Associations between the Timing and Nutritional Characteristics of Bedtime Meals and Sleep Quality for Nurses after a Rotating Night Shift: A Cross-Sectional Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Setting and Participants
2.3. Bedtime Meals
2.4. Sleep Time
2.5. Subjective Sleep Assessment
2.6. Procedure
2.7. Ethical Considerations
2.8. Statistical Analysis
3. Results
3.1. Characteristics of Participants and Their Bedtime Meals and Sleep after Night Shifts
3.2. Differences in Sleep Quality According to Participant Characteristics
3.3. Correlations between Bedtime Meals and Sleep Measures
3.4. Factors Associated with Sleep after a Night Shift
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Savic, M.; Ogeil, R.P.; Sechtig, M.J.; Lee-Tobin, P.; Ferguson, N.; Lubsman, D.I. How Do Nurses Cope with Shift Work? A Qualitative Analysis of Open-Ended Responses from a Survey of Nurses. Int. J. Environ. Res. Public Health 2019, 16, 3821. [Google Scholar] [CrossRef]
- McDowall, K.; Murphy, E.; Anderson, K. The impact of shift work on sleep quality among nurses. Occup. Med. 2017, 67, 621–625. [Google Scholar] [CrossRef]
- Kim, S.J.; Gu, M.O. A study on the relationship among circadian types, sleep quality and adaptation to night shifts among nurses working on two or three day night duties. J. Korean Clin. Nurs. Res. 2013, 19, 309–320. [Google Scholar]
- Organization for Economic Cooperation and Development. Social Protection and Wellbeing. Available online: https://www.oecd.org/gender/data/OECD_1564_TUSupdatePortal.xlsx (accessed on 18 January 2022).
- Kecklund, G.; Axelsson, J. Health consequences of shift work and insufficient sleep. BMJ 2016, 355, i5210. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.C. Negative impacts of shiftwork and long work hours. Rehabil. Nurs. 2014, 39, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.-H.; Kang, J.-S. A study on job involvement according to working pattern and daytime sleepiness among hospital nurses. J. East-West Nurs. Res. 2011, 17, 81–86. [Google Scholar]
- Yang, E.O.; Choi, I.R.; Kim, S.-M. The impact of sleep disorder and job stress on turnover intention of shift-working nurses. Korean J. Stress Res. 2017, 25, 255–264. [Google Scholar] [CrossRef]
- Muecke, S. Effects of rotating night shifts: Literature review. J. Adv. Nurs. 2005, 50, 433–439. [Google Scholar] [CrossRef]
- Chung, M.-H.; Liu, W.-I.; Lee, H.-L.; Hsu, N. Selected neurophysiological, psychological, and behavioral influences on subjective sleep quality in nurses: A structure equation model. PLoS ONE 2013, 8, e79529. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Gu, M.O. Structural equation model for sleep quality of female shift work nurses. J. Korean Acad. Nurs. 2018, 48, 622–635. [Google Scholar] [CrossRef]
