Epigenetics of Trauma Transmission and Fetal Alcohol Spectrum Disorder: What Does the Evidence Support?
Abstract
1. Introduction
2. Methods
3. FASD and Prevalence
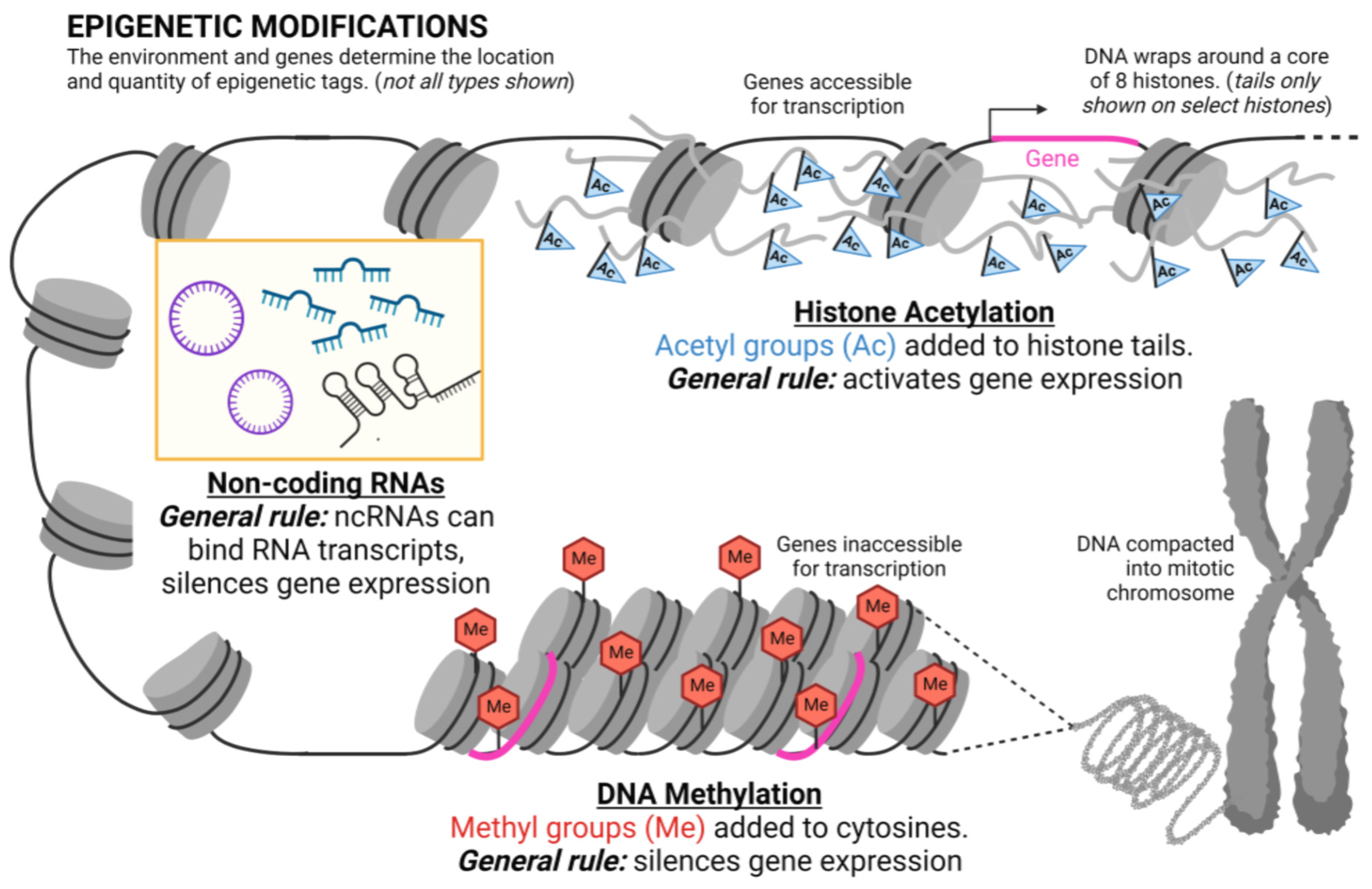
4. What Is Epigenetics?
5. Epigenetic Mechanisms
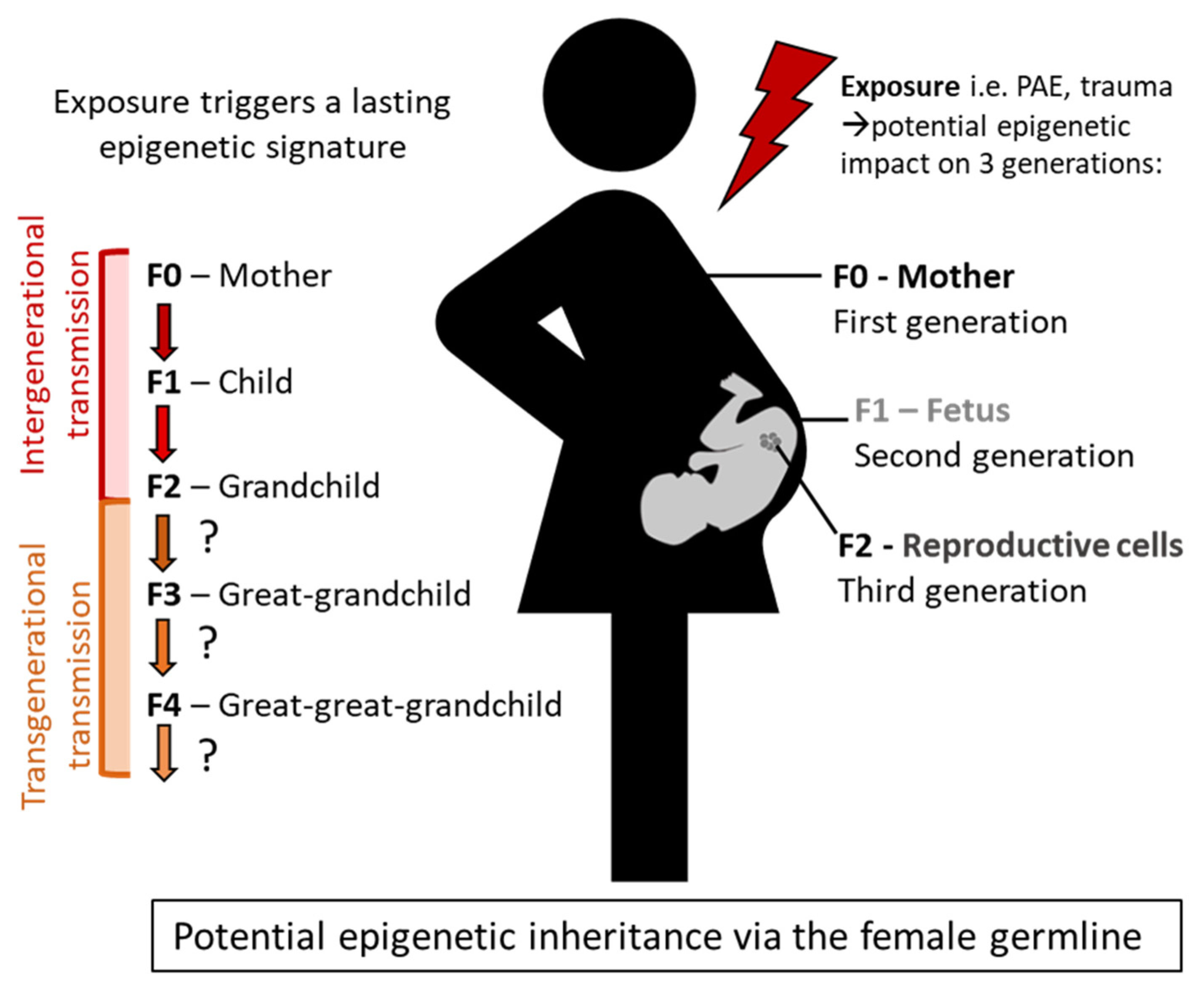
6. What Is Epigenetic Inheritance?
7. Defining Intergenerational and Transgenerational Epigenetic Transmission
8. Epigenetic Effects and Transmission in FASD
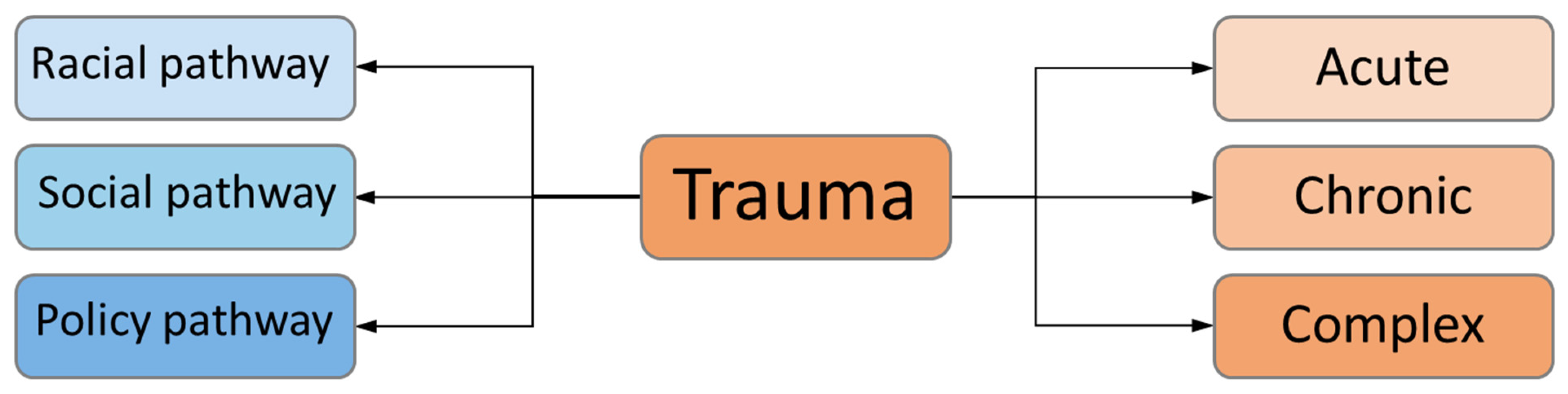
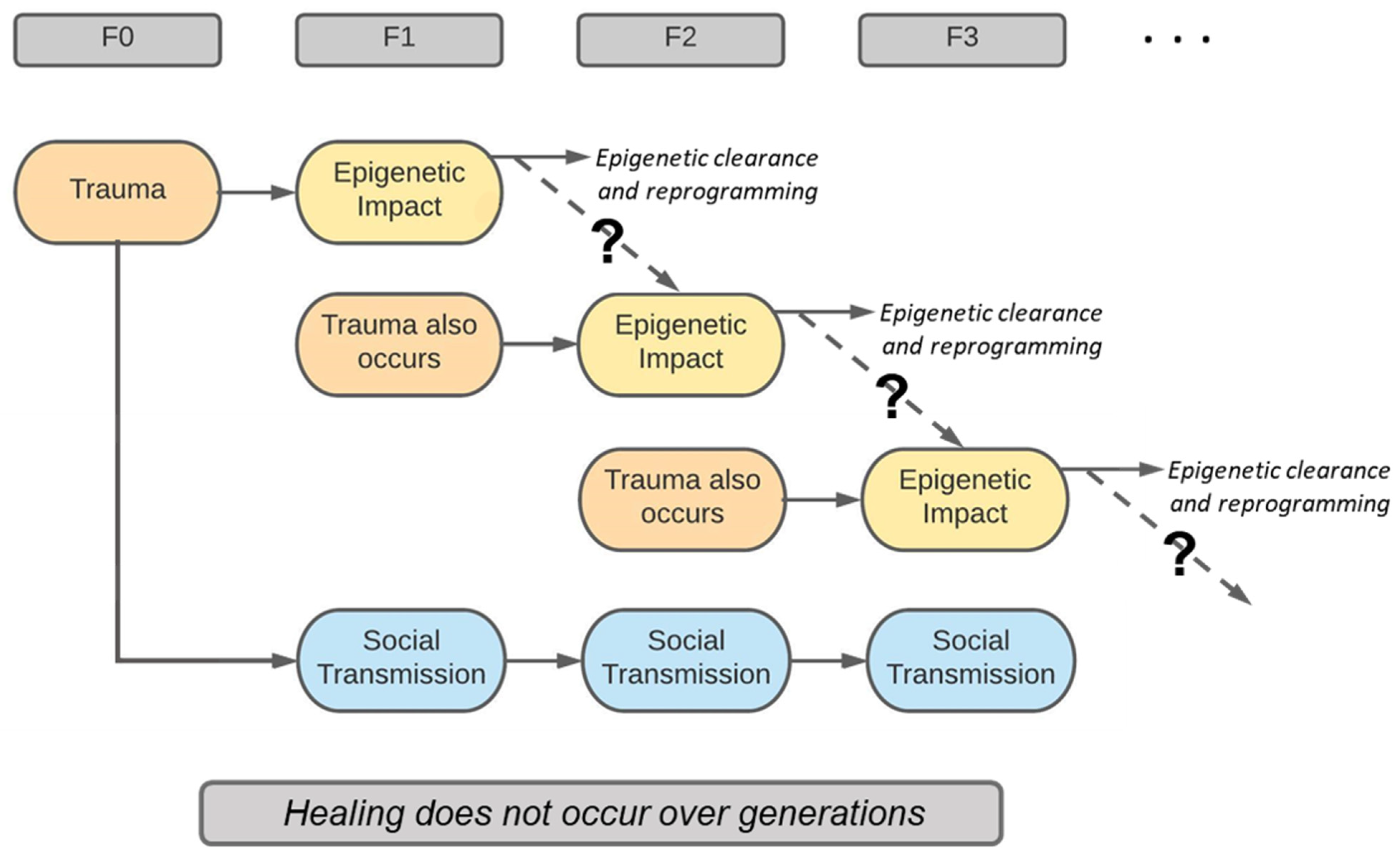
9. Epigenetic Transmission of Trauma
10. Social Transmission of Trauma
11. Challenge of Discerning Social and Epigenetic Transmission
12. Why Is This Important to Social Work?
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, C.H.; Denny, C.H.; Cheal, N.E.; Sniezek, J.E.; Kanny, D. Alcohol Use and Binge Drinking among Women of Childbearing Age—United States, 2011–2013. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Popova, S.; Lange, S.; Probst, C.; Gmel, G.; Rehm, J. Estimation of National, Regional, and Global Prevalence of Alcohol Use during Pregnancy and Fetal Alcohol Syndrome: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2017, 5, e290–e299. [Google Scholar] [CrossRef]
- Popova, S.; Lange, S.; Shield, K.; Burd, L.; Rehm, J. Prevalence of Fetal Alcohol Spectrum Disorder among Special Subpopulations: A Systematic Review and Meta-Analysis. Addiction 2019, 114, 1150–1172. [Google Scholar] [CrossRef] [PubMed]
- Public Health Agency of Canada. Report on the State of Public Health in Canada 2010. Available online: https://www.canada.ca/en/public-health/corporate/publications/chief-public-health-officer-reports-state-public-health-canada/addressing-stigma-toward-more-inclusive-health-system.html (accessed on 2 April 2023).
