Psychological and Behavioral Factors Involved in Temporomandibular Myalgia and Migraine: Common but Differentiated Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample and Physical Examination
2.3. Sample Size
2.4. Evaluation of Psychological, Cognitive, and Emotional Factors
2.4.1. Anxiety Assessment
2.4.2. Depression Assessment
2.4.3. Psychopathological Symptoms Assessment
2.4.4. Stress Coping Assessment
2.5. Statistical Analysis
3. Results
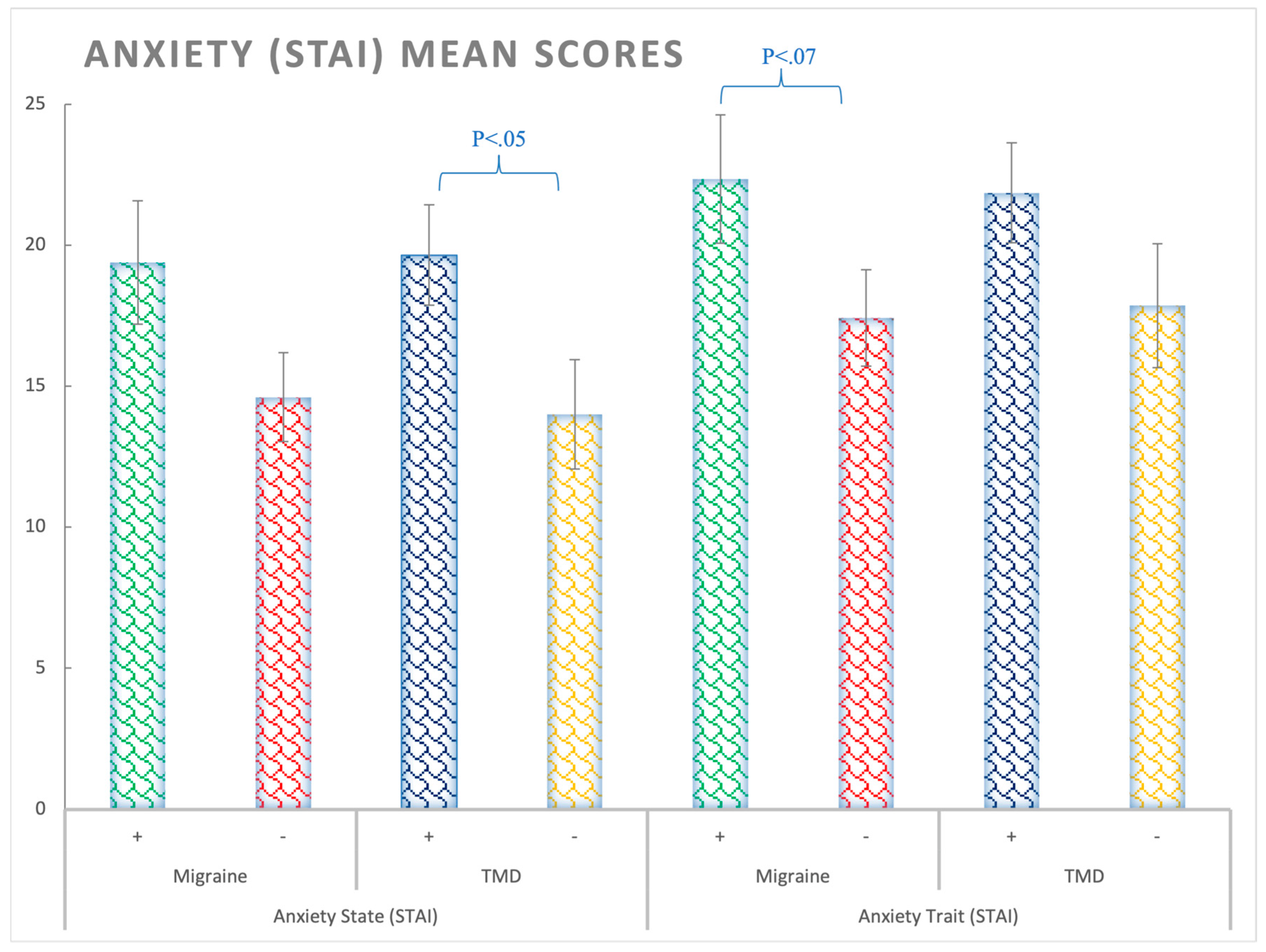
3.1. Anxiety (STAI Questionnaire)
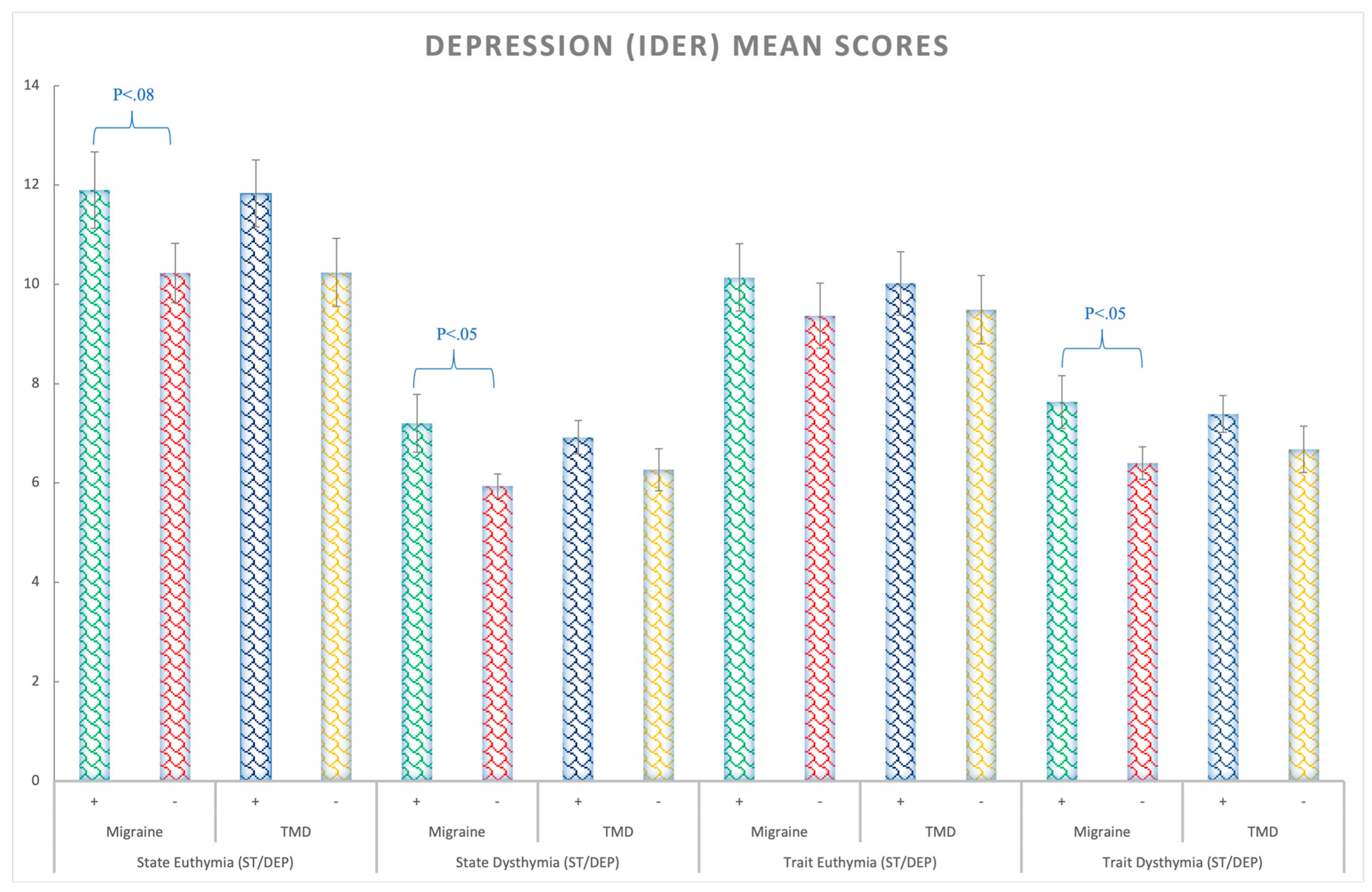
3.2. Depression (ST/DEP Questionnaire)
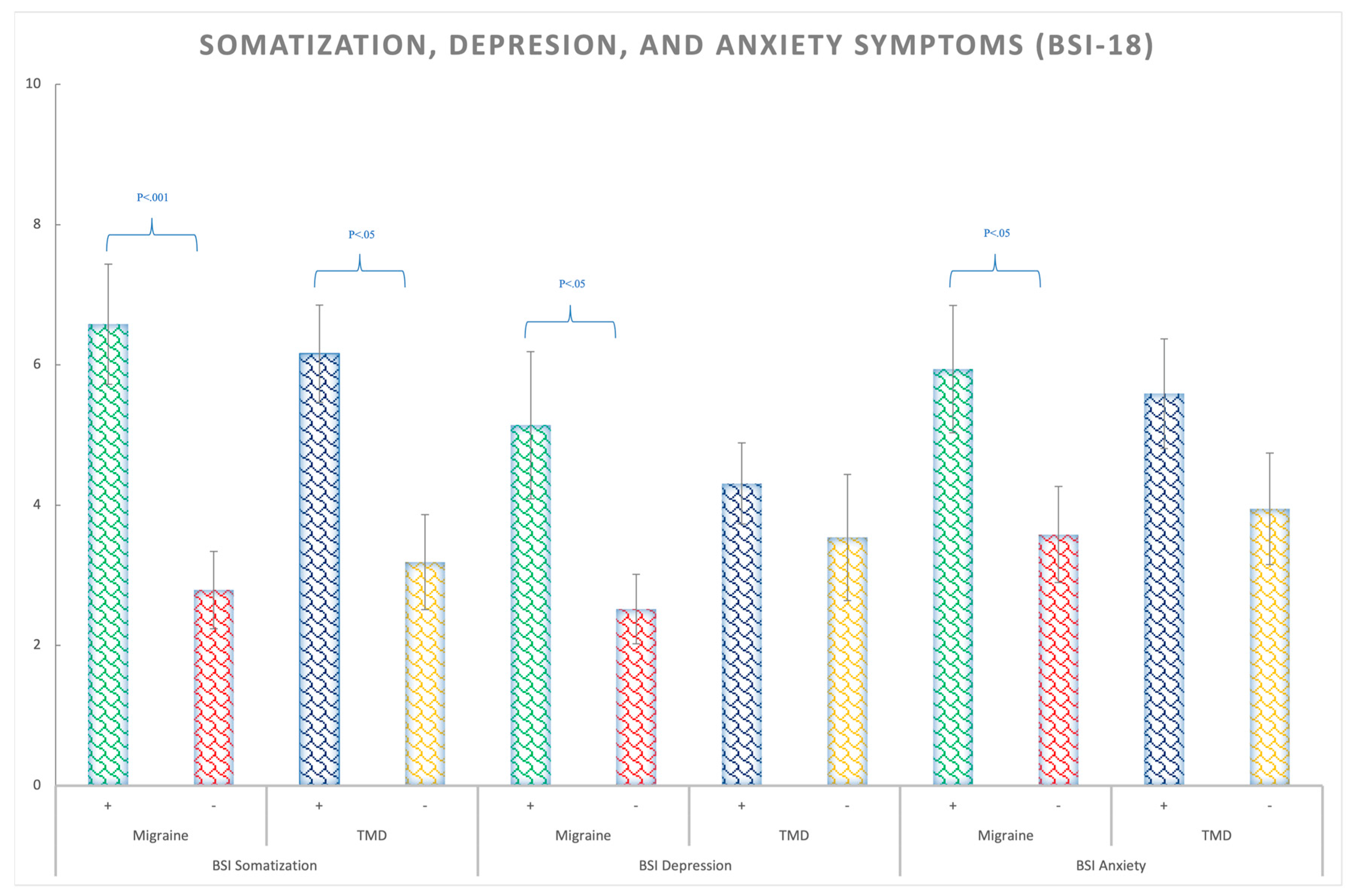
3.3. Somatization, Depression, and Anxiety Symptoms (BSI-18)
3.4. Stress Coping Styles (CRI)
4. Discussion
4.1. Limitations and Strengths
4.2. Highlights and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vale Braido, G.V.d.; Svensson, P.; dos Santos Proença, J.; Mercante, F.G.; Fernandes, G.; de Godoi Gonçalves, D.A. Are central sensitization symptoms and psychosocial alterations interfering in the association between painful TMD, migraine, and headache attributed to TMD? Clin. Oral Investig. 2022, 1–10. [Google Scholar] [CrossRef]
- Romero-Reyes, M.; Uyanik, J.M. Orofacial pain management: Current perspectives. J. Pain Res. 2014, 7, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. International Rdc/Tmd consortium network IafDR, orofacial pain special interest group IAftSoP. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: Recommendations of the international RDC/TMD consortium network* and orofacial pain special interest groupdagger. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar]
- Schiffman, E.; Ohrbach, R. Executive summary of the Diagnostic Criteria for Temporomandibular Disorders for clinical and research applications. J. Am. Dent. Assoc. 2016, 147, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Ohrbach, R.; Dworkin, S.F. AAPT diagnostic criteria for chronic painful temporomandibular disorders. J. Pain 2019, 20, 1276–1292. [Google Scholar] [CrossRef]
- De Leeuw, R.; Klasser, G.D. Orofacial Pain: Guidelines for Assessment, Diagnosis, and Management; Quintessence Publishing Company, Incorporated: Hanover Park, IL, USA, 2018. [Google Scholar]
