Determination of Selected Harmful Substances in Baby Diapers Available on the South African Market
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. pH Determination
2.3. Extractable Heavy Metals Determination
2.3.1. Extraction Using Artificial Urine
2.3.2. Extraction Using Artificial Sweat Solution
2.4. Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) Analysis
3. Results and Discussion
3.1. FTIR Analysis
3.2. Harmful Substances
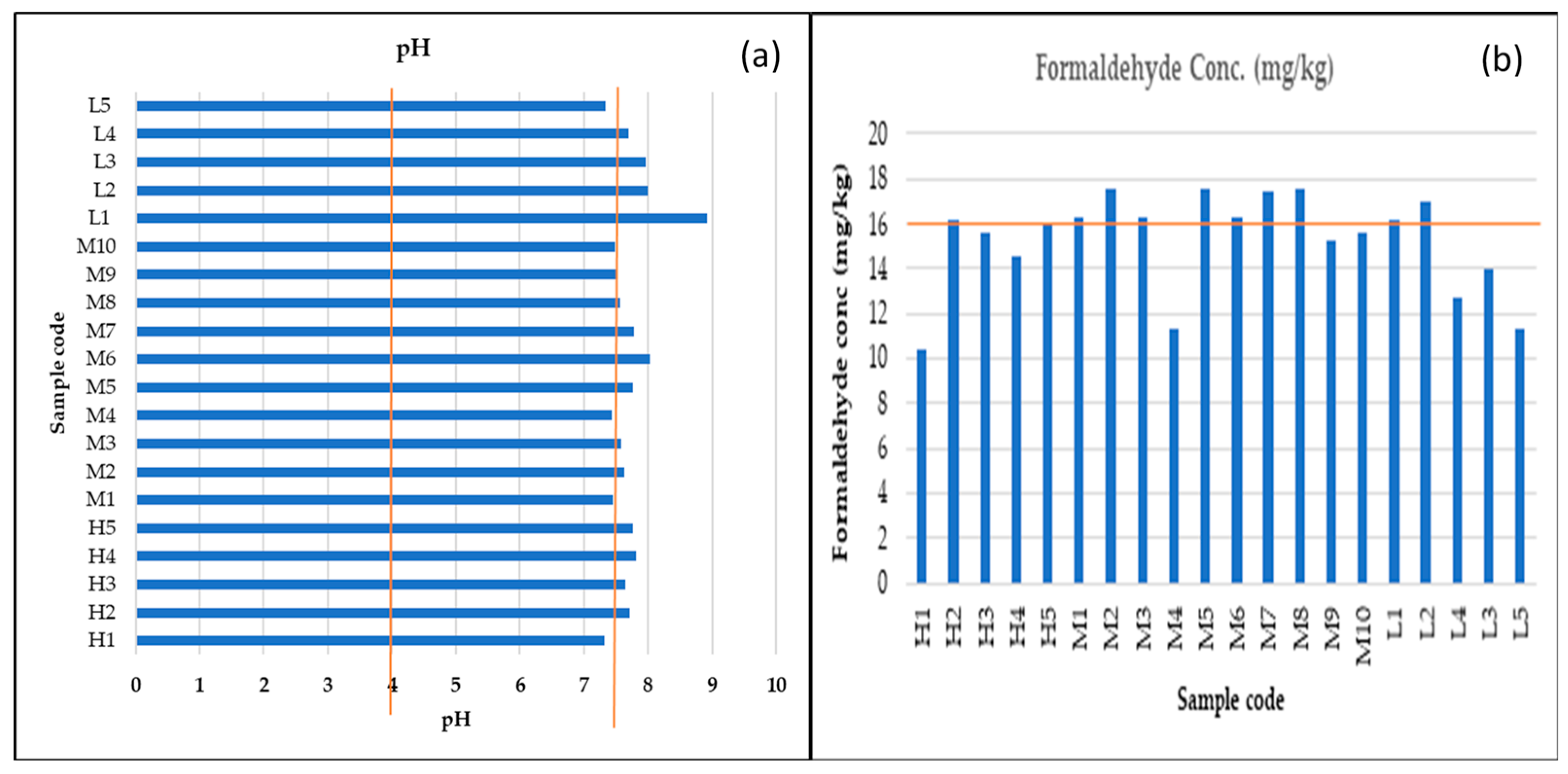
3.2.1. Formaldehyde
3.2.2. pH
3.2.3. pH and Formaldehyde Statistical Analysis
3.2.4. Trace Metals
4. Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bachra, Y.; Grouli, A.; Damiri, F.; Bennamara, A.; Berrada, M. A new approach for assessing the absorption of disposable baby diapers and superabsorbent polymers: A comparative study. Results Mater. 2020, 8, 100156. [Google Scholar] [CrossRef]
- Lam, N. How Do Disposable Diapers Absorb. 2012. Available online: https://www.cwimedical.com/disposable-diapers. (accessed on 20 September 2022).