- Flannigan, K.; Kapasi, A.; Pei, J.; Murdoch, I.; Andrew, G.; Rasmussen, C. Characterizing Adverse Childhood Experiences among Children and Adolescents with Prenatal Alcohol Exposure and Fetal Alcohol Spectrum Disorder. Child Abus. Negl. 2021, 112, 104888. [Google Scholar] [CrossRef] [PubMed]
- Price, A.; Cook, P.A.; Norgate, S.; Mukherjee, R. Prenatal Alcohol Exposure and Traumatic Childhood Experiences: A Systematic Review. Neurosci. Biobehav. Rev. 2017, 80, 89–98. [Google Scholar] [CrossRef]
- Lam, V.Y.Y.; Raineki, C.; Takeuchi, L.E.; Ellis, L.; Woodward, T.S.; Weinberg, J. Chronic Stress Alters Behavior in the Forced Swim Test and Underlying Neural Activity in Animals Exposed to Alcohol Prenatally: Sex- and Time-Dependent Effects. Front. Behav. Neurosci. 2018, 12, 42. [Google Scholar] [CrossRef]
- Anda, R.F.; Felitti, V.J.; Bremner, J.D.; Walker, J.D.; Whitfield, C.; Perry, B.D.; Dube, S.R.; Giles, W.H. The Enduring Effects of Abuse and Related Adverse Experiences in Childhood: A Convergence of Evidence from Neurobiology and Epidemiology. Eur. Arch. Psychiatry Clin. Neurosci. 2006, 256, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Hyter, Y.D. Complex Trauma and Prenatal Alcohol Exposure: Clinical Implications. Perspect. Sch. Issues 2012, 13, 32–42. [Google Scholar] [CrossRef]
- Piras, F.; Vecchio, D.; Assogna, F.; Pellicano, C.; Ciullo, V.; Banaj, N.; Edden, R.A.E.; Pontieri, F.E.; Piras, F.; Spalletta, G. Cerebellar Gaba Levels and Cognitive Interference in Parkinson’s Disease and Healthy Comparators. J. Pers. Med. 2021, 11, 16. [Google Scholar] [CrossRef]