- Calixtre, L.B.; Gruninger, B.L.; Chaves, T.C.; Oliveira, A.B. Is there an association between anxiety/depression and temporomandibular disorders in college students? J. Appl. Oral Sci. 2014, 22, 15–21. [Google Scholar] [CrossRef]
- Sánchez Romero, E.A.; Martínez-Pozas, O.; García-González, M.; de-Pedro, M.; González-Álvarez, M.E.; Esteban-González, P.; Cid-Verdejo, R.; Villafañe, J.H. Association between Sleep Disorders and Sleep Quality in Patients with Temporomandibular Joint Os-teoarthritis: A Systematic Review. Biomedicines 2022, 10, 2143. [Google Scholar] [CrossRef]
- Lipton, R.B.; Bigal, M.E. Migraine: Epidemiology, impact, and risk factors for progression. Headache 2005, 45 (Suppl. 1), S3–S13. [Google Scholar] [CrossRef]
- Di Paolo, C.; D’Urso, A.; Papi, P.; Di Sabato, F.; Rosella, D.; Pompa, G.; Polimeni, A. Temporomandibular Disorders and Headache: A Retrospective Analysis of 1198 Patients. Pain Res. Manag. 2017, 2017, 3203027. [Google Scholar] [CrossRef] [PubMed]
- Cruz, D.; Monteiro, F.; Paço, M.; Vaz-Silva, M.; Lemos, C.; Alves-Ferreira, M.; Pinho, T. Genetic overlap between temporomandibular disorders and primary headaches: A systematic review. Jpn. Dent. Sci. Rev. 2022, 58, 69–88. [Google Scholar] [CrossRef]
- Yakkaphan, P.; Smith, J.G.; Chana, P.; Renton, T.; Lambru, G. Temporomandibular disorder and headache prevalence: A systematic review and meta-analysis. Cephalalgia Rep. 2022, 5, 25158163221097352. [Google Scholar] [CrossRef]
- Nazeri, M.; Ghahrechahi, H.R.; Pourzare, A.; Abareghi, F.; Samiee-Rad, S.; Shabani, M.; Arjmand, S.; Abazarpour, R. Role of anxiety and depression in association with migraine and myofascial pain temporomandibular disorder. Indian J. Dent. Res. 2018, 29, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, M.C.; Florencio, L.L.; Chaves, T.C.; Speciali, J.G.; Bigal, M.E.; Bevilaqua-Grossi, D. Do women with migraine have higher prevalence of temporomandibular disorders? Braz. J. Phys. Ther. 2013, 17, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Réus, J.C.; Polmann, H.; Souza, B.D.M.; Flores-Mir, C.; Gonçalves, D.A.G.; de Queiroz, L.P.; Okeson, J.; Canto, G.D.L. Association between primary headaches and temporomandibular disorders: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2021, 153, 120–131.e6. [Google Scholar] [CrossRef]
