Organophosphate Pesticides and Pyrethroids in Farmland of the Pearl River Delta, China: Regional Residue, Distributions and Risks
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Collection
2.3. Sample Extraction, Cleanup, and Analysis
2.4. Quality Assurance and Quality Control
2.5. Health and Ecological Risk Evaluation
2.6. Statistical Analysis and Spatial Mapping
3. Results and Discussion
3.1. Occurrence of Pesticides in Soils in PRD
3.2. Spatial Distribution of Pesticides
3.3. Vertical Distribution of Pesticides in Soil Profiles
3.4. Ecological and Health Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, T.; Zhong, M.; Lu, M.; Xu, D.; Xue, Y.; Huang, J.; Blaney, L.; Yu, G. Occurrence, spatiotemporal distribution, and risk assessment of current-use pesticides in surface water: A case study near Taihu Lake, China. Sci. Total Environ. 2021, 782, 146826. [Google Scholar] [CrossRef] [PubMed]
- Sabzevari, S.; Hofman, J. A worldwide review of currently used pesticides’ monitoring in agricultural soils. Sci. Total Environ. 2022, 812, 152344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, Y.; Ren, W.; Huang, Y.; Wang, X.; Fu, Z.; Ma, W.; Teng, Y.; Luo, Y. Pesticide residues in soils planted with Panax notoginseng in south China, and their relationships in Panax notoginseng and soil. Ecotoxicol. Environ. Saf. 2020, 201, 110783. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, G.; Atreya, K.; Scheepers, P.T.J.; Geissen, V. Concentration and distribution of pesticide residues in soil: Non-dietary human health risk assessment. Chemosphere 2020, 253, 126594. [Google Scholar] [CrossRef]
- Tsaboula, A.; Papadakis, E.-N.; Vryzas, Z.; Kotopoulou, A.; Kintzikoglou, K.; Papadopoulou-Mourkidou, E. Environmental and human risk hierarchy of pesticides: A prioritization method, based on monitoring, hazard assessment and environmental fate. Environ. Int. 2016, 91, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Hvězdová, M.; Kosubová, P.; Košíková, M.; Scherr, K.E.; Šimek, Z.; Brodský, L.; Šudoma, M.; Škulcová, L.; Sáňka, M.; Svobodová, M.; et al. Currently and recently used pesticides in Central European arable soils. Sci. Total Environ. 2018, 613–614, 361–370. [Google Scholar] [CrossRef]
- Sun, J.; Pan, L.; Li, Z.; Zeng, Q.; Wang, L.; Zhu, L. Comparison of greenhouse and open field cultivations across China: Soil characteristics, contamination and microbial diversity. Environ. Pollut. 2018, 243 Pt B, 1509–1516. [Google Scholar] [CrossRef]
- Degrendele, C.; Klanova, J.; Prokes, R.; Pribylova, P.; Senk, P.; Sudoma, M.; Roosli, M.; Dalvie, M.A.; Fuhrimann, S. Current use pesticides in soil and air from two agricultural sites in South Africa: Implications for environmental fate and human exposure. Sci. Total Environ. 2022, 807 Pt 1, 150455. [Google Scholar] [CrossRef]
- Jokanović, M. Neurotoxic effects of organophosphorus pesticides and possible association with neurodegenerative diseases in man: A review. Toxicology 2018, 410, 125–131. [Google Scholar] [CrossRef]
- Pan, L.L.; Sun, J.T.; Li, Z.; Zhan, Y.; Xu, S.; Zhu, L. Organophosphate pesticide in agricultural soils from the Yangtze River Delta of China: Concentration, distribution, and risk assessment. Environ. Sci. Pollut. Res. 2018, 25, 4–11. [Google Scholar] [CrossRef]
- Velasco, A.; Rodríguez, J.; Castillo, R.; Ortíz, I. Residues of organochlorine and organophosphorus pesticides in sugarcane crop soils and river water. J. Environ. Sci. Health Part B 2012, 47, 833–841. [Google Scholar] [CrossRef]
