The Effects of Exercise for Cognitive Function in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
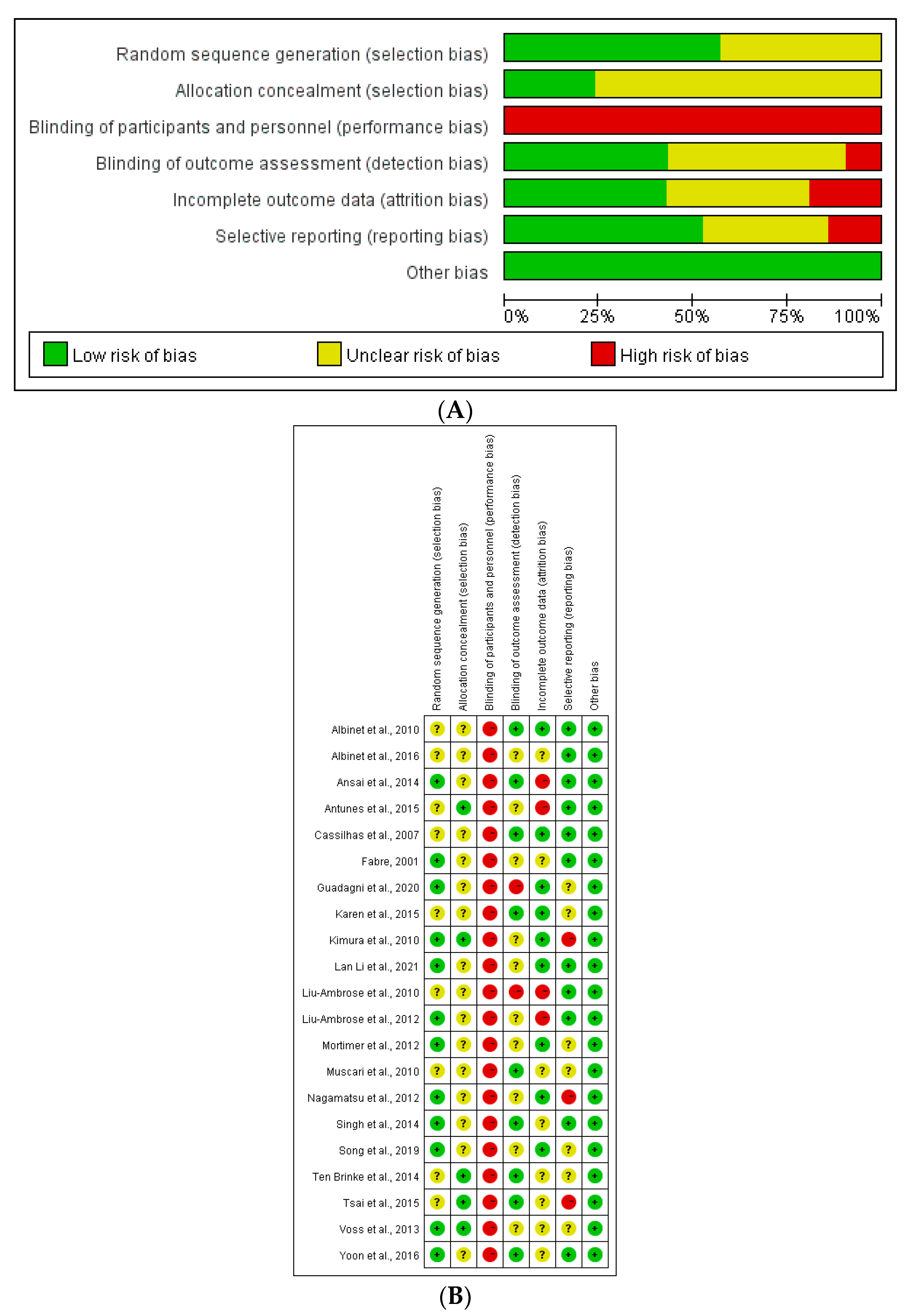
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
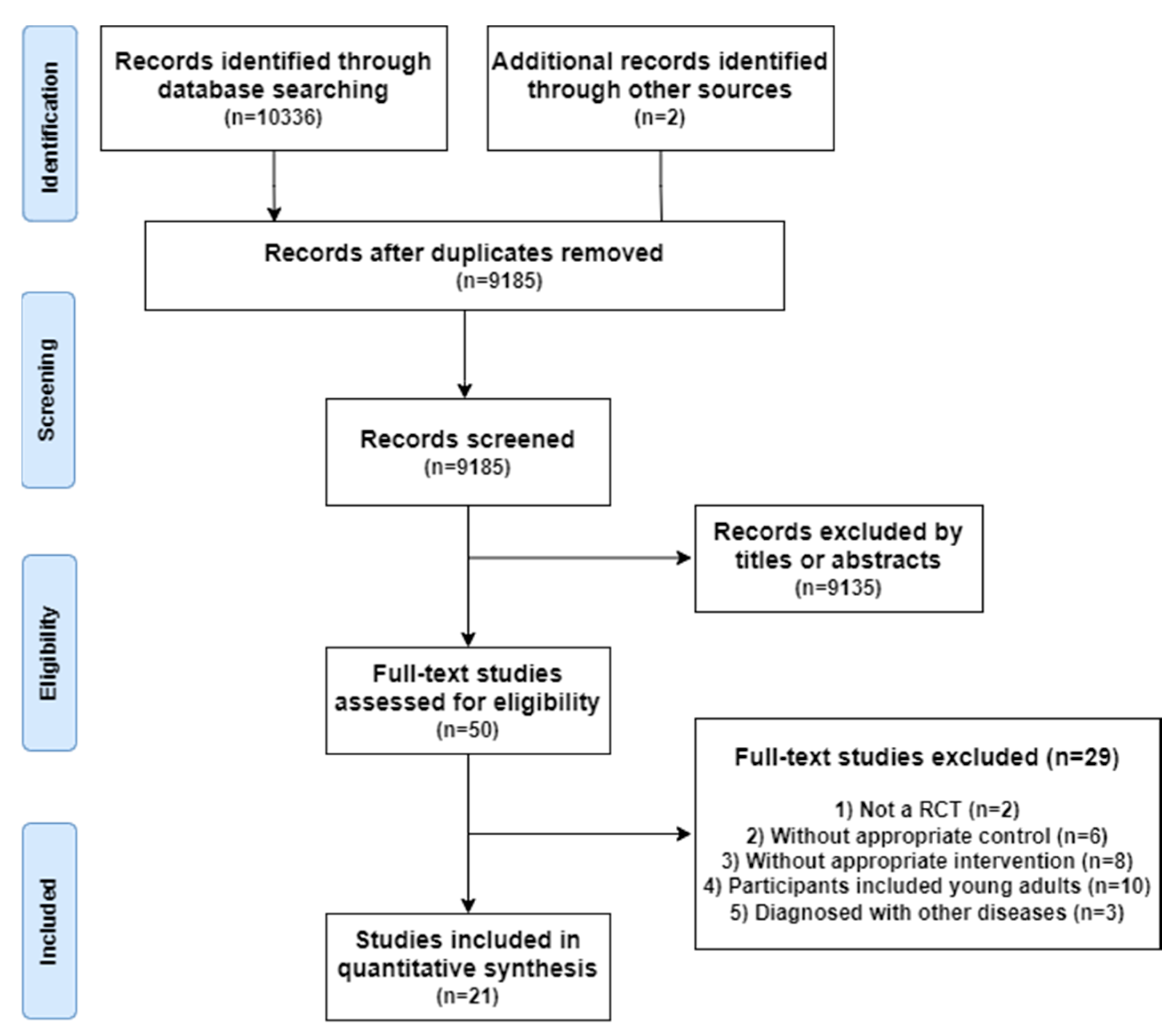
3.1. Search Results
3.2. Studies Characteristics
3.3. Quality Evaluation
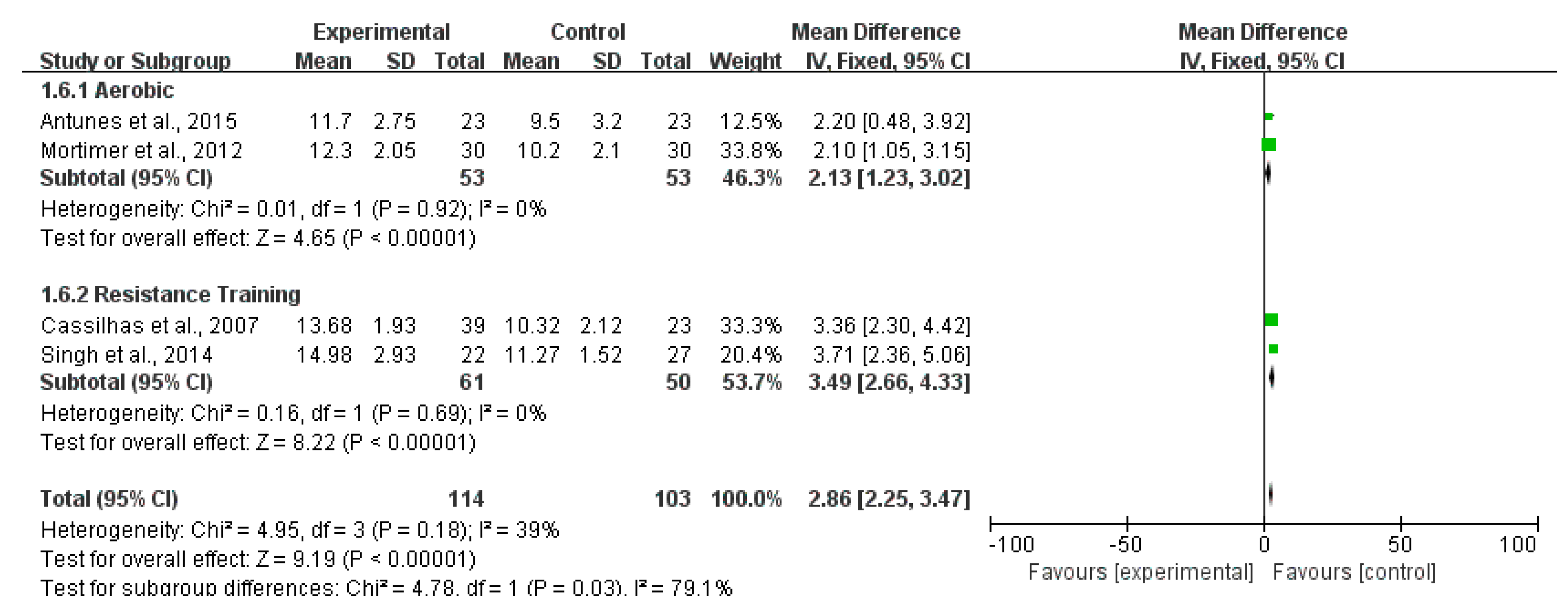
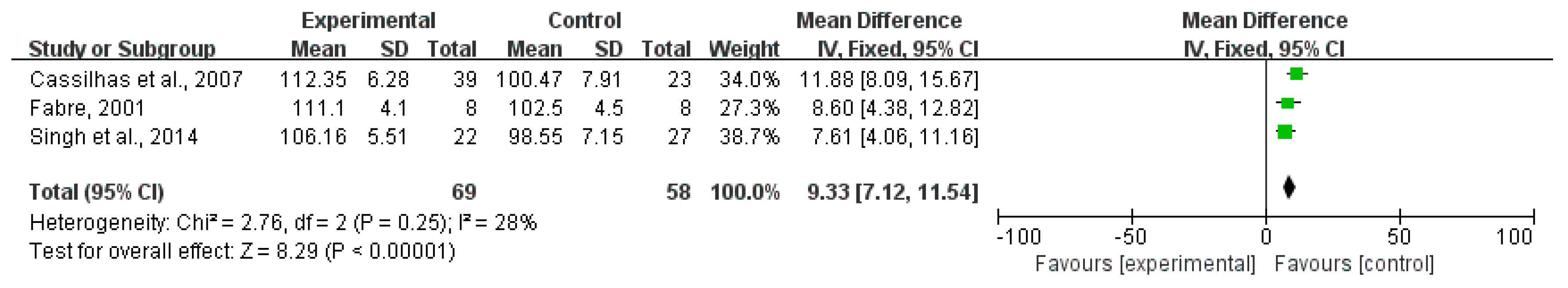
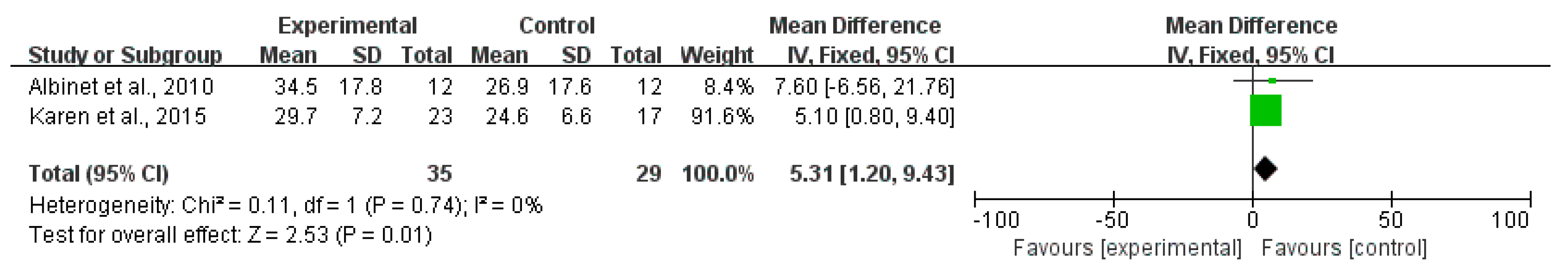
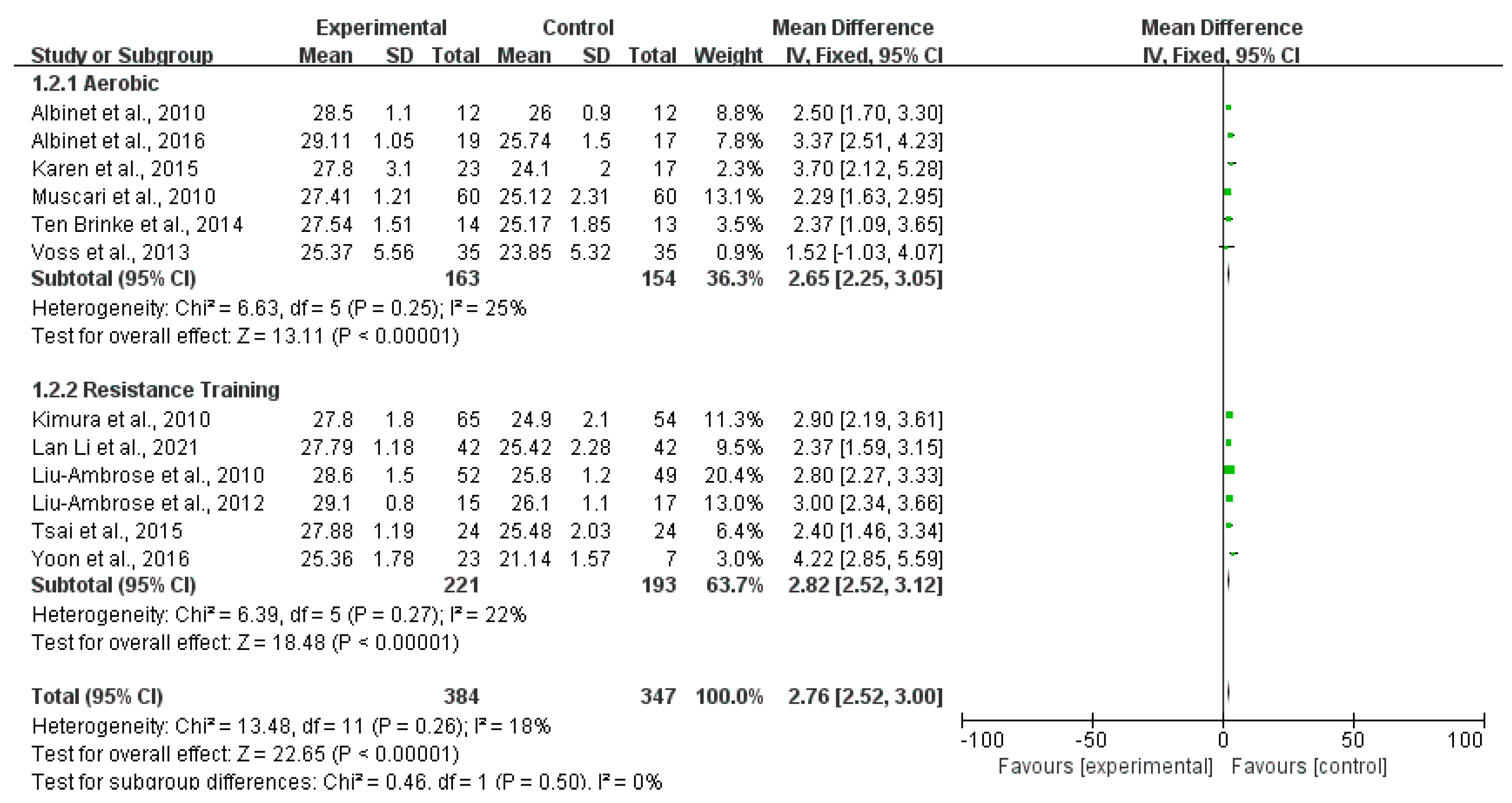
3.4. Effects of Exercise on Cognitive Functions
3.5. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colcombe, S.J.; Erickson, K.I.; Raz, N.; Webb, A.G.; Cohen, N.J.; McAuley, E.; Kramer, A.F. Aerobic fitness reduces brain tissue loss in aging humans. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2003, 58, 176–180. [Google Scholar] [CrossRef]
- Van Dam, P.S.; Aleman, A. Insulin-like growth factor-I, cognition and brain aging. Eur. J. Pharmacol. 2004, 490, 87–95. [Google Scholar] [CrossRef]
- Angevaren, M.; Aufdemkampe, G.; Verhaar, H.J.; Aleman, A.; Vanhees, L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst. Rev. 2008, 3, 538–541. [Google Scholar]
- Kronenberg, G.; Bick-Sander, A.; Bunk, E.; Wolf, C.; Ehninger, D.; Kempermann, G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol. Aging 2006, 27, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Van Uffelen, J.G.; Chin, A.P.M.J.; Hopman-Rock, M.; Van Mechelen, W. The effects of exercise on cognition in older adults with and without cognitive decline: A systematic review. Clin. J. Sport Med. Off. J. Can. Acad. Sport Med. 2008, 18, 486–500. [Google Scholar] [CrossRef] [PubMed]