- Pot, G.K. Sleep and dietary habits in the urban environment: The role of chrono-nutrition. Proc. Nutr. Soc. 2018, 77, 189–198. [Google Scholar] [CrossRef]
- Katagiri, R.; Asakura, K.; Kobayashi, S.; Suga, H.; Sasaki, S. Low intake of vegetables, high intake of confectionary, and unhealthy eating habits are associated with poor sleep quality among middle-aged female Japanese workers. J. Occup. Health 2014, 56, 359–368. [Google Scholar] [CrossRef]
- Tan, X.; Alén, M.; Cheng, S.M.; Mikkola, T.M.; Tenhunen, J.; Lyytikäinen, A.; Wiklund, P.; Cong, F.; Saarinen, A.; Tarkka, I. Associations of disordered sleep with body fat distribution, physical activity and diet among overweight middle-aged men. J. Sleep Res. 2015, 24, 414–424. [Google Scholar] [CrossRef]
- Jaussent, I.; Dauvilliers, Y.; Ancelin, M.-L.; Dartigues, J.-F.; Tavernier, B.; Touchon, J.; Ritchie, K.; Besset, A. Insomnia symptoms in older adults: Associated factors and gender differences. Am. J. Geriatr. Psychiatry 2011, 19, 88–97. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Mikic, A.; Pietrolungo, C.E. Effects of diet on sleep quality. Adv. Nutr. 2016, 7, 938–949. [Google Scholar] [CrossRef]
- Heath, G.; Dorrian, J.; Coates, A. Associations between shift type, sleep, mood, and diet in a group of shift working nurses. Scand. J. Work Environ. Health 2019, 45, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, T.; Kawano, Y.; Noguchi, O.; Onishi, J.; Teramoto, R.; Sunami, A.; Yokoyama, Y.; Tada, Y.; Hida, A.; Togo, F. Association of eating behaviours with diurnal preference and rotating shift work in Japanese female nurses: A cross-sectional study. BMJ Open 2016, 6, e011987. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.J.; Kripke, D.F.; Gruen, W.; Mullaney, D.J.; Gillin, J.C. Automatic sleep/wake identification from wrist activity. Sleep 1992, 15, 461–469. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Bigué, J.L.; Duclos, C.; Dumont, M.; Paquet, J.; Blais, H.; Menon, D.K.; Bernard, F.; Gosselin, N. Validity of actigraphy for nighttime sleep monitoring in hospitalized patients with traumatic injuries. J. Clin. Sleep Med. 2020, 16, 185–192. [Google Scholar] [CrossRef]
- Yoon, E.; Bae, S.; Park, H. Gait Speed and Sleep Duration Is Associated with Increased Risk of MCI in Older Community-Dwelling Adults. Int J Env. Res Public Health 2022, 19, 7625. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Kang, J.-E. The effect of preparatory audiovisual information with videotape influencing on sleep and anxiety of abdominal surgical patients. J. Korean Acad. Fundam. Nurs. 1994, 1, 19–36. [Google Scholar]
- Snyder-Halpern, R.; Verran, J.A. Instrumentation to describe subjective sleep characteristics in healthy subjects. Res Nurs Health 1987, 10, 155–163. [Google Scholar] [CrossRef]
