Association of Hallux Valgus with Degenerative Spinal Diseases: A Population-Based Cohort Study
Abstract
1. Introduction
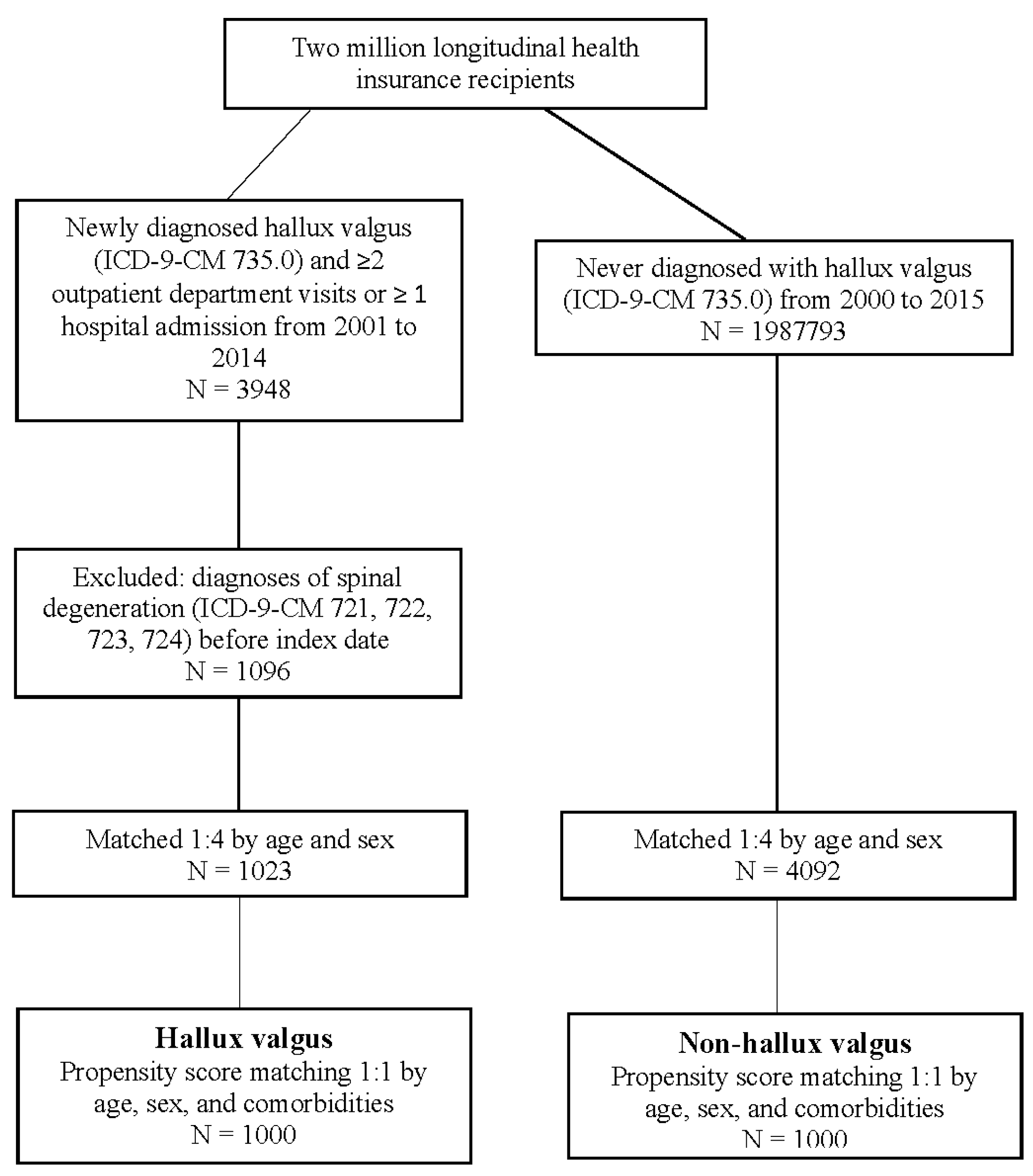
2. Materials and Methods
2.1. Data Sources
2.2. Study Design
2.3. Covariates and Matching
2.4. Statistical Analysis
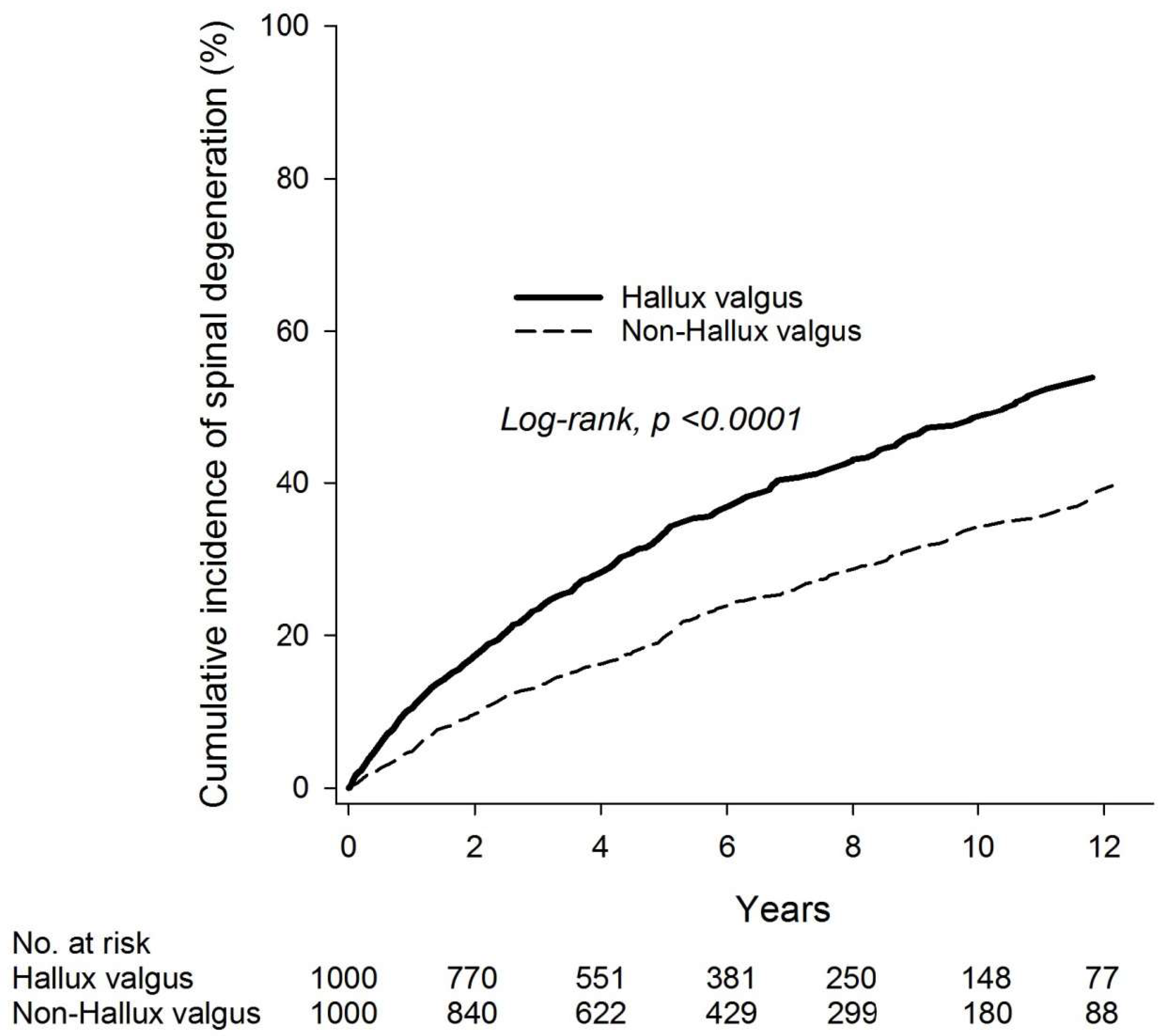
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| All | Female | Male | Age < 40 | Age ≥ 40 | |
|---|---|---|---|---|---|
| 721 Spondylosis | 2.01 (1.55–2.61) | 2.16 (1.62–2.88) | 1.47 (0.80–2.72) | 1.87 (1.05–3.30) | 2.06 (1.54–2.77) |
| 722 Intervertebral disorder | 2.27 (1.62–3.17) | 2.41 (1.65–3.51) | 1.90 (0.92–3.95) | 2.35 (1.36–4.07) | 2.18 (1.43–3.31) |
| 723, 724 Spinal stenosis | 1.47 (1.24–1.76) | 1.43 (1.17–1.74) | 1.64 (1.11–2.41) | 1.42 (1.10–1.84) | 1.53 (1.21–1.94) |
| Cervical stenosis: 723.0 | 1.04 (0.32–3.45) | 1.90 (0.47–7.61) | NA | 0.91 (0.13–6.49) | 1.16 (0.26–5.20) |
| Lumbar stenosis: 722.73, 724.02 | 1.68 (0.87–3.25) | 2.49 (1.13–5.48) | 0.57 (0.13–2.48) | 0.55 (0.13–2.31) | 2.42 (1.11–5.27) |
| Univariate | Multivariate † | |||||
|---|---|---|---|---|---|---|
| n | No. of Events | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Group | ||||||
| Non-hallux valgus | 1000 | 255 | Reference | Reference | ||
| Hallux valgus without OP | 844 | 358 | 1.86 (1.58–2.18) | <0.0001 | 1.95 (1.66–2.29) | <0.0001 |
| Hallux valgus with OP | 156 | 33 | 0.86 (0.60–1.24) | 0.418 | 0.84 (0.58–1.21) | 0.340 |
| Non-Hallux Valgus | Hallux Valgus | |||||
|---|---|---|---|---|---|---|
| n | No. of Spinal Degenerations | n | No. of Spinal Degenerations | HR † (95% CI) | p-Value | |
| Washout period = 1 year | 951 | 208 | 894 | 287 | 1.61 (1.35–1.93) | <0.0001 |
| Washout period = 2 years | 840 | 159 | 770 | 220 | 1.64 (1.34–2.02) | <0.0001 |
References
- Kushchayev, S.V.; Glushko, T.; Jarraya, M.; Schuleri, K.H.; Preul, M.C.; Brooks, M.L.; Teytelboym, O.M. ABCs of the degenerative spine. Insights Imaging 2018, 9, 253–274. [Google Scholar] [CrossRef] [PubMed]