- Kimani, E.W.; Muchiri, J.; Makindi, S. Soiled diapers disposal practices among caregivers in poor and Middle-income urban settings. Int. J. Sci. Res. Pub. 2014, 5, 1–10. [Google Scholar]
- ANSES. Safety of Baby Diapers; Collective Expert Appraisal Report; French Agency for Food, Environmental and Occupational Health & Safety on the Safety of Baby Diapers: Paris, French, 2017; ANSES Opinion Request No. 2017-SA-0019. [Google Scholar]
- Shin, J.H.; Lee, K.K.; Chung, M.H. Determination of Heavy Metals in Sanitary Products of Women. J. Korean Soc. Cloth. Text. 2009, 33, 853–859. [Google Scholar] [CrossRef]
- Alberta, L.; Sweeney, S.M.; Wiss, K. Diaper dye dermatitis. Pediatrics 2005, 116, 450–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klunk, C.; Domingues, E.; Wiss, K. An update on diaper dermatitis. Clin. Dermatol. 2014, 32, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.K.; Faergemann, J.; Sköld, M. Skin Health Connected to the Use of Absorbent Hygiene Products: A Review. Dermatol. Ther. 2017, 7, 319–330. [Google Scholar] [CrossRef] [Green Version]
- Atherton, D.J. Understanding irritant napkin dermatitis. Int. J. Dermatol. 2016, 55, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Šikić Pogačar, M.; Maver, U.; Marčun Varda, N.; Mičetić-Turk, D. Diagnosis and management of diaper dermatitis in infants with emphasis on skin microbiota in the diaper area. Int. J. Dermatol. 2018, 57, 265–275. [Google Scholar] [CrossRef]
- Yu, J.; Treat, J.; Brod, B. Patch Test Series for Allergic Perineal Dermatitis in the Diapered Infant. Dermatitis 2017, 28, 70–75. [Google Scholar] [CrossRef]
- Giusti, F.; Massone, F.; Bertoni, L.; Pellacani, G.; Seidenari, S. Contact sensitization to disperse dyes in children. Pediatr. Dermatol. 2003, 20, 393–397. [Google Scholar] [CrossRef]