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle; Akad Emiai KiadoKiado: Budapest, Hungary, 1973. [Google Scholar]
- Hastie, T.; Tibshirani, R. Generalized additive models for medical research. Stat. Methods Med. Res. 1995, 4, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.A.S.C.; Lopes, T.d.V.C.; Teixeira, K.R.; Mendes, J.A.; de Souza Borba, M.E.; Mota, M.C.; Waterhouse, J.; Crispim, C.A. The association between anxiety, hunger, the enjoyment of eating foods and the satiety after food intake in individuals working a night shift compared with after taking a nocturnal sleep: A prospective and observational study. Appetite 2017, 108, 255–262. [Google Scholar] [CrossRef]
- Makker, H.; Walker, M.; Selsick, H.; Kotecha, B.; Johal, A. Oxford Case Histories in Sleep Medicine; OUP Oxford: Oxford, UK, 2015. [Google Scholar]
- Chnag, S.-B.; Chu, S.-H.; Kim, Y.-I.; Yun, S.-H. The effects of aroma inhalation on sleep and fatigue in night shift nurses. Korean J. Adult Nurs. 2008, 20, 941–949. [Google Scholar]
- Forner-Cordero, A.; Umemura, G.S.; Furtado, F.; Gonçalves, B.d.S.B. Comparison of sleep quality assessed by actigraphy and questionnaires to healthy subjects. Sleep Sci. 2018, 11, 141. [Google Scholar] [CrossRef]
- Ibáñez-del Valle, V.; Silva, J.; Castelló-Domenech, A.-B.; Martinez-Martinez, M.; Verdejo, Y.; Sanantonio-Camps, L.; Cauli, O. Subjective and objective sleep quality in elderly individuals: The role of psychogeriatric evaluation. Arch. Gerontol. Geriatr. 2018, 76, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Slightam, C.; Petrowski, K.; Jamison, A.L.; Keller, M.; Bertram, F.; Kim, S.; Roth, W.T. Assessing sleep quality using self-report and actigraphy in PTSD. J. Sleep Res. 2018, 27, e12632. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.-X. Objective and subjective measures for sleep disorders. Neurosci. Bull. 2007, 23, 236–240. [Google Scholar] [CrossRef]
- Afaghi, A.; O’Connor, H.; Chow, C.M. High-glycemic-index carbohydrate meals shorten sleep onset. Am. J. Clin. Nutr. 2007, 85, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Binks, H.; Vincent, G.E.; Gupta, C.; Irwin, C.; Khalesi, S. Effects of diet on sleep: A narrative review. Nutrients 2020, 12, 936. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.-M.; Ma, J.-H.; Wang, S.-Y.; Huang, Z.; Zhou, Y.; Yu, H. The role of tryptophan metabolism in postpartum depression. Metab. Brain Dis. 2018, 33, 647–660. [Google Scholar] [CrossRef]
- Chaput, J.-P. Sleep patterns, diet quality and energy balance. Physiol. Behav. 2014, 134, 86–91. [Google Scholar] [CrossRef]
- Huo, L.; Li, B.; Wei, F. Maternal nutrition associated with nausea and vomiting during pregnancy: A prospective cohort China study. Biomed. Res. 2017, 28, 4543–4548. [Google Scholar]