- Bogduk, N. Degenerative joint disease of the spine. Radiol. Clin. N. Am. 2012, 50, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Izzo, R.; Guarnieri, G.; Guglielmi, G.; Muto, M. Biomechanics of the spine. Part I: Spinal stability. Eur. J. Radiol. 2013, 82, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Steelman, T.; Lewandowski, L.; Helgeson, M.; Wilson, K.; Olsen, C.; Gwinn, D. Population-based Risk Factors for the Development of Degenerative Disk Disease. Clin. Spine Surg. 2018, 31, E409–E412. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.C.; Huang, J.Y.; Hung, Y.M.; Perng, W.T.; Chang, R.; Wei, J.C. Flat foot and spinal degeneration: Evidence from nationwide population-based cohort study. J. Formos. Med. Assoc. 2021, 120, 1897–1906. [Google Scholar] [CrossRef]
- Roddy, E.; Zhang, W.; Doherty, M. Prevalence and associations of hallux valgus in a primary care population. Arthritis Rheum. 2008, 59, 857–862. [Google Scholar] [CrossRef]
- Ray, J.J.; Friedmann, A.J.; Hanselman, A.E.; Vaida, J.; Dayton, P.D.; Hatch, D.J.; Smith, B.; Santrock, R.D. Hallux Valgus. Foot Ankle Orthop. 2019, 4. [Google Scholar] [CrossRef]
- Nix, S.; Smith, M.; Vicenzino, B. Prevalence of hallux valgus in the general population: A systematic review and meta-analysis. J. Foot Ankle Res. 2010, 3, 21. [Google Scholar] [CrossRef]
- Santos, A.D.; Prado-Rico, J.M.; Cirino, N.T.O.; Perracini, M.R. Are foot deformity and plantar sensitivity impairment associated with physical function of community-dwelling older adults? Braz. J. Phys. Ther. 2021, 25, 846–853. [Google Scholar] [CrossRef]
- Menz, H.B.; Lord, S.R. Gait instability in older people with hallux valgus. Foot Ankle Int. 2005, 26, 483–489. [Google Scholar] [CrossRef]
- Sánchez-Sanjuan, A.; Romero-Morales, C.; Alfaro-Santafé, J.; Almenar-Arasanz, A.-J.; Gómez-Bernal, A.; Pareja-Galeano, H. Foot Anatomical Structural Variations Increase the Risk of Falls in Older Adults. Appl. Sci. 2022, 12, 9825. [Google Scholar] [CrossRef]
- Menz, H.B.; Munteanu, S.E.; Zammit, G.V.; Landorf, K.B. Foot structure and function in older people with radiographic osteoarthritis of the medial midfoot. Osteoarthr. Cartil. 2010, 18, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Cheney, N.; Rockwell, K.; Long, J.; Weis, J.; Lewis, D.; Law, T.; Carr, A. Is a Flatfoot Associated with a Hallux Valgus Deformity? Foot Ankle Orthop. 2017, 2. [Google Scholar] [CrossRef]