- Pedroli, E.; Greci, L.; Colombo, D.; Serino, S.; Cipresso, P.; Arlati, S.; Mondellini, M.; Boilini, L.; Giussani, V.; Goulene, K.; et al. Characteristics, Usability, and Users Experience of a System Combining Cognitive and Physical Therapy in a Virtual Environment: Positive Bike. Sensors 2018, 18, 2343. [Google Scholar] [CrossRef]
- Kramer, A.F.; Hahn, S.; Cohen, N.J.; Banich, M.T.; McAuley, E.; Harrison, C.R.; Chason, J.; Vakil, E.; Bardell, L.; Boileau, R.A. Ageing, fitness and neurocognitive function. Nature 1999, 400, 418–429. [Google Scholar] [CrossRef]
- Colcombe, S.; Kramer, A.F. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef]
- Mitrushina, M.; Satz, P. Reliability and validity of the Mini-Mental State Exam in neurologically intact elderly. J. Clin. Psychol. 1991, 47, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Albinet, C.T.; Abou-Dest, A.; André, N.; Audiffren, M. Executive functions improvement following a 5-month aquaerobics program in older adults: Role of cardiac vagal control in inhibition performance. Biol. Psychol. 2016, 115, 69–77. [Google Scholar] [CrossRef]
- Albinet, C.T.; Boucard, G.; Bouquet, C.A.; Audiffren, M. Increased heart rate variability and executive performance after aerobic training in the elderly. Eur. J. Appl. Physiol. 2010, 109, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Antunes, H.K.; De Mello, M.T.; Santos-Galduróz, R.F.; Galduróz, J.C.; Lemos, V.A.; Tufik, S.; Bueno, O.F. Effects of a physical fitness program on memory and blood viscosity in sedentary elderly men. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Med. Biol. 2015, 48, 805–812. [Google Scholar] [CrossRef]