- Crispim, C.A.; Mota, M.C. New perspectives on chrononutrition. Biol. Rhythm Res. 2019, 50, 63–77. [Google Scholar] [CrossRef]
- Borbely, A.A.; Daan, S.; Wirz-Justice, A.; Deboer, T. The two-process model of sleep regulation: A reappraisal. J. Sleep Res. 2016, 25, 131–143. [Google Scholar] [CrossRef]
- Nguyen, J.; Wright, K.P., Jr. Influence of weeks of circadian misalignment on leptin levels. Nat Sci Sleep 2010, 2, 9–18. [Google Scholar] [CrossRef]
- Spiegel, K.; Leproult, R.; Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999, 354, 1435–1439. [Google Scholar] [CrossRef]
- Pan, A.; Schernhammer, E.S.; Sun, Q.; Hu, F.B. Rotating night shift work and risk of type 2 diabetes: Two prospective cohort studies in women. PLoS Med. 2011, 8, e1001141. [Google Scholar] [CrossRef]
- Munoz, J.S.G.; Canavate, R.; Hernandez, C.M.; Cara-Salmeron, V.; Morante, J.J.H. The association among chronotype, timing of food intake and food preferences depends on body mass status. Eur. J. Clin. Nutr. 2017, 71, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Booker, L.A.; Magee, M.; Rajaratnam, S.M.W.; Sletten, T.L.; Howard, M.E. Individual vulnerability to insomnia, excessive sleepiness and shift work disorder amongst healthcare shift workers. A systematic review. Sleep Med. Rev. 2018, 41, 220–233. [Google Scholar] [CrossRef]
- Juda, M.; Vetter, C.; Roenneberg, T. Chronotype modulates sleep duration, sleep quality, and social jet lag in shift-workers. J. Biol. Rhythm. 2013, 28, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Togo, F.; Yoshizaki, T.; Komatsu, T. Association between depressive symptoms and morningness-eveningness, sleep duration and rotating shift work in Japanese nurses. Chronobiol. Int. 2017, 34, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.S.; Scheer, F.A.; Jacques, P.F.; Lamon-Fava, S.; Ordovas, J.M. Short sleep duration and dietary intake: Epidemiologic evidence, mechanisms, and health implications. Adv. Nutr. 2015, 6, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, T.; Komatsu, T.; Tada, Y.; Hida, A.; Kawano, Y.; Togo, F. Association of habitual dietary intake with morningness-eveningness and rotating shift work in Japanese female nurses. Chronobiol. Int. 2018, 35, 392–404. [Google Scholar] [CrossRef]
- Crispim, C.A.; Zimberg, I.Z.; dos Reis, B.G.; Diniz, R.M.; Tufik, S.; de Mello, M.T. Relationship between food intake and sleep pattern in healthy individuals. J. Clin. Sleep Med. 2011, 7, 659–664. [Google Scholar] [CrossRef]