- Wirantari, N.; Astari, L.; Zulkarnain, I. pH value of infant’s skin is higher on diaper area compared to nondiaper area. Dermatol. Rep. 2019, 11, 8056. [Google Scholar] [CrossRef]
- Nyamukamba, P.; Bantom, C.; Mququ, Z.; Ngcobo, T.; Isaacs, S. Determination of the Levels of Heavy Metals and Formaldehyde in Baby Clothes in South Africa: A Case Study of Stores in the Greater Cape Town Region. J. Spectrosc. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Shmaefsky, B.R. Artificial urine for laboratory testing. Am. Biol. Teach. 1990, 52, 170–172. [Google Scholar] [CrossRef]
- Behrouzi, M.; Moghadam, P.N. Synthesis of a new superabsorbent copolymer based on acrylic acid grafted onto carboxymethyl tragacanth. Carbohydr. Polymer 2018, 202, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Damiri, F.; Bachra, Y.; Bounacir, C.; Laaraibi, A.; Berrada, M. Synthesis and characterization of lyophilized chitosan-based hydrogels cross-linked with benzaldehyde for controlled drug release. J. Chem. 2020, 2020, 10. [Google Scholar] [CrossRef]
- Kim, Y.J.; Hong, S.J.; Shin, W.S.; Kwon, Y.R.; Lim, S.H.; Kim, J.S.; Choi, J.; Kim, J.W.; Kim, D.H. Preparation and performance of superabsorbent polymer with cellulose additives. Fibers Polym. 2020, 21, 2448–2455. [Google Scholar] [CrossRef]
- Zohourian, M.M.; Kabiri, K. Superabsorbent Polymer Materials: A Review. Iran. Polym. J. 2008, 17, 451–477. [Google Scholar]
- Makoś-Chełstowska, P.; Kurowska-Susdorf, A.; Płotka-Wasylka, J. Environmental problems and health risks with disposable baby diapers: Monitoring of toxic compounds by application of analytical techniques and need of education. TrAC Trends Analyt. Chem. 2021, 143, 116408. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Chemical Agents and Related Occupations—A Review of Humans Carcinogens, IARC Monographs on the Evaluation of Carcinogenic Risk to Humans; World Human Organization (WHO): Lyon, France, 2012. [Google Scholar]