- Antunes, H.K.; Santos-Galduroz, R.F.; De Aquino Lemos, V.; Bueno, O.F.; Rzezak, P.; De Santana, M.G.; De Mello, M.T. The influence of physical exercise and leisure activity on neuropsychological functioning in older adults. Age 2015, 37, 981–995. [Google Scholar] [CrossRef] [PubMed]
- Fabre, C.; Chamari, K.; Mucci, P.; Massé-Biron, J.; Préfaut, C. Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. Int. J. Sport. Med. 2002, 23, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Guadagni, V.; Drogos, L.L.; Tyndall, A.V.; Davenport, M.H.; Anderson, T.J.; Eskes, G.A.; Longman, R.S.; Hill, M.D.; Hogan, D.B.; Poulin, M.J. Aerobic exercise improves cognition and cerebrovascular regulation in older adults. Neurology 2020, 94, 2245–2257. [Google Scholar] [CrossRef]
- Mortimer, J.A.; Ding, D.; Borenstein, A.R.; DeCarli, C.; Guo, Q.; Wu, Y.; Zhao, Q.; Chu, S. Changes in brain volume and cognition in a randomized trial of exercise and social interaction in a community-based sample of non-demented Chinese elders. J. Alzheimer’s Dis. JAD 2012, 30, 757–766. [Google Scholar] [CrossRef]
- Muscari, A.; Giannoni, C.; Pierpaoli, L.; Berzigotti, A.; Maietta, P.; Foschi, E.; Ravaioli, C.; Poggiopollini, G.; Bianchi, G.; Magalotti, D. Chronic endurance exercise training prevents aging-related cognitive decline in healthy older adults: A randomized controlled trial. Int. J. Geriatr. Psychiatry 2010, 25, 1055–1064. [Google Scholar] [CrossRef]
- Nagamatsu, L.S.; Handy, T.C.; Hsu, C.L.; Voss, M.; Liu-Ambrose, T. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch. Intern. Med. 2012, 172, 666–678. [Google Scholar] [CrossRef]
- Song, D.; Yu, D.S.F. Effects of a moderate-intensity aerobic exercise programme on the cognitive function and quality of life of community-dwelling elderly people with mild cognitive impairment: A randomised controlled trial. Int. J. Nurs. Stud. 2019, 93, 97–105. [Google Scholar] [CrossRef]