- McGregor, A.H.; Hukins, D.W. Lower limb involvement in spinal function and low back pain. J. Back Musculoskelet. Rehabil. 2009, 22, 219–222. [Google Scholar] [CrossRef]
- Cebulski-Delebarre, A.; Boutry, N.; Szymanski, C.; Maynou, C.; Lefebvre, G.; Amzallag-Bellenger, E.; Cotten, A. Correlation between primary flat foot and lower extremity rotational misalignment in adults. Diagn. Interv. Imaging 2016, 97, 1151–1157. [Google Scholar] [CrossRef]
- Steinberg, N.; Siev-Ner, I.; Zeev, A.; Dar, G. The association between hallux valgus and proximal joint alignment in young female dancers. Int. J. Sport. Med. 2015, 36, 67–74. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Su, C.C.; Shao, S.C.; Sung, S.F.; Lin, S.J.; Kao Yang, Y.H.; Lai, E.C. Taiwan’s National Health Insurance Research Database: Past and future. Clin. Epidemiol. 2019, 11, 349–358. [Google Scholar] [CrossRef]
- Kawaguchi, Y. Genetic background of degenerative disc disease in the lumbar spine. Spine Surg. Relat. Res. 2018, 2, 98–112. [Google Scholar] [CrossRef]
- Kodithuwakku Arachchige, S.N.K.; Chander, H.; Knight, A. Flatfeet: Biomechanical implications, assessment and management. Foot 2019, 38, 81–85. [Google Scholar] [CrossRef]
- Sato, K.; Kikuchi, S.; Yonezawa, T. In vivo intradiscal pressure measurement in healthy individuals and in patients with ongoing back problems. Spine 1999, 24, 2468–2474. [Google Scholar] [CrossRef]
- Tsai, T.T.; Ho, N.Y.; Lin, Y.T.; Lai, P.L.; Fu, T.S.; Niu, C.C.; Chen, L.H.; Chen, W.J.; Pang, J.H. Advanced glycation end products in degenerative nucleus pulposus with diabetes. J. Orthop. Res. 2014, 32, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Kosashvili, Y.; Fridman, T.; Backstein, D.; Safir, O.; Bar Ziv, Y. The correlation between pes planus and anterior knee or intermittent low back pain. Foot Ankle Int. 2008, 29, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Klugarova, J.; Janura, M.; Svoboda, Z.; Sos, Z.; Stergiou, N.; Klugar, M. Hallux valgus surgery affects kinematic parameters during gait. Clin. Biomech. 2016, 40, 20–26. [Google Scholar] [CrossRef]