- Goncalves, D.A.; Camparis, C.M.; Franco, A.L.; Fernandes, G.; Speciali, J.G.; Bigal, M.E. How to investigate and treat: Migraine in patients with temporomandibular disorders. Curr. Pain Headache Rep. 2012, 16, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Bevilaqua-Grossi, D.; Lipton, R.; Napchan, U.; Grosberg, B.; Ashina, S.; Bigal, M. Temporomandibular disorders and cutaneous allodynia are associated in individuals with migraine. Cephalalgia 2010, 30, 425–432. [Google Scholar] [CrossRef]
- Tchivileva, I.E.; Ohrbach, R.; Fillingim, R.B.; Greenspan, J.D.; Maixner, W.; Slade, G.D. Temporal change in headache and its contribution to the risk of developing first-onset temporomandibular disorder in the Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) study. Pain 2017, 158, 120–129. [Google Scholar] [CrossRef]
- Fillingim, R.B.; Slade, G.D.; Diatchenko, L.; Dubner, R.; Greenspan, J.D.; Knott, C.; Ohrbach, R.; Maixner, W. Summary of findings from the OPPERA baseline case-control study: Implications and future directions. J. Pain 2011, 12, T102–T107. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Lobbezoo, F.; van Wijk, A.; van der Heijden, G.J.; Visscher, C.M. Associations of pain intensity and pain-related disability with psychological and socio-demographic factors in patients with temporomandibular disorders: A cross-sectional study at a specialised dental clinic. J. Oral Rehabil. 2017, 44, 187–196. [Google Scholar] [CrossRef]
- Bigal, M.E.; Lipton, R.B. Migraine chronification. Curr. Neurol. Neurosci. Rep. 2011, 11, 139–148. [Google Scholar] [CrossRef]
- Pistoia, F.; Salfi, F.; Saporito, G.; Ornello, R.; Frattale, I.; D’Aurizio, G.; Tempesta, D.; Ferrara, M.; Sacco, S. Behavioral and psychological factors in individuals with migraine without psychiatric comorbidities. J. Headache Pain 2022, 23, 1. [Google Scholar] [CrossRef]