- Ten Brinke, L.F.; Bolandzadeh, N.; Nagamatsu, L.S.; Hsu, C.L.; Davis, J.C.; Miran-Khan, K.; Liu-Ambrose, T. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: A 6-month randomised controlled trial. Br. J. Sport. Med. 2015, 49, 248–254. [Google Scholar] [CrossRef]
- Voss, M.W.; Heo, S.; Prakash, R.S.; Erickson, K.I.; Alves, H.; Chaddock, L.; Szabo, A.N.; Mailey, E.L.; Wójcicki, T.R.; White, S.M. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: Results of a one-year exercise intervention. Hum. Brain Mapp. 2013, 34, 2972–2985. [Google Scholar] [CrossRef]
- Ansai, J.H.; Rebelatto, J.R. Effect of two physical exercise protocols on cognition and depressive symptoms in oldest-old people: A randomized controlled trial. Geriatr. Gerontol. Int. 2015, 15, 1127–1134. [Google Scholar] [CrossRef]
- Cassilhas, R.C.; Viana, V.A.; Grassmann, V.; Santos, R.T.; Santos, R.F.; Tufik, S.; Mello, M.T. The impact of resistance exercise on the cognitive function of the elderly. Med. Sci. Sport. Exerc. 2007, 39, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Fiatarone Singh, M.A.; Gates, N.; Saigal, N.; Wilson, G.C.; Meiklejohn, J.; Brodaty, H.; Wen, W.; Singh, N.; Baune, B.T.; Suo, C. The Study of Mental and Resistance Training (SMART) study—Resistance training and/or cognitive training in mild cognitive impairment: A randomized, double-blind, double-sham controlled trial. J. Am. Med. Dir. Assoc. 2014, 15, 873–880. [Google Scholar] [CrossRef]