- González-Elena, M.L.; Castro-Méndez, A.; Coheña-Jiménez, M.; Córdoba-Fernández, A. Relationship of the Use of Short Footwear with the Development of Hallux Valgus in a Sample of Andalusian Schoolchildren. Int. J. Environ. Res. Public Health 2021, 18, 11244. [Google Scholar] [CrossRef] [PubMed]
- Arinci Incel, N.; Genc, H.; Erdem, H.R.; Yorgancioglu, Z.R. Muscle imbalance in hallux valgus: An electromyographic study. Am. J. Phys. Med. Rehabil. 2003, 82, 345–349. [Google Scholar] [CrossRef]
- Fraissler, L.; Konrads, C.; Hoberg, M.; Rudert, M.; Walcher, M. Treatment of hallux valgus deformity. EFORT Open Rev. 2016, 1, 295–302. [Google Scholar] [CrossRef]
- Koski, K.; Luukinen, H.; Laippala, P.; Kivela, S.L. Physiological factors and medications as predictors of injurious falls by elderly people: A prospective population-based study. Age Ageing 1996, 25, 29–38. [Google Scholar] [CrossRef]
- Nix, S.E.; Vicenzino, B.T.; Collins, N.J.; Smith, M.D. Gait parameters associated with hallux valgus: A systematic review. J. Foot Ankle Res. 2013, 6, 9. [Google Scholar] [CrossRef]
- Perera, A.M.; Mason, L.; Stephens, M.M. The pathogenesis of hallux valgus. J Bone Joint Surg Am 2011, 93, 1650–1661. [Google Scholar] [CrossRef]
- Cibulka, M.T. Low back pain and its relation to the hip and foot. J. Orthop. Sport. Phys. Ther. 1999, 29, 595–601. [Google Scholar] [CrossRef]
- Shih, K.S.; Chien, H.L.; Lu, T.W.; Chang, C.F.; Kuo, C.C. Gait changes in individuals with bilateral hallux valgus reduce first metatarsophalangeal loading but increase knee abductor moments. Gait Posture 2014, 40, 38–42. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, C.B.; Cahill, C.R.; Robinson, A.W.; Barnes, M.J.; Hong, J. A systematic review: The effects of podiatrical deviations on nonspecific chronic low back pain. J. Back Musculoskelet. Rehabil. 2013, 26, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.; Wagner, P. Metatarsal Pronation in Hallux Valgus Deformity: A Review. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2020, 4, e20.00091. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.S.; Koohpayehzadeh, J.; Kadkhodaei, H.; Ehsani, A.A. The effect of foot hyperpronation on spine alignment in standing position. Med. J. Islam. Repub. Iran 2016, 30, 466. [Google Scholar] [PubMed]