- Blumenthal, H.; Blanchard, L.; Feldner, M.; Babson, K.; Leen-Feldner, E.; Dixon, L. Traumatic Event Exposure, Posttraumatic Stress, and Substance Use Among Youth: A Critical Review of the Empirical Literature. Curr. Psychiatry Rev. 2008, 4, 228–254. [Google Scholar] [CrossRef]
- Jaenisch, R.; Bird, A. Epigenetic Regulation of Gene Expression: How the Genome Integrates Intrinsic and Environmental Signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef]
- Chudley, A.E. Genetic Factors Contributing to FASD. In Fetal Alcohol Spectrum Disorder: Management and Policy Perspectives of FASD; Riley, E.P., Clarren, S., Weinberg, J., Jonsson, E., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 109–126. ISBN 978-3-527-32839-0. [Google Scholar]
- Yehuda, R.; Lehrner, A. Intergenerational Transmission of Trauma Effects: Putative Role of Epigenetic Mechanisms. World Psychiatry 2018, 17, 243–257. [Google Scholar] [CrossRef]
- Mead, E.A.; Sarkar, D.K. Fetal Alcohol Spectrum Disorders and Their Transmission through Genetic and Epigenetic Mechanisms. Front. Genet. 2014, 5, 154. [Google Scholar] [CrossRef] [PubMed]
- Henriques, M. Can the Legacy of Trauma Be Passed down the Generations? Available online: https://www.bbc.com/future/article/20190326-what-is-epigenetics (accessed on 26 March 2023).
- DeAngelis, T. The Legacy of Trauma. Am. Psychol. Assoc. 2019, 50, 36. [Google Scholar]
- Gupta, K.K.; Gupta, V.K.; Shirasaka, T. An Update on Fetal Alcohol Syndrome—Pathogenesis, Risks, and Treatment. Alcohol. Clin. Exp. Res. 2016, 40, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Ungerer, M.; Knezovich, J.; Ramsay, M. In Utero Alcohol Exposure, Epigenetic Changes, and Their Consequences. Alcohol Res. Curr. Rev. 2013, 35, 37–46. [Google Scholar]