- Inci, M.; Zararsız, I.; Davarci, M.; Gorur, S. Toxic effects of formaldehyde on the urinary system. Turk. J. Urol. 2013, 39, 48–52. [Google Scholar] [CrossRef]
- Rai, P.; Lee, B.M.; Liu, T.Y.; Yuhui, Q.; Krause, E.; Marsman, D.S.; Felter, S. Safety Evaluation of Disposable Baby Diapers Using Principles of Quantitative Risk Assessment. J. Toxicol. Environ. Health Part A 2009, 72, 1262–1271. [Google Scholar] [CrossRef]
- Xue, J.; Liu, W.; Kannan, K. Bisphenols, Benzophenones, and Bisphenol A Diglycidyl Ethers in Textiles and Infant Clothing. Environ. Sci. Technol. 2017, 51, 5279–5286. [Google Scholar] [CrossRef] [PubMed]
- Matousek, J.L.; Campbell, K.L. A comparative reveiw of cutaneous pH. Vet. Dermatol. 2002, 13, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17, 679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negev, M.; Berman, T.; Reicher, S.; Sadeh, M.; Ardi, R.; Shammai, Y. Concentrations of trace metals, phthalates, bisphenol A and flame-retardants in toys and other children’s products in Israel. Chemosphere 2018, 192, 217–224. [Google Scholar] [CrossRef] [PubMed]
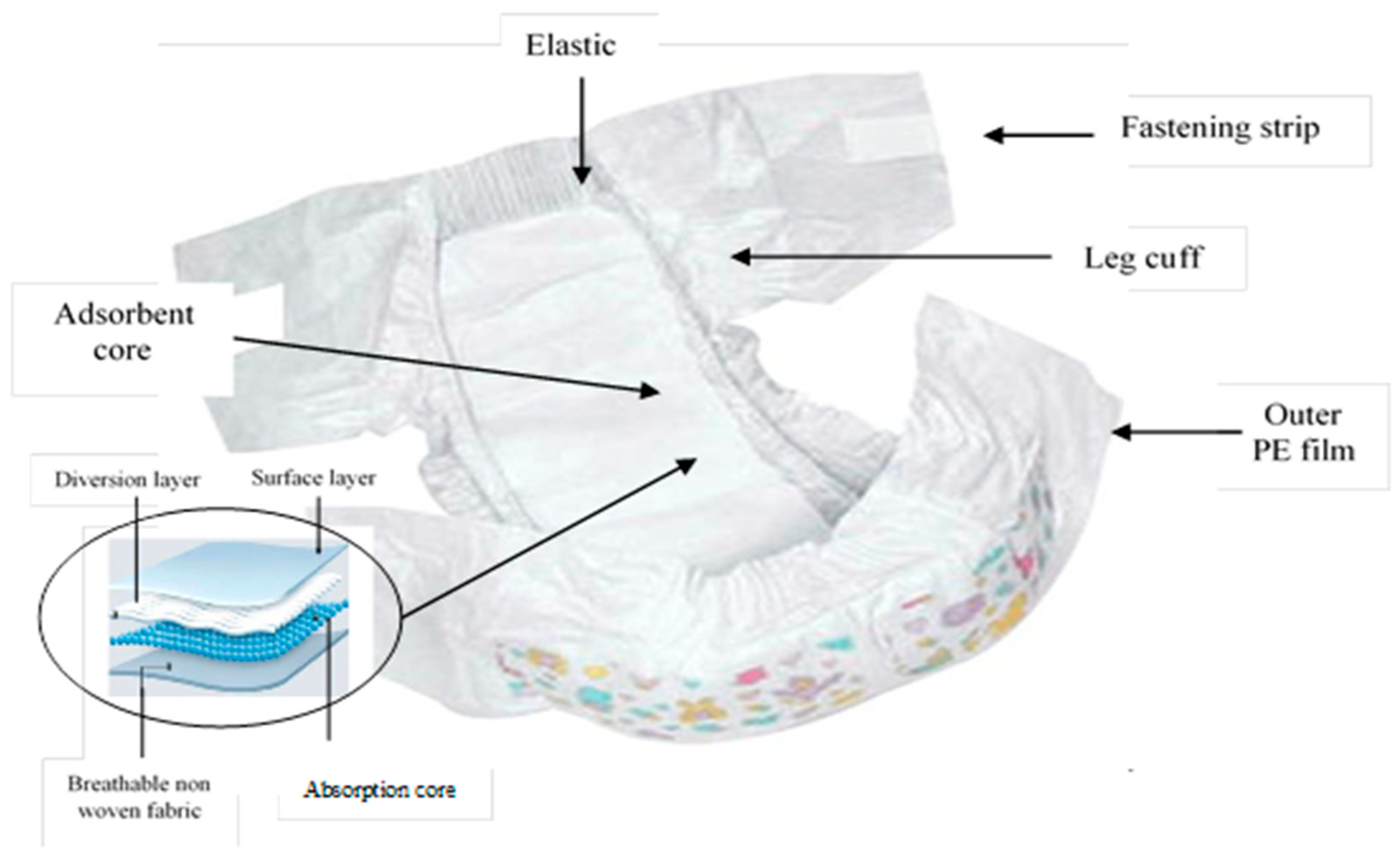
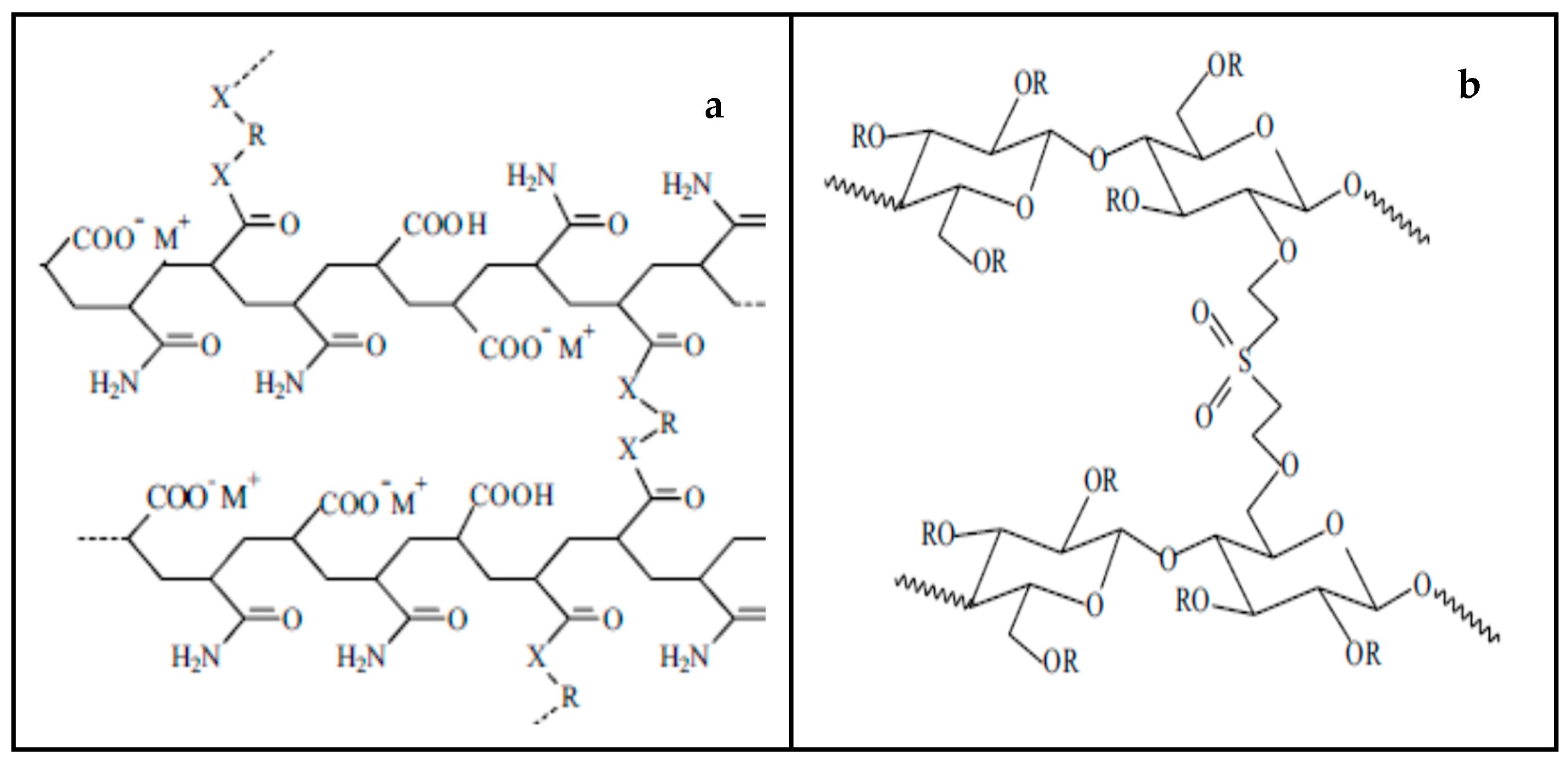
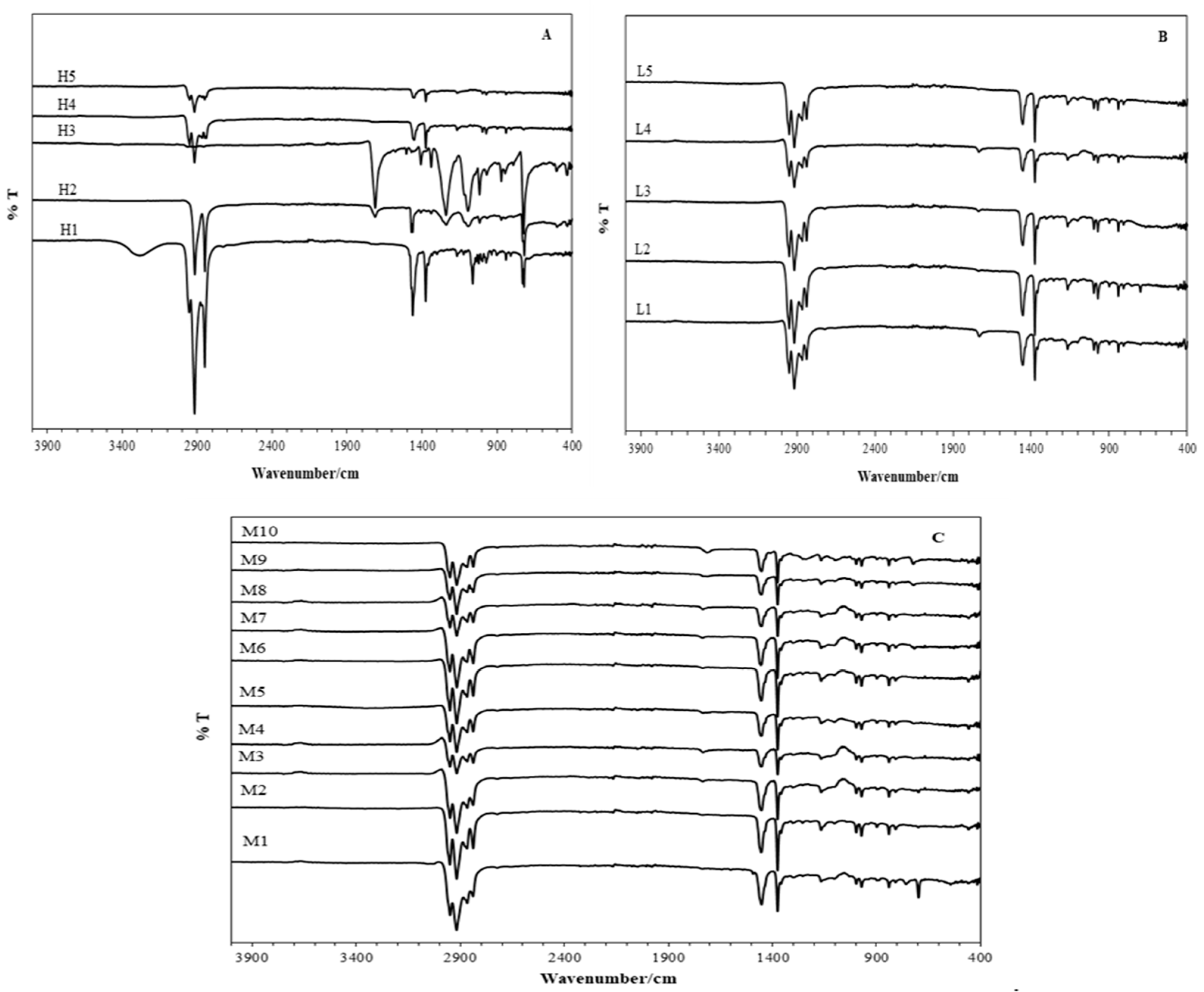
| Sample Code | Sample Description | Colur of the Section in Direct Contact with Skin | Made in |
|---|---|---|---|
| H1 | Ingredients include petroleum, stearyl alcohol, paraffinum, liquidium, aloe barbadensis leaf extract. | Slightly blue | Poland |
| H2 | Has wetness indicator, dual leak guard, soft cotton feel. | white | China |
| H3 | SA material, fluff pulp, non-woven, leakage protection. | white | South Africa |