- Murray, K.J.; Le Grande, M.R.; Ortega de Mues, A.; Azari, M.F. Characterisation of the correlation between standing lordosis and degenerative joint disease in the lower lumbar spine in women and men: A radiographic study. BMC Musculoskelet. Disord. 2017, 18, 330. [Google Scholar] [CrossRef]
- Heineman, N.; Liu, G.; Pacicco, T.; Dessouky, R.; Wukich, D.K.; Chhabra, A. Clinical and imaging assessment and treatment of hallux valgus. Acta Radiol. 2020, 61, 56–66. [Google Scholar] [CrossRef]
- Pique-Vidal, C.; Sole, M.T.; Antich, J. Hallux valgus inheritance: Pedigree research in 350 patients with bunion deformity. J. Foot Ankle Surg. 2007, 46, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Perez Giraldo, G.S.; Ortiz Garcia, J.G. Immune-Mediated Disorders Affecting the Spinal Cord and the Spine. Curr. Neurol. Neurosci. Rep. 2021, 21, 3. [Google Scholar] [CrossRef] [PubMed]
| Before PSM Matching | After PSM Matching | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-Hallux Valgus (n = 4092) | Hallux Valgus (n = 1023) | Non-Hallux Valgus (n = 1000) | Hallux Valgus (n = 1000) | |||||||
| n | % | n | % | p-Value | n | % | n | % | p-Value | |
| Age (years) | 1 | 0.9914 | ||||||||
| <20 | 684 | 16.7 | 171 | 16.7 | 176 | 17.6 | 171 | 17.1 | ||
| 20–39 | 1660 | 40.6 | 415 | 40.6 | 408 | 40.8 | 411 | 41.1 | ||
| 40–64 | 1448 | 35.4 | 362 | 35.4 | 348 | 34.8 | 351 | 35.1 | ||
| ≥65 | 300 | 7.3 | 75 | 7.3 | 68 | 6.8 | 67 | 6.7 | ||
| Mean ± SD | 37.11 ± 17.51 | 37.11 ± 17.52 | 1 | 36.47 ± 17.52 | 36.71 ± 17.31 | 0.757 | ||||
| Sex | 1 | 0.6428 | ||||||||
| Female | 3040 | 74.3 | 760 | 74.3 | 753 | 75.3 | 744 | 74.4 | ||
| Male | 1052 | 25.7 | 263 | 25.7 | 247 | 24.7 | 256 | 25.6 | ||
| Hypertension | 316 | 7.7 | 72 | 7.0 | 0.460 | 73 | 7.3 | 68 | 6.8 | 0.6623 |
| Hyperlipidemia | 105 | 2.6 | 45 | 4.4 | 0.0019 | 42 | 4.2 | 40 | 4.0 | 0.8216 |
| Chronic liver disease | 77 | 1.9 | 22 | 2.2 | 0.577 | 24 | 2.4 | 22 | 2.2 | 0.7654 |
| Diabetes | 159 | 3.9 | 18 | 1.8 | 0.0009 | 18 | 1.8 | 18 | 1.8 | 1.0000 |