- Young, J.K.; Giesbrecht, H.E.; Eskin, M.N.; Aliani, M.; Suh, M. Nutrition Implications for Fetal Alcohol Spectrum Disorder. Adv. Nutr. 2014, 5, 675–692. [Google Scholar] [CrossRef]
- O’Malley, K.D.; Rich, S.D. Clinical Implications of a Link Between Fetal Alcohol Spectrum Disorders (FASD) and Autism or Asperger’s Disorder—A Neurodevelopmental Frame for Helping Understanding and Management. Intech 2013, 11, 806. [Google Scholar] [CrossRef][Green Version]
- Jones, K.I.; Smith, D.W.; Ulleland, C.N.; Pykowicz Streissguth, A. Pattern of Malformation in Offspring of Chronic Alcoholic Mothers. Lancet 1973, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Himmelreich, M.; Lutke, C.J.; Travis Hargrove, E. Lay of the Land: Fetal Alcohol Spectrum Disorder (FASD) as a Whole Body Diagnosis. In The Routledge Handbook of Social Work and Addictive Behaviors; Begun, A.L., Murray, M.M., Eds.; Routledge: Abingdon, UK, 2020; ISBN 978-0-367-19554-0. [Google Scholar]
- Burd, L.; Martsolf, J.T.; Klug, M.G.; Kerbeshian, J. Diagnosis of FAS: A Comparison of the Fetal Alcohol Syndrome Diagnostic Checklist and the Institute of Medicine Criteria for Fetal Alcohol Syndrome. Neurotoxicol. Teratol. 2003, 25, 719–724. [Google Scholar] [CrossRef]
- O’Leary, C. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, and Developmental Outcomes. J. Paediatr. Child Health 2004, 40, 2–7. [Google Scholar] [CrossRef]