- Seng, E.K.; Buse, D.C.; Klepper, J.E.; Mayson, S.; Grinberg, A.S.; Grosberg, B.M.; Pavlovic, J.M.; Robbins, M.S.; Vollbracht, S.E.; Lipton, R.B. Psychological factors associated with chronic migraine and severe migraine-related disability: An observational study in a tertiary headache center. Headache J. Head Face Pain 2017, 57, 593–604. [Google Scholar] [CrossRef]
- Perez-Munoz, A.; Buse, D.C.; Andrasik, F. Behavioral Interventions for Migraine. Neurol. Clin. 2019, 37, 789–813. [Google Scholar] [CrossRef]
- Antonaci, F.; Nappi, G.; Galli, F.; Manzoni, G.C.; Calabresi, P.; Costa, A. Migraine and psychiatric comorbidity: A review of clinical findings. J. Headache Pain 2011, 12, 115–125. [Google Scholar] [CrossRef]
- Bouteloup, M.; Belot, R.A.; Noiret, N.; Sylvestre, G.; Bertoux, M.; Magnin, E.; Vuillier, F. Social and emotional cognition in patients with severe migraine consulting in a tertiary headache center: A preliminary study. Rev. Neurol. 2021, 177, 955–1000. [Google Scholar] [CrossRef]
- Ballegaard, V.; Thede-Schmidt-Hansen, P.; Svensson, P.; Jensen, R. Are headache and temporomandibular disorders related? A blinded study. Cephalalgia 2008, 28, 832–841. [Google Scholar] [CrossRef]
- Garrigós-Pedrón, M.; La Touche, R.; Navarro-Desentre, P.; Gracia-Naya, M.; Segura-Ortí, E. Widespread mechanical pain hypersensitivity in patients with chronic migraine and temporomandibular disorders: Relationship and correlation between psychological and sensorimotor variables. Acta Odontol. Scand. 2019, 77, 224–231. [Google Scholar] [CrossRef]
- Gonzalez, Y.; Castrillon, E.E.; Oyarzo, J.F.; Espinoza de Santillana, I.; Ortiz, F.; Velasco Neri, J.; Leyva, E. Criterios Diagnósticos para Trastornos Temporomandibulares: Instrumentos de Evaluación; UB WordPress: Buffalo, NY, USA, 2016. [Google Scholar]
- Headache Classification Committee. The International Classification of Headache Disorders, 3rd ed.; SAGE Publications Inc.: London, UK.
- Naing, N.N. Determination of sample size. Malays. J. Med. Sci. 2003, 10, 84–86. [Google Scholar]
- Spielberger, C.; Gorsuch, R.; Lushene, R. STAI: Cuestionario de Ansiedad Estado-Rasgo, 9th ed.; TEA Ediciones: Madrid, Spain, 2011. [Google Scholar]