- Das, S.; Hageman, K.J.; Taylor, M.; Michelsen-Heath, S.; Stewart, I. Fate of the organophosphate insecticide, chlorpyrifos, in leaves, soil, and air following application. Chemosphere 2020, 243, 125194. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Tan, P.; Wang, R.; Li, S.; Liu, H.; Yang, Y.; Wu, Z. Advances in organophosphorus pesticides pollution: Current status and challenges in ecotoxicological, sustainable agriculture, and degradation strategies. J. Hazard. Mater. 2022, 424 Pt B, 127494. [Google Scholar] [CrossRef]
- Cecchi, A.; Rovedatti, M.G.; Sabino, G.; Magnarelli, G.G. Environmental exposure to organophosphate pesticides: Assessment of endocrine disruption and hepatotoxicity in pregnant women. Ecotoxicol. Environ. Saf. 2012, 80, 280–287. [Google Scholar] [CrossRef]
- Czajka, M.; Matysiak-Kucharek, M.; Jodłowska-Jędrych, B.; Sawicki, K.; Fal, B.; Drop, B.; Kruszewski, M.; Kapka-Skrzypczak, L. Organophosphorus pesticides can influence the development of obesity and type 2 diabetes with concomitant metabolic changes. Environ. Res. 2019, 178, 108685. [Google Scholar] [CrossRef] [PubMed]
- Li, A.J.; Kannan, K. Urinary concentrations and profiles of organophosphate and pyrethroid pesticide metabolites and phenoxyacid herbicides in populations in eight countries. Environ. Int. 2018, 121 Pt 2, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, T.O.; Oladoye, P.O.; Olanrewaju, C.A.; Akinsola, G.O. Organophosphorus pesticides: Impacts, detection and removal strategies. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100655. [Google Scholar] [CrossRef]
- Sidhu, G.K.; Singh, S.; Kumar, V.; Dhanjal, D.S.; Datta, S.; Singh, J. Toxicity, monitoring and biodegradation of organophosphate pesticides: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1135–1187. [Google Scholar] [CrossRef]
- Han, Y.; Mo, R.; Yuan, X.; Zhong, D.; Tang, F.; Ye, C.; Liu, Y. Pesticide residues in nut-planted soils of China and their relationship between nut/soil. Chemosphere 2017, 180, 42–47. [Google Scholar] [CrossRef]
- Tang, W.; Wang, D.; Wang, J.; Wu, Z.; Li, L.; Huang, M.; Xu, S.; Yan, D. Pyrethroid pesticide residues in the global environment: An overview. Chemosphere 2018, 191, 990–1007. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Sun, J.; Dou, R.; Yu, X.; Wei, Z.; Yang, C.; Zeng, X.; Zhu, L. Contamination of pyrethroids in agricultural soils from the Yangtze River Delta, China. Sci. Total Environ. 2020, 731, 139181. [Google Scholar] [CrossRef] [PubMed]
- Brander, S.M.; Gabler, M.K.; Fowler, N.L.; Connon, R.E.; Schlenk, D. Pyrethroid Pesticides as Endocrine Disruptors: Molecular Mechanisms in Vertebrates with a Focus on Fishes. Environ. Sci. Technol. 2016, 50, 8977–8992. [Google Scholar] [CrossRef] [PubMed]
- Gajendiran, A.; Abraham, J. An overview of pyrethroid insecticides. Front. Biol. 2018, 13, 79–90. [Google Scholar] [CrossRef]
- Yu, Z.; Li, X.F.; Wang, S.; Liu, L.Y.; Zeng, E.Y. The human and ecological risks of neonicotinoid insecticides in soils of an agricultural zone within the Pearl River Delta, South China. Environ. Pollut. 2021, 284, 117358. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.L.; Bao, L.J.; Wu, C.C.; He, Z.C.; Zeng, E.Y. Assessing the effects of urbanization on the environment with soil legacy and current-use insecticides: A case study in the Pearl River Delta, China. Sci. Total Environ. 2015, 514, 409–417. [Google Scholar] [CrossRef] [PubMed]
- National Agro-Tech Extension and Service Center of China. In Proceedings of the Chinese Plant Protection Conference, Nanjing, China, 11–13 November 2022.