| H4 | Ingredients include petroleum, stearyl alcohol, paraffinum, liquidium, aloe barbadensis leaf extract. | White and blue | Poland |
| H5 | Has active channels to absorb wetness and distribute it evenly, SA core. | Slightly blue | South Africa |
| M1 | I.N.A. | White | South Africa |
| M2 | SA core, leak protection and breathable back sheet. | Light green | South Africa |
| M3 | Has wetness indicator, multicore with supergel, double leakage barrier. | Purple | South Africa |
| M4 | SA core, anti-leak guard, breathable cover. | Light green | South Africa |
| M5 | Diamond embossed core with SA, dry guard layer, and anti-leak plastics. | White | South Africa |
| M6 | SA gel, multicore with supergel, extra soft, wetness indicator. | Purple | South Africa |
| M7 | SA gel, multicore with supergel, wetness indicator. | Purple | South Africa |
| M8 | I.N.A. | Light green | South Africa |
| M9 | SA lockgel core, stretchy waistband. | white | South Africa |
| M10 | Two-dimensional absorbent core with side leak guard. | Light green | South Africa |
| L1 | I.N.A. | Malaysia | |
| L2 | SA gel, dual air leakage. | Blue | South Africa |
| L3 | I.N.A. | white | I.N.A |
| L4 | I.N.A. | white | South Africa |
| L5 | I.N.A. | white | I.N.A |
| Sample Code | Formaldehyde Conc. (mg/kg) | Recommended (mg/kg) |
|---|---|---|
| H1 | 10.438 | <16 mg/kg |
| H2 | 16.205 | <16 mg/kg |
| H3 | 15.616 | <16 mg/kg |
| H4 | 14.557 | <16 mg/kg |
| H5 | 15.969 | <16 mg/kg |
| M1 | 16.322 | <16 mg/kg |
| M2 | 17.617 | <16 mg/kg |
| M3 | 16.322 | <16 mg/kg |
| M4 | 11.380 | <16 mg/kg |
| M5 | 17.617 | <16 mg/kg |
| M6 | 16.322 | <16 mg/kg |
| M7 | 17.499 | <16 mg/kg |
| M8 | 17.617 | <16 mg/kg |
| M9 | 15.263 | <16 mg/kg |
| M10 | 15.616 | <16 mg/kg |
| L1 | 16.205 | <16 mg/kg |
| L2 | 17.029 | <16 mg/kg |
| L3 | 12.674 | <16 mg/kg |
| L4 | 13.969 | <16 mg/kg |
| L5 | 11.380 | <16 mg/kg |