- Spielberger, C. IDER. Inventario de Depresión Estado-Rasgo; TEA Ediciones: Madrid, Spain, 2008. [Google Scholar]
- Derogatis, L. Brief Symptom Inventory (BSI)-18. In Administration, Scoring and Procedures Manual; NCS Pearson: Minneapolis, MN, USA, 2001. [Google Scholar]
- Derogatis, L.R.; Melisaratos, N. The Brief Symptom Inventory: An introductory report. Psychol. Med. 1983, 13, 595–605. [Google Scholar] [CrossRef]
- Moos, R. Inventario de Respuestas de Afrontamiento para Adultos; TEA Ediciones: Madrid, Spain, 2010. [Google Scholar]
- Moos, R.H.; Brennan, P.L.; Fondacaro, M.R.; Moos, B.S. Approach and avoidance coping responses among older problem and nonproblem drinkers. Psychol. Aging 1990, 5, 31–40. [Google Scholar] [CrossRef]
- Korkmaz, S.; Goksuluk, D.; Zararsiz, G. MVN: An R Package for Assessing Multivariate Normality. R J. 2014, 6, 151–162. [Google Scholar] [CrossRef]
- Resende, C.; Rocha, L.; Paiva, R.P.; Cavalcanti, C.D.S.; Almeida, E.O.; Roncalli, A.G.; Barbosa, G.A.S. Relationship between anxiety, quality of life, and sociodemographic characteristics and temporomandibular disorder. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 125–132. [Google Scholar] [CrossRef]
- Fernandes Azevedo, A.B.; Camara-Souza, M.B.; Dantas, I.S.; de Resende, C.; Barbosa, G.A.S. Relationship between anxiety and temporomandibular disorders in dental students. Cranio 2018, 36, 300–303. [Google Scholar] [CrossRef]
- Jung, W.; Lee, K.E.; Suh, B.J. Influence of psychological factors on the prognosis of temporomandibular disorders pain. J. Dent. Sci. 2021, 16, 349–355. [Google Scholar] [CrossRef]
- Canales, G.T.; Guarda-Nardini, L.; Rizzatti-Barbosa, C.M.; Conti, P.C.R.; Manfredini, D. Distribution of depression, somatization and pain-related impairment in patients with chronic temporomandibular disorders. J. Appl. Oral Sci. 2019, 27, e20180210. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, D.; Winocur, E.; Ahlberg, J.; Guarda-Nardini, L.; Lobbezoo, F. Psychosocial impairment in temporomandibular disorders patients. RDC/TMD axis II findings from a multicentre study. J. Dent. 2010, 38, 765–772. [Google Scholar] [CrossRef]