- Lowden, A.; Moreno, C.; Holmbäck, U.; Lennernäs, M.; Tucker, P. Eating and shift work—Effects on habits, metabolism, and performance. Scand. J. Work Environ. Health 2010, 36, 150–162. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare. 2020 Dietary Reference Intakes for Koreans: Energy and Macronutrients; Ministry of Health and Welfare: Sejong, Republic of Korea, 2020. [Google Scholar]
- Centofanti, S.; Dorrian, J.; Hilditch, C.; Grant, C.; Coates, A.; Banks, S. Eating on nightshift: A big vs small snack impairs glucose response to breakfast. Neurobiol. Sleep Circadian Rhythm. 2018, 4, 44–48. [Google Scholar] [CrossRef]
- Acebo, C.; Sadeh, A.; Seifer, R.; Tzischinsky, O.; Wolfson, A.R.; Hafer, A.; Carskadon, M.A. Estimating sleep patterns with activity monitoring in children and adolescents: How many nights are necessary for reliable measures? Sleep 1999, 22, 95–103. [Google Scholar] [CrossRef]
- Shin, S.; Kim, S.H.; Jeon, B. Objective Assessment of Sleep Patterns among Night-Shift Workers: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 13236. [Google Scholar] [CrossRef] [PubMed]
| Variables | Categories | n (%) | Mean ± SD |
|---|---|---|---|
| Gender | Man | 8 (6.3) | |
| Woman | 120 (93.8) | ||
| Age (years) | <27 | 85 (66.4) | 26.90 ± 5.69 |
| ≥27 | 43 (33.6) | ||
| Body Mass Index (kg/m2) | <18.5 | 20 (15.6) | 21.00 ± 3.06 |
| 18.5~23 | 84 (65.6) | ||
| ≥23 | 24 (18.8) | ||
| Marital status | Married | 24 (18.8) | |
| Unmarried | 104 (81.3) | ||
| Clinical experience (months) | <36 | 68 (53.1) | 55.40 ± 62.72 |
| ≥36 | 60 (46.9) | ||
| Number of night shifts per month | <7 | 74 (69.8) | 6.50 ± 1.24 |
| ≥7 | 32 (30.2) | ||
| Position | Staff nurse | 126 (98.4) | |
| Charge nurse | 2 (1.6) | ||
| Department | Inpatient units | 56 (14.8) | |
| Intensive care units | 61 (47.7) | ||
| Emergency rooms | 9 (7.0) | ||
| Bedtime meal | Protein (%) | 12.82 ± 6.56 | |
| Fat (%) | 27.53 ± 15.31 | ||
| Carbohydrate (%) | 59.54 ± 18.53 | ||
| Total Calorie (Kcal) | 428.20 ± 262.23 | ||
| Time interval between meal and sleep (hours) | 1.43 ± 1.18 | ||
| Accelerometer measured sleep times | Total sleep time (minutes) | 334.97 ± 102.44 | |
| Wake After Sleep Onset (minutes) | 17.64 ± 19.07 | ||
| Sleep Efficiency (%) | 95.35 ± 4.69 | ||
| Subjective sleep assessment | VSH sleep scale score | 38.96 ± 10.71 |
| Variables | Categories | Total Sleep Time | WASO | Sleep Efficiency | VSH Sleep Scale Score | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | t or F | Mean ± SD | t or F | Mean ± SD | t or F | Mean ± SD | t or F | ||
| Gender | Man | 432.50 ± 129.8 | 2.42 * | 18.86 ± 23.18 | 0.17 | 94.20 ± 6.48 | −0.70 | 40.00 ± 14.04 | 0.14 |
| Woman | 332.41 ± 96.97 | 17.57 ± 18.90 | 95.47 ± 4.58 | 39.44 ± 10.29 | |||||
| Age (years) | <27 | 333.49 ± 95.75 | −0.60 | 18.71 ± 20.36 | 0.83 | 95.50 ± 4.82 | 0.31 | 40.87 ± 10.00 | 2.14 * |
| ≥27 | 345.38 ± 110.4 | 15.67 ± 16.44 | 95.21 ± 4.47 | 36.66 ± 10.94 | |||||