- Kimura, K.; Obuchi, S.; Arai, T.; Nagasawa, H.; Shiba, Y.; Watanabe, S.; Kojima, M. The influence of short-term strength training on health-related quality of life and executive cognitive function. J. Physiol. Anthropol. 2010, 29, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, M.; Zeng, H.; Pan, L. Multi-component exercise training improves the physical and cognitive function of the elderly with mild cognitive impairment: A six-month randomized controlled trial. Ann. Palliat. Med. 2021, 10, 8919–8929. [Google Scholar] [CrossRef] [PubMed]
- Liu-Ambrose, T.; Nagamatsu, L.S.; Graf, P.; Beattie, B.L.; Ashe, M.C.; Handy, T.C. Resistance training and executive functions: A 12-month randomized controlled trial. Arch. Intern. Med. 2010, 170, 170–178. [Google Scholar] [CrossRef]
- Liu-Ambrose, T.; Nagamatsu, L.S.; Voss, M.W.; Khan, K.M.; Handy, T.C. Resistance training and functional plasticity of the aging brain: A 12-month randomized controlled trial. Neurobiol. Aging 2012, 33, 1690–1698. [Google Scholar] [CrossRef]
- Tsai, C.L.; Wang, C.H.; Pan, C.Y.; Chen, F.C. The effects of long-term resistance exercise on the relationship between neurocognitive performance and GH, IGF-1, and homocysteine levels in the elderly. Front. Behav. Neurosci. 2015, 9, 23–33. [Google Scholar] [CrossRef]
- Yoon, D.H.; Kang, D.; Kim, H.J.; Kim, J.S.; Song, H.S.; Song, W. Effect of elastic band-based high-speed power training on cognitive function, physical performance and muscle strength in older women with mild cognitive impairment. Geriatr. Gerontol. Int. 2017, 17, 765–772. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71–80. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, 5928–5936. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sport. Med. 2018, 52, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Gates, N.; Fiatarone Singh, M.A.; Sachdev, P.S.; Valenzuela, M. The effect of exercise training on cognitive function in older adults with mild cognitive impairment: A meta-analysis of randomized controlled trials. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2013, 21, 1086–1097. [Google Scholar] [CrossRef]