- Zampieri, M.A.; Tognola, W.A.; Galego, J.C. Patients with chronic headache tend to have more psychological symptoms than those with sporadic episodes of pain. Arq. Neuropsiquiatr. 2014, 72, 598–602. [Google Scholar] [CrossRef]
- Radat, F. What is the link between migraine and psychiatric disorders? From epidemiology to therapeutics. Rev. Neurol. 2021, 177, 821–826. [Google Scholar] [CrossRef]
- Ashina, S.; Bendtsen, L.; Buse, D.C.; Lyngberg, A.C.; Lipton, R.B.; Jensen, R. Neuroticism, depression and pain perception in migraine and tension-type headache. Acta Neurol. Scand. 2017, 136, 470–476. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, H.; Heath, A.C.; Madden, P.A.; Martin, N.G.; Nyholt, D.R. Shared Genetic Factors Underlie Migraine and Depression. Twin Res. Hum. Genet. 2016, 19, 341–350. [Google Scholar] [CrossRef]
- Derogatis, L.R.; Rickels, K.; Rock, A.F. The SCL-90 and the MMPI: A step in the validation of a new self-report scale. Br. J. Psychiatry 1976, 128, 280–289. [Google Scholar] [CrossRef]
- Jeremic-Knezevic, M.; Knezevic, A.; Boban, N.; Djurovic Koprivica, D.; Boban, J. Correlation of somatization, depression, and chronic pain with clinical findings of the temporomandibular disorders in asymptomatic women. Cranio 2021, 39, 17–23. [Google Scholar] [CrossRef]
- Carver, C.S.; Scheier, M.F.; Weintraub, J.K. Assessing coping strategies: A theoretically based approach. J. Pers. Soc. Psychol. 1989, 56, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Reissmann, D.R.; John, M.T.; Schierz, O.; Seedorf, H.; Doering, S. Stress-related adaptive versus maladaptive coping and temporomandibular disorder pain. J. Orofac. Pain 2012, 26, 181–190. [Google Scholar] [PubMed]
- Hasanoglu Erbasar, G.N.; Alpaslan, C. Influence of coping strategies on oral health-related quality of life in patients with myalgia. Cranio 2019, 37, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Soto-Goni, X.A.; Viñals, A.C.; Pérex-González, M.; Sánchez-Labrador, L.; Domínguez-Gordillo, A.; Sanchez-Sanchez, T.; Ardizone-Garcia, I.; Jimenez-Ortega, L. Stress-related coping styles, anxiety, and neuroticism in university students with myalgia temporomandibular: A case control study. Rev. Psicol. Salud 2021, 8, 36–49. [Google Scholar] [CrossRef]