- Doyle, L.R.; Mattson, S.N. Neurobehavioral Disorder Associated with Prenatal Alcohol Exposure (ND-PAE): Review of Evidence and Guidelines for Assessment. Curr. Dev. Disord. Rep. 2015, 2, 175–186. [Google Scholar] [CrossRef]
- Lynch, M.E.; Kable, J.A.; Coles, C.D. Prenatal Alcohol Exposure, Adaptive Function, and Enty into Adult Roles in Prospective Study of Young Adults. Neurotoxicol. Teratol. 2015, 51, 52–60. [Google Scholar] [CrossRef]
- Moore, E.M.; Riley, E.P. What Happens When Children with Fetal Alcohol Spectrum Disorders Become Adults? Curr. Dev. Disord. Rep. 2015, 3, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Panczakiewicz, A.L.; Glass, L.; Coles, C.D.; Kable, J.A.; Sowell, E. lizabet. R.; Wozniak, J.R.; Jones, K.L.; Riley, E.P.; Mattson, S.N. Neurobehavioral Deficits Consistent across Age and Sex in Youth with Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2016, 40, 1971–1981. [Google Scholar] [CrossRef]
- Carter, C.R.; Jacobson, J.L.; Molteno, C.D.; Dodge, N.C.; Meintjes, E.M.; Jacobson, S.W. Fetal Alcohol Growth Restriction and Cognitive Impairment. Pediatrics 2016, 138, e20160775. [Google Scholar] [CrossRef] [PubMed]
- May, P.A.; Chambers, C.D.; Kalberg, W.O.; Zellner, J.; Feldman, H.; Buckley, D.; Kopald, D.; Hasken, J.M.; Xu, R.; Honerkamp-Smith, G.; et al. Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities. JAMA-J. Am. Med. Assoc. 2018, 319, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Coles, C.D. Discriminating the Effects of Prenatal Alcohol Exposure from Other Behavioral and Learning Disorders. Alcohol Res. Health 2011, 34, 42–50. [Google Scholar] [PubMed]
- Stevens, S.A.; Nash, K.; Koren, G.; Rovet, J. Autism Characteristics in Children with Fetal Alcohol Spectrum Disorders. Child Neuropsychol. 2013, 19, 579–587. [Google Scholar] [CrossRef]