- Liu, L.; Jia, L.; Jian, P.; Zhou, Y.; Zhou, J.; Wu, F.; Tang, Y. The Effects of Benzodiazepine Use and Abuse on Cognition in the Elders: A Systematic Review and Meta-Analysis of Comparative Studies. Front. Psychiatry 2020, 11, 755–768. [Google Scholar] [CrossRef]

| # | Searches | Results |
|---|---|---|
| 1 | ((((“exercise*”[Title/Abstract]) OR (“sport*”[Title/Abstract])) OR (“physical exercise”[Title/Abstract])) OR (“exercise intervention”[Title/Abstract])) OR (“intervention*”[Title/Abstract]) | 1,586,474 |
| 2 | (((“older adults*”[Title/Abstract]) OR (“aged*”[Title/Abstract])) OR (“old people”[Title/Abstract])) OR (“elderly people”[Title/Abstract]) | 930,566 |
| 3 | (((“cognitive*”[Title/Abstract]) OR (“cognitive ability “[Title/Abstract])) OR (“cognitive function”[Title/Abstract])) OR (“cognitive decline”[Title/Abstract]) | 443,939 |
| 4 | 1 and 2 | 127,165 |
| 5 | 3 and 4 | 10,645 |
| 6 | Limit 5 to (English language and humans and “all aged (60 and over)”) | 2045 |
| Included Studies | Mean Age (Years) | Participants (M/F) | Sample Size (N) | Intervention | Intervention Duration (Weeks) | Session Duration | Session Frequency | Outcome Measure |
|---|---|---|---|---|---|---|---|---|
| Guadagni et al., 2020 [15] | 65.9 | 206 (101/105) | IG = 103; CG = 103 | Aerobic | 48 | 60 min | 3 times/week | MoCA |
| Song et al., 2019 [19] | 75.78 | 120 (30/90) | IG = 60; CG = 60 | Aerobic | 16 | 60 min | 3 times/week | MoCA |