| Sample Code | pH | Recommended |
|---|---|---|
| H1 | 7.32 | 4.0–7.5 |
| H2 | 7.71 | 4.0–7.5 |
| H3 | 7.65 | 4.0–7.5 |
| H4 | 7.81 | 4.0–7.5 |
| H5 | 7.77 | 4.0–7.5 |
| M1 | 7.46 | 4.0–7.5 |
| M2 | 7.63 | 4.0–7.5 |
| M3 | 7.58 | 4.0–7.5 |
| M4 | 7.44 | 4.0–7.5 |
| M5 | 7.76 | 4.0–7.5 |
| M6 | 8.03 | 4.0–7.5 |
| M7 | 7.78 | 4.0–7.5 |
| M8 | 7.57 | 4.0–7.5 |
| M9 | 7.52 | 4.0–7.5 |
| M10 | 7.49 | 4.0–7.5 |
| L1 | 8.93 | 4.0–7.5 |
| L2 | 8.00 | 4.0–7.5 |
| L3 | 7.96 | 4.0–7.5 |
| L4 | 7.70 | 4.0–7.5 |
| L5 | 7.34 | 4.0–7.5 |
| Heavy Metal | Pb | Cu | As | Zn | Co | Ni | Cr | Cd | Mn | Se |
|---|---|---|---|---|---|---|---|---|---|---|
| H1 | 0.031 | 0.009 | 0.001 | 0.040 | 0.013 | 0.059 | 0.040 | 0.006 | 0.024 | 0.005 |
| H2 | 0.016 | 0.011 | 0.013 | 0.644 | 0.002 | 0.197 | 0.042 | 0.001 | 0.014 | 0.005 |
| H3 | 0.013 | 0.013 | 0.002 | 0.079 | 0.013 | 0.053 | 0.038 | 0.006 | 0.022 | 0.006 |
| H4 | 0.045 | 0.005 | 0.002 | 0.252 | 0.013 | 0.056 | 0.119 | 0.006 | 0.018 | 0.005 |
| H5 | 0.018 | 0.011 | 0.002 | 0.292 | 0.013 | 0.049 | 0.082 | 0.006 | 0.030 | 0.038 |
| M1 | 0.004 | 0.019 | 0.003 | 0.025 | 0.013 | 0.022 | 0.050 | 0.006 | 0.034 | 0.004 |
| M2 | 0.094 | 0.001 | n.d. | 0.131 | 0.013 | 0.056 | 0.039 | 0.006 | 0.026 | 0.005 |
| M3 | 0.027 | 0.008 | 0.001 | 0.115 | 0.013 | 0.055 | 0.040 | 0.006 | 0.025 | 0.006 |
| M4 | 0.056 | 0.010 | n.d. | 0.072 | 0.013 | 0.053 | 0.023 | 0.006 | 0.026 | 0.002 |
| M5 | 0.022 | 0.012 | 0.001 | 0.093 | 0.013 | 0.052 | 0.041 | 0.006 | 0.027 | 0.005 |
| M6 | 0.041 | 0.010 | 0.001 | 0.154 | 0.013 | 0.057 | 0.032 | 0.006 | 0.023 | 0.005 |
| M7 | 0.034 | 0.014 | 0.001 | 0.208 | 0.013 | 0.055 | 0.029 | 0.006 | 0.024 | 0.006 |
| M8 | 0.035 | 0.005 | 0.001 | 0.223 | 0.013 | 0.053 | 0.020 | 0.006 | 0.022 | 0.006 |