| Body Mass Index (kg/m2) | <18.5 | 339.84 ± 80.63 | 0.09 | 19.45 ± 24.67 | 0.14 | 95.85 ± 3.37 | 0.16 | 41.00 ± 12.68 | 0.25 |
| 18.5~23 | 334.87 ± 103.8 | 17.04 ± 17.31 | 95.38 ± 4.95 | 39.11 ± 10.01 | |||||
| ≥23 | 345.38 ± 109.4 | 18.19 ± 20.28 | 95.01 ± 4.89 | 39.50 ± 10.44 | |||||
| Marital status | Married | 338.04 ± 106.0 | 0.03 | 19.04 ± 17.71 | 0.40 | 95.24 ± 3.44 | −0.18 | 36.71 ± 10.66 | −1.45 |
| Unmarried | 337.47 ± 99.87 | 17.29 ± 19.46 | 95.43 ± 4.96 | 40.14 ± 10.36 | |||||
| Clinical experience (months) | < 36 | 334.40 ± 101.2 | −0.36 | 18.81 ± 20.03 | 0.69 | 95.29 ± 4.98 | −0.25 | 41.63 ± 10.04 | 2.10 * |
| ≥36 | 341.24 ± 101.0 | 16.40 ± 18.06 | 95.50 ± 4.38 | 37.71 ± 10.54 | |||||
| Number of night shifts per month | < 7 | 324.38 ± 93.41 | −0.71 | 19.94 ± 19.86 | 2.47 * | 94.92 ± 4.98 | −1.67 | 39.36 ± 11.28 | −0.15 |
| ≥7 | 340.41 ± 114.3 | 10.10 ± 12.31 | 96.67 ± 4.04 | 39.65 ± 9.28 | |||||
| Position | Staff nurse | 337.74 ± 101.4 | 0.12 | 17.46 ± 18.83 | −0.81 | 95.39 ± 4.71 | −0.11 | 39.48 ± 10.55 | −0.01 |
| Charge nurse | 329.00 ± 65.05 | 28.50 ± 38.89 | 95.76 ± 4.11 | 39.50 ± 0.71 | |||||
| Department | Inpatient units | 347.14 ± 93.27 | 0.48 | 17.94 ± 19.83 | 0.38 | 95.45 ± 4.70 | 0.06 | 39.75 ± 10.91 | 0.04 |
| Intensive care units | 332.02 ± 107.5 | 18.19 ± 18.97 | 95.27 ± 4.60 | 39.24 ± 9.81 | |||||
| Emergency rooms | 318.11 ± 103.7 | 12.33 ± 15.91 | 95.82 ± 5.60 | 39.33 ± 12.90 | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Time between meals and sleep | 1 | ||||||||
| 2. Protein ratio | 0.19 | 1 | |||||||
| 3. Fat ratio | 0.16 | 0.32 ** | 1 | ||||||
| 4. Carbohydrate ratio | −0.20 | −0.62 *** | −0.94 *** | 1 | |||||
| 5. Total calories | 0.16 | 0.11 | 0.10 | −0.12 | 1 | ||||
| 6. Total Sleep Time | −0.36 ** | −0.00 | 0.01 | −0.01 | −0.07 | 1 | |||
| 7. Wake After Sleep Onset | −0.11 | −0.14 | 0.02 | 0.03 | 0.04 | 0.04 | 1 | ||
| 8. Sleep efficiency | 0.19 | 0.28 * | −0.10 | −0.01 | −0.00 | −0.12 | −0.70 *** | 1 | |
| 9. VSH sleep scale score | −0.12 | −0.01 | −0.12 | 0.10 | 0.19 | 0.18 | 0.14 | −0.09 | 1 |
| Sleep | Factors | B | SE | β | t | p | VIF |
|---|---|---|---|---|---|---|---|
| Total Sleep Time R2 = 0.151 F = 2.532 p = 0.036 | (constant) | 500.21 | 110.57 | 4.52 | <0.001 | ||
| Gender | −62.03 | 51.48 | −0.14 | −1.20 | 0.232 | 1.12 | |
| Time interval between meals and sleep (hour) | −30.73 | 9.39 | −0.37 | −3.27 | 0.002 | 1.06 | |
| Protein ratio (%) | 0.89 | 1.89 | 0.05 | 0.47 | 0.640 | 1.10 | |
| Fat ratio (%) | 0.24 | 0.75 | 0.04 | 0.32 | 0.752 | 1.07 | |
| Total calories (Kcal) | −0.03 | 0.05 | −0.07 | −0.56 | 0.579 | 1.18 | |
| Wake After Sleep Onset R2 = 0.035 F = 0.545 p = 0.741 | (constant) | 21.88 | 13.27 | 1.65 | 0.103 | ||