| COPD | 43 | 1.1 | 14 | 1.4 | 0.3866 | 9 | 0.9 | 10 | 1.0 | 0.8177 |
| Rheumatoid arthritis | 5 | 0.1 | 16 | 1.6 | <0.0001 | 4 | 0.4 | 5 | 0.5 | 1 ¶ |
| Heart failure | 12 | 0.3 | 6 | 0.6 | 0.157 | 3 | 0.3 | 3 | 0.3 | 1 ¶ |
| Hyperthyroidism | 9 | 0.2 | 6 | 0.6 | 0.0958 ¶ | 4 | 0.4 | 6 | 0.6 | 0.5261 |
| Cancer | 51 | 1.2 | 17 | 1.7 | 0.2994 | 16 | 1.6 | 14 | 1.4 | 0.7129 |
| Lower-limb fracture | 26 | 0.6 | 11 | 1.1 | 0.1376 | 13 | 1.3 | 11 | 1.1 | 0.6813 |
| Osteoarthritis | 59 | 1.4 | 48 | 4.7 | <0.0001 | 34 | 3.4 | 33 | 3.3 | 0.9011 |
| Non-Hallux Valgus | Hallux Valgus | |
|---|---|---|
| n | 1000 | 1000 |
| Person-years | 5982 | 5349 |
| No. of spinal degeneration | 255 | 391 |
| ID (95% CI) | 42.63 (37.70–48.19) | 73.10 (66.20–80.72) |
| Relative risk (95% CI) | Reference | 1.72 (1.46–2.01) |
| Univariate | Multivariate † | |||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Group | ||||
| Non-hallux valgus | Reference | Reference | ||
| Hallux valgus | 1.69 (1.45–1.98) | <0.0001 | 1.75 (1.50–2.05) | <0.0001 |
| Age | ||||
| <20 | Reference | Reference | ||
| 20–39 | 1.99 (1.47–2.69) | <0.0001 | 1.99 (1.47–2.69) | <0.0001 |
| 40–64 | 3.72 (2.77–4.99) | <0.0001 | 3.61 (2.68–4.86) | <0.0001 |
| ≥65 | 3.90 (2.70–5.62) | <0.0001 | 3.43 (2.28–5.14) | <0.0001 |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 0.83 (0.69–1.00) | 0.046 | 0.86 (0.71–1.04) | 0.111 |
| Hypertension | 1.89 (1.47–2.44) | <0.0001 | 1.25 (0.92–1.70) | 0.158 |
| Hyperlipidemia | 1.69 (1.21–2.37) | 0.002 | 1.07 (0.74–1.53) | 0.734 |
| Chronic liver disease | 2.23 (1.53–3.23) | <0.0001 | 1.69 (1.15–2.50) | 0.008 |
| Diabetes | 1.14 (0.67–1.94) | 0.626 | 0.63 (0.35–1.12) | 0.115 |
| COPD | 2.15 (1.19–3.91) | 0.012 | 1.14 (0.57–2.30) | 0.709 |
| Rheumatoid arthritis | 0.35 (0.05–2.45) | 0.287 | 0.26 (0.04–1.88) | 0.184 |
| Heart failure | 6.86 (2.84–16.58) | <0.0001 | 4.07 (1.44–11.47) | 0.008 |
| Hyperthyroidism | 0.50 (0.13–2.01) | 0.331 | 0.40 (0.10–1.63) | 0.203 |
| Cancer | 1.20 (0.64–2.23) | 0.575 | 0.79 (0.42–1.50) | 0.475 |
| Lower-limb fracture | 1.27 (0.68–2.37) | 0.453 | 1.25 (0.66–2.39) | 0.495 |
| Osteoarthritis | 1.79 (1.26–2.55) | 0.001 | 1.48 (1.03–2.13) | 0.033 |