| M9 | 0.023 | 0.014 | 0.001 | 0.167 | 0.013 | 0.050 | 0.028 | 0.006 | 0.024 | 0.005 |
| M10 | 0.042 | 0.010 | 0.001 | 0.138 | 0.013 | 0.022 | 0.040 | 0.006 | 0.026 | 0.002 |
| L1 | 0.042 | 0.008 | 0.002 | 0.040 | 0.013 | 0.069 | 0.042 | 0.006 | 0.022 | 0.006 |
| L2 | 0.011 | 0.011 | 0.013 | 0.014 | 0.002 | 0.003 | 0.013 | 0.001 | 0.002 | 0.055 |
| L3 | 0.002 | 0.011 | 0.002 | 0.087 | 0.013 | 0.060 | 0.024 | 0.006 | 0.027 | 0.006 |
| L4 | 0.042 | 0.013 | 0.002 | 0.107 | 0.013 | 0.058 | 0.042 | 0.006 | 0.025 | 0.005 |
| L5 | 0.081 | 0.007 | 0.004 | 0.091 | 0.013 | 0.050 | 0.041 | 0.006 | 0.026 | 0.005 |
| Min | 0.002 | 0.001 | 0.001 | 0.014 | 0.002 | 0.022 | 0.013 | 0.001 | 0.002 | 0.002 |
| Max | 0.094 | 0.019 | 0.013 | 0.644 | 0.013 | 0.197 | 0.119 | 0.006 | 0.034 | 0.038 |
| Heavy Metal | Pb | Cu | As | Zn | Co | Ni | Cr | Cd | Mn | Se |
|---|---|---|---|---|---|---|---|---|---|---|
| H1 | 0.011 | 0.010 | 0.007 | 0.040 | n.d. | 0.009 | 0.036 | 0.001 | 0.005 | 0.023 |
| H2 | 0.002 | 0.015 | n.d. | 0.006 | 0.013 | 0.022 | 0.008 | 0.006 | 0.030 | 0.005 |
| H3 | 0.011 | 0.018 | n.d. | 0.021 | 0.013 | 0.019 | 0.045 | 0.006 | 0.031 | 0.006 |
| H4 | 0.006 | 0.011 | 0.005 | 0.071 | n.d. | 0.016 | 0.078 | 0.001 | 0.009 | 0.032 |
| H5 | 0.006 | 0.011 | 0.013 | 0.032 | n.d. | 0.001 | 0.077 | 0.001 | 0.002 | 0.035 |
| M1 | 0.008 | 0.014 | n.d. | 0.022 | 0.013 | 0.019 | 0.050 | 0.006 | 0.030 | 0.005 |
| M2 | 0.011 | 0.019 | 0.004 | 0.029 | 0.013 | 0.024 | 0.049 | 0.006 | 0.034 | 0.005 |
| M3 | 0.007 | 0.013 | 0.001 | 0.005 | 0.013 | 0.004 | 0.045 | 0.006 | 0.030 | 0.005 |
| M4 | 0.008 | 0.014 | 0.001 | 0.015 | 0.013 | 0.018 | 0.023 | 0.039 | 0.018 | 0.004 |
| M5 | 0.008 | 0.008 | 0.014 | 0.036 | n.d. | 0.014 | 0.034 | 0.001 | 0.008 | 0.044 |
| M6 | 0.007 | 0.011 | 0.031 | 0.009 | 0.009 | 0.002 | 0.001 | 0.001 | 0.003 | 0.038 |