| Monthly number of night shifts | 0.01 | 1.68 | 0.00 | 0.01 | 0.996 | 1.03 | |
| Time interval between meals and sleep (hour) | −1.62 | 1.88 | −0.10 | −0.86 | 0.391 | 1.07 | |
| Protein ratio (%) | −0.47 | 0.39 | −0.15 | −1.22 | 0.227 | 1.10 | |
| Fat ratio (%) | 0.08 | 0.16 | 0.06 | 0.50 | 0.622 | 1.11 | |
| Total calories (Kcal) | 0.01 | 0.01 | 0.08 | 0.68 | 0.500 | 1.05 | |
| Sleep Efficacy R2 = 0.140 F = 3.040 p = 0.022 | (constant) | 93.15 | 1.56 | 59.68 | <0.001 | ||
| Time interval between meals and sleep (hour) | 0.76 | 0.45 | 0.19 | 1.71 | 0.091 | 1.06 | |
| Protein ratio (%) | 0.26 | 0.09 | 0.31 | 2.77 | 0.007 | 1.09 | |
| Fat ratio (%) | −0.06 | 0.04 | −0.17 | −1.53 | 0.130 | 1.05 | |
| Total calories (Kcal) | −0.00 | 0.00 | −0.11 | −0.97 | 0.337 | 1.09 | |
| Subjective Assessment R2 = 0.206 F = 2.981 p = 0.012 | (constant) | 49.97 | 5.26 | 9.51 | <.001 | ||
| Age (year) | −5.18 | 3.13 | −0.25 | −1.65 | 0.103 | 1.93 | |
| Clinical experience (month) | −1.40 | 3.03 | −0.07 | −0.46 | 0.646 | 1.87 | |
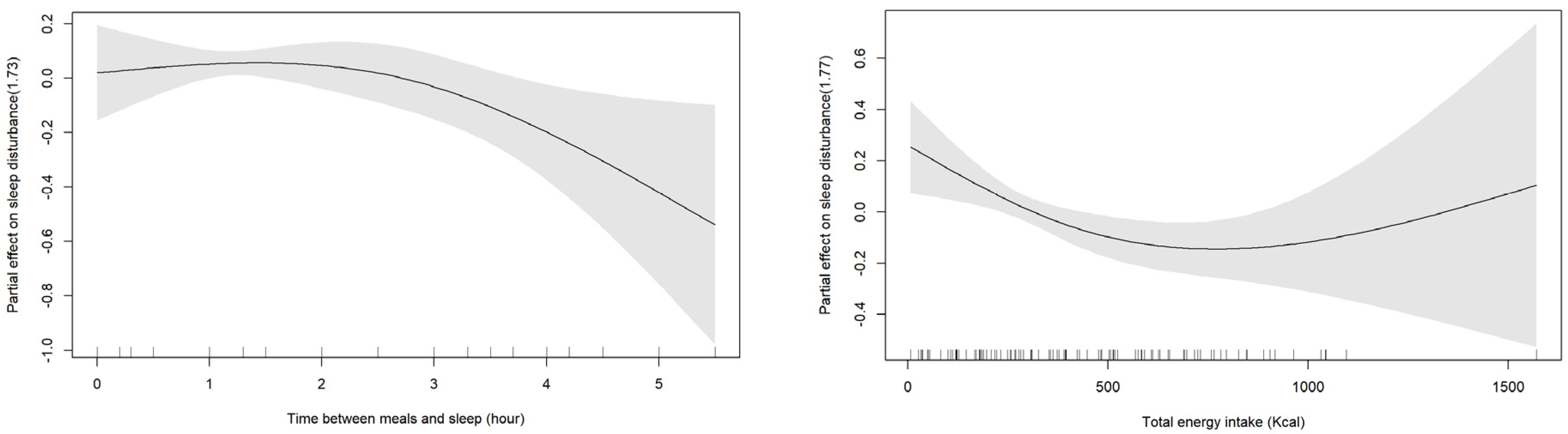
| Time interval between meals and sleep (hour) | −1.89 | 0.94 | −0.23 | −2.02 | 0.048 | 1.11 | |
| Protein ratio (%) | 0.07 | 0.19 | 0.04 | 0.36 | 0.724 | 1.10 | |
| Fat ratio (%) | −0.13 | 0.08 | −0.19 | −1.66 | 0.102 | 1.12 | |
| Total calories (Kcal) | 0.01 | 0.01 | 0.23 | 2.06 | 0.043 | 1.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.H.; Park, H.; Bae, S.; Kang, J. Associations between the Timing and Nutritional Characteristics of Bedtime Meals and Sleep Quality for Nurses after a Rotating Night Shift: A Cross-Sectional Analysis. Int. J. Environ. Res. Public Health 2023, 20, 1489. https://doi.org/10.3390/ijerph20021489
Park JH, Park H, Bae S, Kang J. Associations between the Timing and Nutritional Characteristics of Bedtime Meals and Sleep Quality for Nurses after a Rotating Night Shift: A Cross-Sectional Analysis. International Journal of Environmental Research and Public Health. 2023; 20(2):1489. https://doi.org/10.3390/ijerph20021489
Chicago/Turabian StylePark, Jung Hoon, Hyuntae Park, Seongryu Bae, and Jiyeon Kang. 2023. "Associations between the Timing and Nutritional Characteristics of Bedtime Meals and Sleep Quality for Nurses after a Rotating Night Shift: A Cross-Sectional Analysis" International Journal of Environmental Research and Public Health 20, no. 2: 1489. https://doi.org/10.3390/ijerph20021489
APA StylePark, J. H., Park, H., Bae, S., & Kang, J. (2023). Associations between the Timing and Nutritional Characteristics of Bedtime Meals and Sleep Quality for Nurses after a Rotating Night Shift: A Cross-Sectional Analysis. International Journal of Environmental Research and Public Health, 20(2), 1489. https://doi.org/10.3390/ijerph20021489







