | |
|---|---|---|---|
| Correlation between the changes (pre- and postoperative values) in the different variables | |||
| 6-month follow-up | |||
| Change in headache | - | −0.060, p = 0.681 | −0.083, p = 0.615 |
| Change in eyebrow height | −0.060, p = 0.681 | - | 0.080, p = 0.627 |
| Change in EMG frontalis muscle | −0.083, p = 0.615 | 0.080, p = 0.627 | - |
| Correlation between baseline values and pre- and postoperative changes in the variables | |||
| Baseline headache | - | −0.123, p = 0.394 | −0.078, p = 0.653 |
| Baseline eyebrow height | 0.367, p = 0.009 | - | 0.133, p = 0.420 |
| Baseline EMG frontalis muscle | 0.292, p = 0.071 | −0.134, p = 0.414 | - |
| Correlation between the changes (pre- and postoperative values) in the different variables | |||
| 12-month follow-up | |||
| Change in headache | - | −0.043, p = 0.814 | −0.115, p = 0.630 |
| Change in eyebrow height | −0.043, p = 0.814 | - | 0.136, p = 0.465 |
| Change in EMG frontalis muscle | −0.115, p = 0.630 | 0.136, p = 0.465 | - |
| Correlation between baseline values and pre- and postoperative changes in the variables | |||
| Baseline headache | - | 0.097, p = 0.518 | 0.193, p = 0.291 |
| Baseline eyebrow height | 0.088, p = 0.617 | - | 0.056, p = 0.760 |
| Baseline EMG frontalis muscle | 0.154, p = 0.473 | −0.195, p = 0.261 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hollander, M.H.J.; van der Hoeven, J.H.; Verdonschot, K.H.M.; Delli, K.; Vissink, A.; Jansma, J.; Schepers, R.H. Effects of Upper Blepharoplasty Techniques on Headaches, Eyebrow Position, and Electromyographic Outcomes: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2023, 20, 1559. https://doi.org/10.3390/ijerph20021559
Hollander MHJ, van der Hoeven JH, Verdonschot KHM, Delli K, Vissink A, Jansma J, Schepers RH. Effects of Upper Blepharoplasty Techniques on Headaches, Eyebrow Position, and Electromyographic Outcomes: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2023; 20(2):1559. https://doi.org/10.3390/ijerph20021559
Chicago/Turabian StyleHollander, Maria H.J., Johannes H. van der Hoeven, Koen H.M. Verdonschot, Konstantina Delli, Arjan Vissink, Johan Jansma, and Rutger H. Schepers. 2023. "Effects of Upper Blepharoplasty Techniques on Headaches, Eyebrow Position, and Electromyographic Outcomes: A Randomized Controlled Trial" International Journal of Environmental Research and Public Health 20, no. 2: 1559. https://doi.org/10.3390/ijerph20021559
APA StyleHollander, M. H. J., van der Hoeven, J. H., Verdonschot, K. H. M., Delli, K., Vissink, A., Jansma, J., & Schepers, R. H. (2023). Effects of Upper Blepharoplasty Techniques on Headaches, Eyebrow Position, and Electromyographic Outcomes: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 20(2), 1559. https://doi.org/10.3390/ijerph20021559