| Nagamatsu et al., 2012 [18] | 75.36 | 58 (0/58) | IG = 30; CG = 28 | Aerobic | 26 | 60 min | 2 times/week | TMT |
| Ten Brinke et al., 2015 [20] | 75.78 | 27 (0/27) | IG = 14; CG = 13 | Aerobic | 26 | 60 min | 2 times/week | MMSE; MoCA |
| Voss et al., 2013 [21] | 64.87 | 70 (25/45) | IG = 35; CG = 35 | Aerobic | 52 | 40 min | 3 times/week | MMSE |
| Albinet et al., 2010 [11] | 70.65 | 24 (11/13) | IG = 12; CG = 12 | Aerobic | 12 | 60 min | 3 times/week | MMSE; WCST |
| Fabre, 2002 [14] | 65.55 | 16 (3/13) | IG = 8; CG = 8 | Aerobic | 8 | 60 min | 2 times/week | WMS |
| Mortimer et al., 2012 [16] | 68 | 60 (20/40) | IG = 30; CG = 30 | Aerobic | 40 | 50min | 3 times/week | TMT; WAIS; SCWT |
| Muscari et al., 2010 [17] | 69.2 | 120 (62/58) | IG = 60; CG = 60 | Aerobic | 52 | 60 min | 3 times/week | MMSE |
| Albinet et al., 2016 [10] | 66.53 | 36 (10/26) | IG = 19; CG = 17 | Aerobic | 21 | 60 min | 2 times/week | MMSE; SCWT |
| Antunes et al., 2015 [12] | 66.97 | 46 (46/0) | IG = 23; CG = 23 | Aerobic | 26 | 60 min | 3 times/week | WAIS |
| Karen et al., 2015 [13] | 64.58 | 40 (0/40) | IG = 23; CG = 17 | Aerobic | 26 | 60 min | 3 times/week | MMSE; WCST |
| Lan Li et al., 2021 [26] | 70.48 | 84 (33/51) | IG = 42; CG = 42 | Resistance | 24 | 30min | 5 times/week | MMSE; MoCA |
| Tsai et al., 2015 [29] | 71.4 | 48 (48/0) | IG = 24; CG = 24 | Resistance | 52 | 60 min | 3 times/week | MMSE |
| Yoon et al., 2017 [30] | 76 | 30 (not stated) | IG = 23; CG = 7 | Resistance | 12 | 60 min | 2 times/week | MMSE; MoCA |
| Liu-Ambrose et al., 2010 [27] | 69.62 | 101 (0/101) | IG = 52; CG = 49 | Resistance | 52 | 60 min | 2 times/week | MMSE; TMT; SCWT |
| Liu-Ambrose et al., 2012 [28] | 69.31 | 52 (0/52) | IG = 15; CG = 17 | Resistance | 52 | 60 min | 2 times/week | MMSE |