- Li, Z.; Sun, J.; Zhu, L. Organophosphorus pesticides in greenhouse and open-field soils across China: Distribution characteristic, polluted pathway and health risk. Sci. Total Environ. 2021, 765, 142757. [Google Scholar] [CrossRef]
- Dou, R.; Sun, J.; Deng, F.; Wang, P.; Zhou, H.; Wei, Z.; Chen, M.; He, Z.; Lai, M.; Ye, T.; et al. Contamination of pyrethroids and atrazine in greenhouse and open-field agricultural soils in China. Sci. Total Environ. 2020, 701, 134916. [Google Scholar] [CrossRef]
- USEPA. Mid-Atlantic RISK Assessment; Untied States Environmental Protection Agency: Washington, DC, USA, 2015. [Google Scholar]
- Xiao, K.; Zhu, N.; Lu, Z.; Zheng, H.; Cai, M. Distribution of eight organophosphorus pesticides and their oxides in surface water of the East China Sea based on high volume solid phase extraction method. Environ. Pollut. 2021, 279, 116886. [Google Scholar] [CrossRef]
- Cao, S.; Zhang, P.; Cai, M.; Yang, Y.; Liu, Y.; Ge, L.; Ma, H. Occurrence, spatial distributions, and ecological risk of pyrethroids in coastal regions of South Yellow and East China Seas. Mar. Pollut. Bull. 2022, 179, 113725. [Google Scholar] [CrossRef]
- Calow, P.; Forbes, V.E. Peer Reviewed: Does Ecotoxicology Inform Ecological Risk Assessment? Environ. Sci. Technol. 2003, 37, 146A–151A. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhao, Q.; Wang, C.; Cui, Y.; Li, J.; Chen, A.; Liang, G.; Jiao, B. Occurrence, temporal variation, quality and safety assessment of pesticide residues on citrus fruits in China. Chemosphere 2020, 258, 127381. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, D.; Li, S.; Ni, Z.; Ding, M.; Ye, C.; Tang, F. Residue levels and risk assessment of pesticides in nuts of China. Chemosphere 2016, 144, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, F.; Wei, Y.; Lydy, M.J.; You, J. Global occurrence of pyrethroid insecticides in sediment and the associated toxicological effects on benthic invertebrates: An overview. J. Hazard. Mater. 2016, 324, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Malhat, F.M.; Haggag, M.N.; Loutfy, N.M.; Osman, M.A.; Ahmed, M.T. Residues of organochlorine and synthetic pyrethroid pesticides in honey, an indicator of ambient environment, a pilot study. Chemosphere 2015, 120, 457–461. [Google Scholar] [CrossRef]
- Chang, J.; Wang, Y.; Wang, H.; Li, J.; Xu, P. Bioaccumulation and enantioselectivity of type I and type II pyrethroid pesticides in earthworm. Chemosphere 2016, 144, 1351–1357. [Google Scholar] [CrossRef]
- Li, H.; Wei, Y.; Lydy, M.J.; You, J. Inter-compartmental transport of organophosphate and pyrethroid pesticides in South China: Implications for a regional risk assessment. Environ. Pollut. 2014, 190, 19–26. [Google Scholar] [CrossRef]
- Lozowicka, B. Health risk for children and adults consuming apples with pesticide residue. Sci. Total Environ. 2015, 502, 184–198. [Google Scholar] [CrossRef]
- Taniwiryono, D.; Van den Brink, P.J.; Rietjens, I.M.; Murk, A.J. A case study on Bangka Island, Indonesia on the habits and consequences of pesticide use in pepper plantations. Environ. Toxicol. 2007, 22, 405–414. [Google Scholar]
- Prates, L.H.F.; Faroni, L.R.D.A.; Heleno, F.F.; de Queiroz, M.E.L.R.; de Sousa, A.H.; Silva, M.V.D.A. Eugenol diffusion coefficient and its potential to control Sitophilus zeamais in rice. Sci. Rep. 2019, 9, 11161. [Google Scholar] [CrossRef]