| M7 | 0.009 | 0.017 | 0.001 | 0.027 | 0.013 | 0.024 | 0.046 | 0.006 | 0.013 | 0.004 |
| M8 | 0.009 | 0.015 | 0.001 | 0.011 | 0.013 | 0.020 | 0.028 | 0.006 | 0.030 | 0.006 |
| M9 | 0.011 | 0.011 | 0.014 | 0.014 | 0.004 | 0.012 | 0.017 | 0.001 | 0.006 | 0.037 |
| M10 | 0.009 | 0.013 | n.d. | 0.023 | 0.013 | 0.055 | 0.025 | 0.006 | 0.032 | 0.001 |
| L1 | 0.001 | 0.013 | 0.002 | 0.012 | 0.013 | 0.021 | 0.028 | 0.006 | 0.031 | 0.005 |
| L2 | 0.009 | 0.008 | 0.014 | 0.230 | 0.002 | 0.224 | 0.021 | 0.001 | 0.012 | 0.004 |
| L3 | 0.008 | 0.012 | 0.004 | 0.002 | n.d. | 0.001 | 0.013 | n.d. | n.d. | 0.061 |
| L4 | 0.008 | 0.014 | n.d. | 0.016 | 0.013 | 0.020 | 0.040 | 0.006 | 0.030 | 0.004 |
| L5 | 0.010 | 0.013 | 0.002 | 0.014 | 0.013 | 0.018 | 0.041 | 0.006 | 0.031 | 0.004 |
| Min | 0.001 | 0.008 | 0.001 | 0.002 | 0.004 | 0.001 | 0.001 | 0.001 | 0.002 | 0.004 |
| Max | 0.011 | 0.019 | 0.031 | 0.230 | 0.013 | 0.224 | 0.078 | 0.006 | 0.034 | 0.061 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyamukamba, P.; Mququ, Z.; Nkosi, S.; Isaacs, S. Determination of Selected Harmful Substances in Baby Diapers Available on the South African Market. Int. J. Environ. Res. Public Health 2023, 20, 1023. https://doi.org/10.3390/ijerph20021023
Nyamukamba P, Mququ Z, Nkosi S, Isaacs S. Determination of Selected Harmful Substances in Baby Diapers Available on the South African Market. International Journal of Environmental Research and Public Health. 2023; 20(2):1023. https://doi.org/10.3390/ijerph20021023
Chicago/Turabian StyleNyamukamba, Pardon, Zethu Mququ, Sandile Nkosi, and Shamil Isaacs. 2023. "Determination of Selected Harmful Substances in Baby Diapers Available on the South African Market" International Journal of Environmental Research and Public Health 20, no. 2: 1023. https://doi.org/10.3390/ijerph20021023