- Soto-Goni, X.A.; Alen, F.; Buiza-Gonzalez, L.; Marcolino-Cruz, D.; Sanchez-Sanchez, T.; Ardizone-Garcia, I.; Aneiros-Lopez, F.; Jimenez-Ortega, L. Adaptive Stress Coping in Awake Bruxism. Front. Neurol. 2020, 11, 564431. [Google Scholar] [CrossRef] [PubMed]
- GaldOn, M.J.; Dura, E.; Andreu, Y.; Ferrando, M.; Poveda, R.; Bagan, J.V. Multidimensional approach to the differences between muscular and articular temporomandibular patients: Coping, distress, and pain characteristics. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2006, 102, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Sauro, K.M.; Becker, W.J. The stress and migraine interaction. Headache 2009, 49, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Bigal, M.E.; Lipton, R.B. Modifiable risk factors for migraine progression (or for chronic daily headaches)—Clinical lessons. Headache 2006, 46 (Suppl. 3), S144–S146. [Google Scholar] [CrossRef]
- Bavia, P.F.; Muller, C.V.; Meirelles, L.; Vilanova, L.S.; Silva Rdos, S. Is migraine a complicating factor for evidence-based therapy for masticatory myofascial pain? A case-control study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Katsarava, Z.; Buse, D.C.; Manack, A.N.; Lipton, R.B. Defining the differences between episodic migraine and chronic migraine. Curr. Pain Headache Rep. 2012, 16, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, A.; Varon, S.; Wilcox, T.; Buse, D.; Kawata, A.; Manack, A.; Goadsby, P.; Lipton, R. Disability, HRQoL and resource use among chronic and episodic migraineurs: Results from the International Burden of Migraine Study (IBMS). Cephalalgia 2011, 31, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Florencio, L.L.; de Oliveira, A.S.; Carvalho, G.F.; Dach, F.; Bigal, M.E.; Fernandez-de-Las-Penas, C.; Bevilaqua-Grossi, D. Association Between Severity of Temporomandibular Disorders and the Frequency of Headache Attacks in Women With Migraine: A Cross-Sectional Study. J. Manip. Physiol. Ther. 2017, 40, 250–254. [Google Scholar] [CrossRef]