- Li, B.-X.; Liu, Y.; Zhang, P.; Li, X.-X.; Pang, X.-Y.; Zhao, Y.-H.; Li, H.; Liu, F.; Lin, J.; Mu, W. Selection of organosilicone surfactants for tank-mixed pesticides considering the balance between synergistic effects on pests and environmental risks. Chemosphere 2019, 217, 591–598. [Google Scholar] [CrossRef]
- Nakanishi, K.; Yokomizo, H.; Hayashi, T.I. Population model analyses of the combined effects of insecticide use and habitat degradation on the past sharp declines of the dragonfly Sympetrum frequens. Sci. Total Environ. 2021, 787, 147526. [Google Scholar] [CrossRef] [PubMed]
- Szpyrka, E.; Podbielska, M.; Zwolak, A.; Piechowicz, B.; Siebielec, G.; Słowik-Borowiec, M. Influence of a Commercial Biological Fungicide containing Trichoderma harzianum Rifai T-22 on Dissipation Kinetics and Degradation of Five Herbicides in Two Types of Soil. Molecules 2020, 25, 1391. [Google Scholar] [CrossRef] [PubMed]
- Osesua, B.A.; Tsafe, A.I.; Birnin-Yauri, U.A.; Sahabi, D.M. Determination of pesticide residues in soil samples collected from Wurno Irrigation farm, Sokoto State, Nigeria. Cont. J. Agric. Sci. 2017, 11, 40–52. [Google Scholar]
- Zhang, Y.; Hou, Y.; Chen, F.; Xiao, Z.; Zhang, J.; Hu, X. The degradation of chlorpyrifos and diazinon in aqueous solution by ultrasonic irradiation: Effect of parameters and degradation pathway. Chemosphere 2011, 82, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, C.; Liao, X.; Luo, Y.; Wu, S.; Wang, J. Hydrolysis mechanism of methyl parathion evidenced by Q-Exactive mass spectrometry. Environ. Sci. Pollut. Res. 2015, 22, 19747–19755. [Google Scholar] [CrossRef]
- Broznic, D.; Didovic, M.P.; Rimac, V.; Marinic, J. Sorption and leaching potential of organophosphorus insecticide dimethoate in Croatian agricultural soils. Chemosphere 2021, 273, 128563. [Google Scholar] [CrossRef]