| Cassilhas et al., 2007 [23] | 68.08 | 62 (62/0) | IG = 39; CG = 23 | Resistance | 24 | 60 min | 3 times/week | WMS; WAIS |
| Kimura et al., 2010 [25] | 74.33 | 119 (49/70) | IG = 65; CG = 54 | Resistance | 12 | 90 min | 2 times/week | MMSE |
| Ansai et al., 2015 [22] | 82.7 | 46 (16/30) | IG = 23; CG = 23 | Resistance | 16 | 60 min | 3 times/week | MoCA |
| Singh et al., 2014 [24] | 70.1 | 49 (33/16) | IG = 22; CG = 27 | Resistance | 26 | 75min | 2–3 times/week | WMS; WAIS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Gu, H.; Cai, X.; Zhang, Y.; Hou, X.; Yu, J.; Sun, T. The Effects of Exercise for Cognitive Function in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Environ. Res. Public Health 2023, 20, 1088. https://doi.org/10.3390/ijerph20021088
Xu L, Gu H, Cai X, Zhang Y, Hou X, Yu J, Sun T. The Effects of Exercise for Cognitive Function in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. International Journal of Environmental Research and Public Health. 2023; 20(2):1088. https://doi.org/10.3390/ijerph20021088
Chicago/Turabian StyleXu, Liya, Hongyi Gu, Xiaowan Cai, Yimin Zhang, Xiao Hou, Jingjing Yu, and Tingting Sun. 2023. "The Effects of Exercise for Cognitive Function in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" International Journal of Environmental Research and Public Health 20, no. 2: 1088. https://doi.org/10.3390/ijerph20021088
APA StyleXu, L., Gu, H., Cai, X., Zhang, Y., Hou, X., Yu, J., & Sun, T. (2023). The Effects of Exercise for Cognitive Function in Older Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. International Journal of Environmental Research and Public Health, 20(2), 1088. https://doi.org/10.3390/ijerph20021088