| TMD−/MIGRAINE− | TMD−/MIGRAINE+ | TMD+/MIGRAINE− | TMD+/MIGRAINE+ | |
|---|---|---|---|---|
| Participants (n) | 39 | 20 | 23 | 60 |
| Females (males) | 27 (12) | 16 (4) | 19 (4) | 52 (8) |
| Mean Age | 37.8 | 34.2 | 35.1 | 42 |
| Episodic Mig. | NA | 18 | NA | 26 |
| Chronic Mig. | NA | 2 | NA | 34 |
| Local myalgia | NA | NA | 2 | 13 |
| Myofascial pain | NA | NA | 14 | 6 |
| Myofascial pain with referral | NA | NA | 7 | 34 |
| Headache attributed to TMD | NA | NA | 0 | 5 |
| Arthralgia | NA | NA | 0 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viñals Narváez, A.C.; Sánchez-Sánchez, T.; García-González, M.; Ardizone García, I.; Cid-Verdejo, R.; Sánchez Romero, E.A.; Jiménez-Ortega, L. Psychological and Behavioral Factors Involved in Temporomandibular Myalgia and Migraine: Common but Differentiated Profiles. Int. J. Environ. Res. Public Health 2023, 20, 1545. https://doi.org/10.3390/ijerph20021545
Viñals Narváez AC, Sánchez-Sánchez T, García-González M, Ardizone García I, Cid-Verdejo R, Sánchez Romero EA, Jiménez-Ortega L. Psychological and Behavioral Factors Involved in Temporomandibular Myalgia and Migraine: Common but Differentiated Profiles. International Journal of Environmental Research and Public Health. 2023; 20(2):1545. https://doi.org/10.3390/ijerph20021545
Chicago/Turabian StyleViñals Narváez, Ana Cristina, Teresa Sánchez-Sánchez, Maria García-González, Ignacio Ardizone García, Rosana Cid-Verdejo, Eleuterio A. Sánchez Romero, and Laura Jiménez-Ortega. 2023. "Psychological and Behavioral Factors Involved in Temporomandibular Myalgia and Migraine: Common but Differentiated Profiles" International Journal of Environmental Research and Public Health 20, no. 2: 1545. https://doi.org/10.3390/ijerph20021545
APA StyleViñals Narváez, A. C., Sánchez-Sánchez, T., García-González, M., Ardizone García, I., Cid-Verdejo, R., Sánchez Romero, E. A., & Jiménez-Ortega, L. (2023). Psychological and Behavioral Factors Involved in Temporomandibular Myalgia and Migraine: Common but Differentiated Profiles. International Journal of Environmental Research and Public Health, 20(2), 1545. https://doi.org/10.3390/ijerph20021545