- Papa, E.; Castiglioni, S.; Gramatica, P.; Nikolayenko, V.; Kayumov, O.; Calamari, D. Screening the leaching tendency of pesticides applied in the Amu Darya Basin (Uzbekistan). Water Res. 2004, 38, 3485–3494. [Google Scholar] [CrossRef]
- El-Saeid, M.H.; Al-Turki, A.M.; Al-Wable, M.I.; Abdel-Nasser, G. Evaluation of Pesticide Residues in Saudi Arabia Ground Water. Res. J. Environ. Earth Sci. 2011, 5, 171–178. [Google Scholar]
- Ensminger, M.; Bergin, R.; Spurlock, F.; Goh, K.S. Pesticide concentrations in water and sediment and associated invertebrate toxicity in Del Puerto and Orestimba Creeks, California, 2007-2008. Environ. Monit. Assess. 2011, 175, 573–587. [Google Scholar] [CrossRef]
- Gao, J.; Liu, L.; Liu, X.; Zhou, H.; Lu, J.; Huang, S.; Wang, Z. The occurrence and spatial distribution of organophosphorous pesticides in Chinese surface water. Bull. Environ. Contam. Toxicol. 2009, 82, 223–229. [Google Scholar] [CrossRef]
- Rasmussen, P.E.; Subramanian, K.S.; Jessiman, B.J. A multi-element profile of house dust in relation to exterior dust and soils in the city of Ottawa, Canada. Sci. Total Environ. 2001, 267, 145–160. [Google Scholar] [CrossRef] [PubMed]


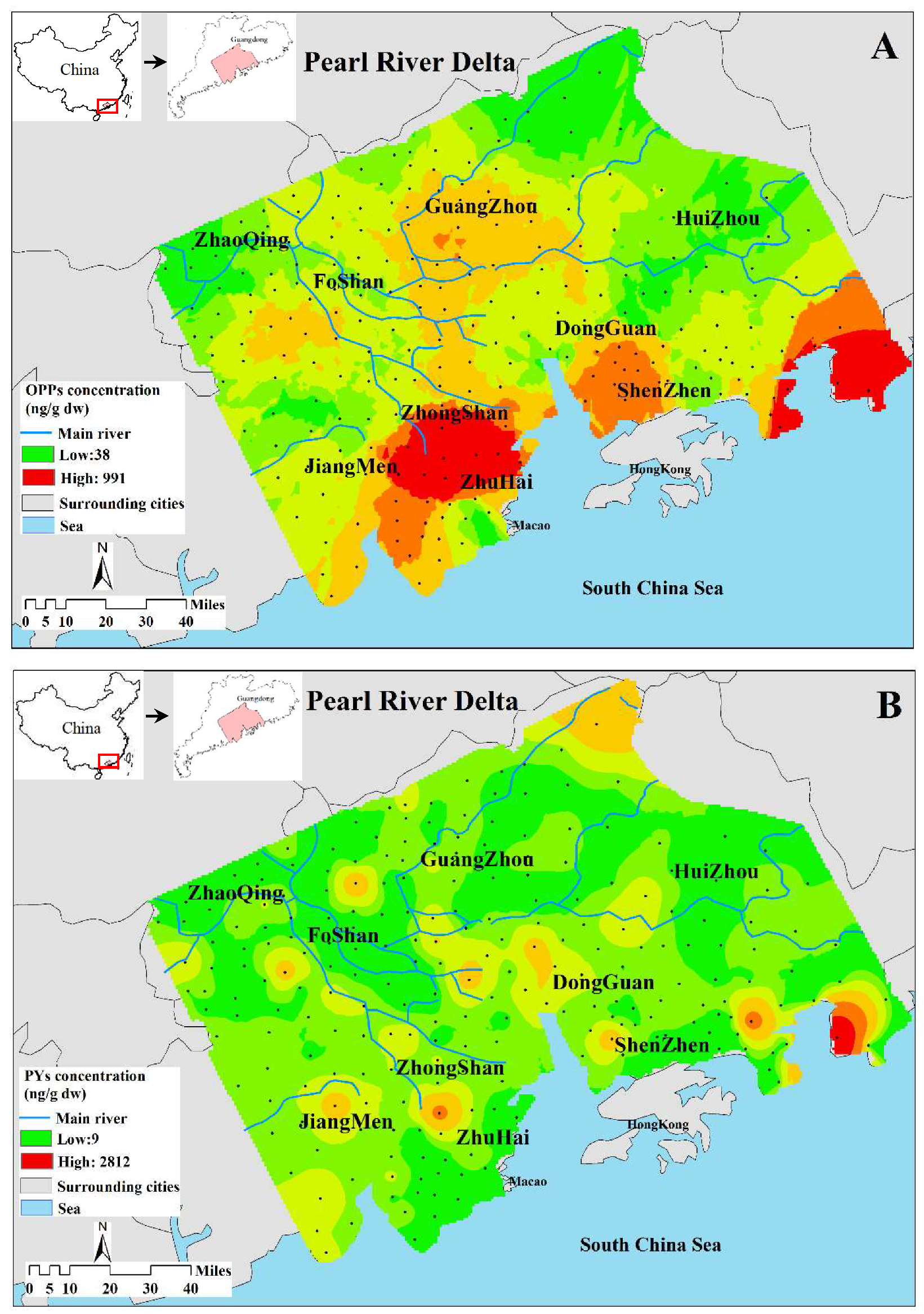
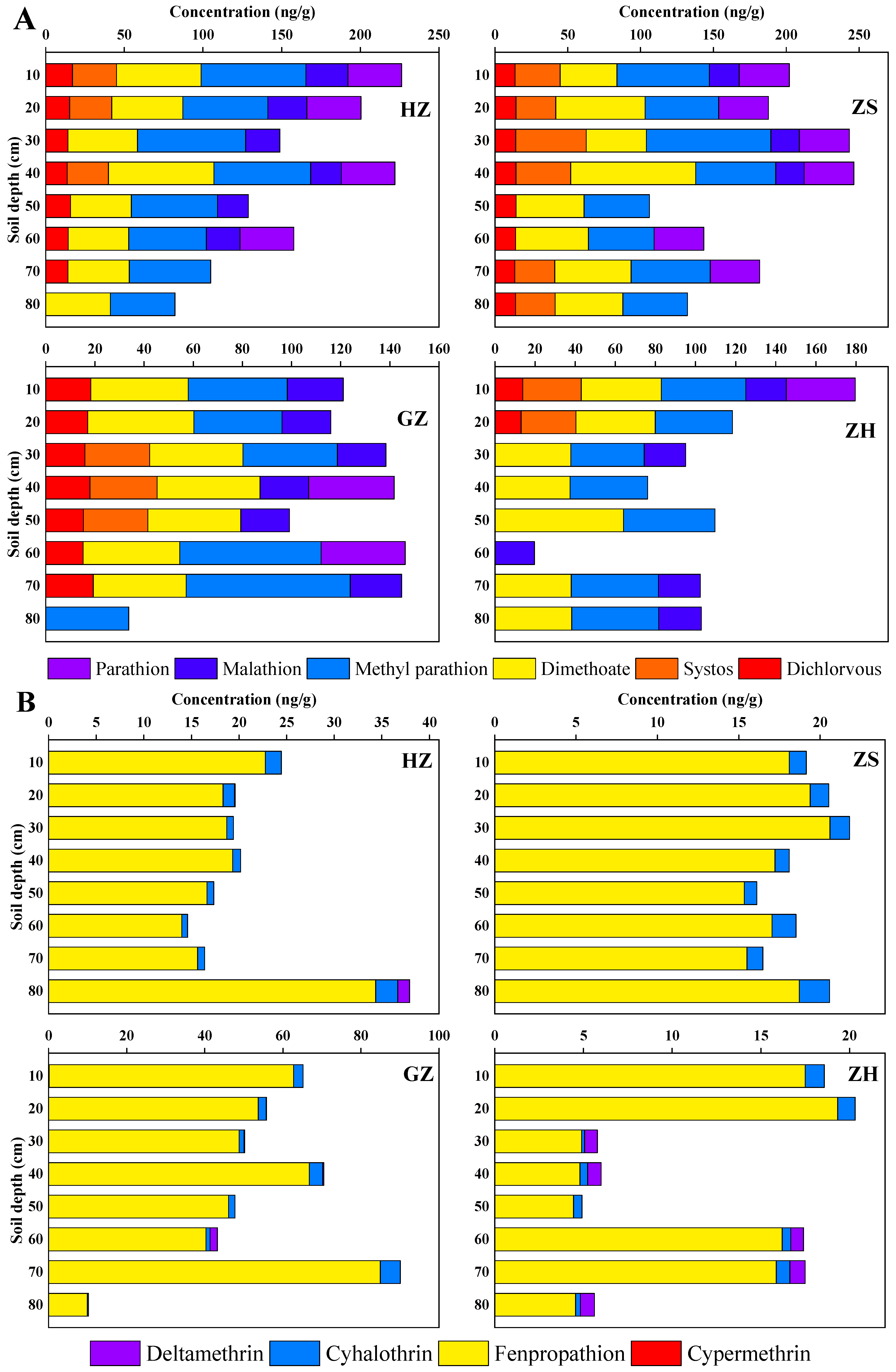
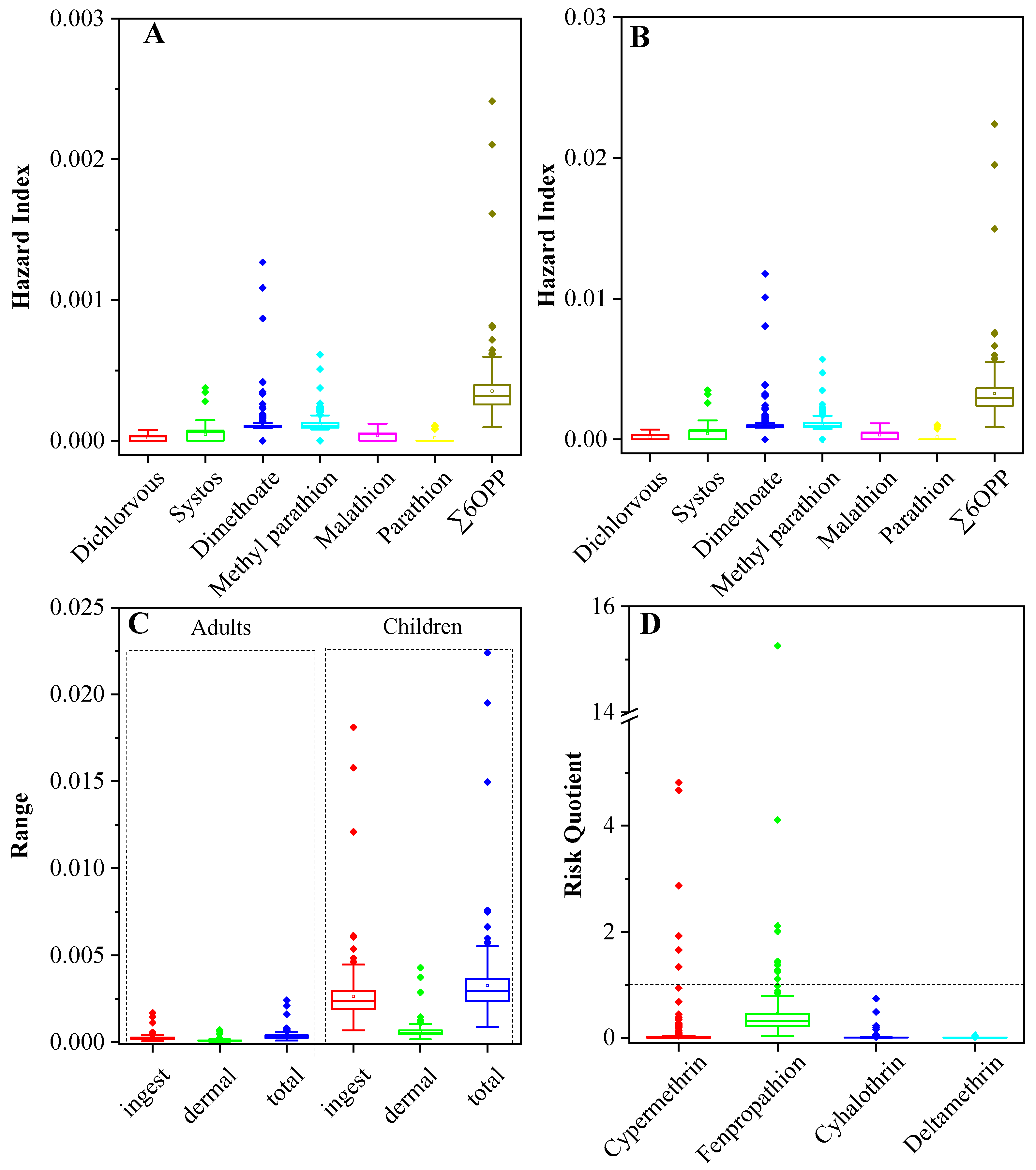
| Compound | Detection Rate (%) | Mean (ng/g) | Min. (ng/g) | Max. (ng/g) | Median (ng/g) | CV (%) |
|---|---|---|---|---|---|---|
| Dichlorvos | 52.1 | 7.34 | ND | 31.0 | 13.6 | 98.9 |
| Demeton | 50.8 | 18.2 | ND | 155 | 29.2 | 121 |
| Dimethoate | 97.1 | 49.7 | ND | 521 | 40.3 | 98.1 |
| Malathion | 63.3 | 14.1 | ND | 50.4 | 21.1 | 78.4 |