| Non-Hallux Valgus | Hallux Valgus | Multivariate † | ||||
|---|---|---|---|---|---|---|
| n | No. of Spinal Degenerations | n | No. of Spinal Degenerations | HR † (95% CI) | p-Value | |
| Age | ||||||
| <20 | 176 | 19 | 171 | 33 | 1.90 (1.08–3.34) | 0.026 |
| 20–39 | 408 | 90 | 411 | 131 | 1.55 (1.19–2.03) | 0.001 |
| 40–64 | 348 | 121 | 351 | 188 | 1.84 (1.46–2.31) | <0.0001 |
| ≥65 | 68 | 25 | 67 | 39 | 1.78 (1.07–2.94) | 0.026 |
| p for interaction = | 0.7842 | |||||
| Sex | ||||||
| Female | 753 | 201 | 744 | 302 | 1.70 (1.42–2.04) | <0.0001 |
| Male | 247 | 54 | 256 | 89 | 1.79 (1.28–2.51) | <0.001 |
| p for interaction = | 0.9188 | |||||
| Lower-limb fracture | ||||||
| No | 987 | NA | 989 | 383 | 1.69 (1.44–1.98) | <0.0001 |
| Yes | 13 | NA | 11 | 8 | 7.50 (1.43–39.37) | 0.017 |
| p for interaction = | 0.0333 | |||||
| Osteoarthritis | ||||||
| No | 966 | 240 | 967 | 373 | 1.71 (1.46–2.01) | <0.0001 |
| Yes | 34 | 15 | 33 | 18 | 2.28 (1.12–4.66) | 0.024 |
| p for interaction = | 0.0937 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, T.-L.; Lee, Y.-H.; Wang, Y.-H.; Chang, R.; Wei, J.C.-C. Association of Hallux Valgus with Degenerative Spinal Diseases: A Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2023, 20, 1152. https://doi.org/10.3390/ijerph20021152
Hsu T-L, Lee Y-H, Wang Y-H, Chang R, Wei JC-C. Association of Hallux Valgus with Degenerative Spinal Diseases: A Population-Based Cohort Study. International Journal of Environmental Research and Public Health. 2023; 20(2):1152. https://doi.org/10.3390/ijerph20021152
Chicago/Turabian StyleHsu, Ta-Li, Yung-Heng Lee, Yu-Hsun Wang, Renin Chang, and James Cheng-Chung Wei. 2023. "Association of Hallux Valgus with Degenerative Spinal Diseases: A Population-Based Cohort Study" International Journal of Environmental Research and Public Health 20, no. 2: 1152. https://doi.org/10.3390/ijerph20021152
APA StyleHsu, T.-L., Lee, Y.-H., Wang, Y.-H., Chang, R., & Wei, J. C.-C. (2023). Association of Hallux Valgus with Degenerative Spinal Diseases: A Population-Based Cohort Study. International Journal of Environmental Research and Public Health, 20(2), 1152. https://doi.org/10.3390/ijerph20021152