| Parathion | 21.7 | 7.52 | ND | 44.8 | 34.3 | 50.7 |
| Methyl parathion | 96.7 | 47.7 | ND | 251 | 21.1 | 191 |
| Σ6OPPs | 100 | 145 | 38.4 | 991 | 130 | 63.8 |
| Cypermethrin | 37.5 | 10.1 | ND | 481 | 2.51 | 20.0 |
| Fenpropathion | 100 | 85.3 | 5.25 | 2800 | 57.9 | 45.1 |
| Cyhalothoin | 99.6 | 11.6 | ND | 739 | 2.96 | 19.3 |
| Deltamethrin | 68.8 | 1.52 | ND | 32.0 | 0.579 | 45.2 |
| Σ4PYs | 100 | 109 | 8.76 | 2810 | 65.3 | 52.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, R.; Yao, S.; Ai, T.; Huang, J.; Liu, Y.; Sun, J. Organophosphate Pesticides and Pyrethroids in Farmland of the Pearl River Delta, China: Regional Residue, Distributions and Risks. Int. J. Environ. Res. Public Health 2023, 20, 1017. https://doi.org/10.3390/ijerph20021017
Yao R, Yao S, Ai T, Huang J, Liu Y, Sun J. Organophosphate Pesticides and Pyrethroids in Farmland of the Pearl River Delta, China: Regional Residue, Distributions and Risks. International Journal of Environmental Research and Public Health. 2023; 20(2):1017. https://doi.org/10.3390/ijerph20021017
Chicago/Turabian StyleYao, Runlin, Siyu Yao, Tao Ai, Jiahui Huang, Yang Liu, and Jianteng Sun. 2023. "Organophosphate Pesticides and Pyrethroids in Farmland of the Pearl River Delta, China: Regional Residue, Distributions and Risks" International Journal of Environmental Research and Public Health 20, no. 2: 1017. https://doi.org/10.3390/ijerph20021017
APA StyleYao, R., Yao, S., Ai, T., Huang, J., Liu, Y., & Sun, J. (2023). Organophosphate Pesticides and Pyrethroids in Farmland of the Pearl River Delta, China: Regional Residue, Distributions and Risks. International Journal of Environmental Research and Public Health, 20(2), 1017. https://doi.org/10.3390/ijerph20021017