- McLachlan, K.; Flannigan, K.; Temple, V.; Unsworth, K.; Cook, J.L. Difficulties in Daily Living Experienced by Adolescents, Transition-Aged Youth, and Adults with Fetal Alcohol Spectrum Disorder. Alcohol. Clin. Exp. Res. 2020, 44, 1609–1624. [Google Scholar] [CrossRef]
- Temple, V.; Cook, J.L.; Unsworth, K.; Roberts, N. Prenatal Alcohol Exposure and Autism Spectrum Disorder in 39 Children and Adults: Examination of Behavioural and Cognitive Profiles. J. Ment. Health Res. Intellect. Disabil. 2021, 14, 107–121. [Google Scholar] [CrossRef]
- Mukherjee, R.; Layton, M.; Yacoub, E.; Turk, J. Autism and Autistic Traits in People Exposed to Heavy Prenatal Alcohol: Data from a Clinical Series of 21 Individuals and Nested Case Control Study. Adv. Ment. Health Intellect. Disabil. 2011, 5, 42–49. [Google Scholar] [CrossRef]
- Bishop, S.; Gahagan, S.; Lord, C. Re-Examining the Core Features of Autism: A Comparison of Autism Spectrum Disorder and Fetal Alcohol Spectrum Disorder. J. Child Psychol. Psychiatry Allied Discip. 2007, 48, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Choate, P.; Badry, D. Stigma as a Dominant Discourse in Fetal Alcohol Spectrum Disorder. Adv. Dual Diagn. 2019, 12, 36–52. [Google Scholar] [CrossRef]
- Barker, C.; Kulyk, J.; Knorr, L.; Brenna, B. Open Inclusion or Shameful Secret: A Comparison of Characters with Fetal Alcohol Spectrum Disorders (FASD) and Characters with Autism Spectrum Disorders (ASD) in a North American Sample of Books for Children and Young Adults. Int. J. Spec. Educ. 2011, 26, 171–180. [Google Scholar]
- Lange, S.; Probst, C.; Gmel, G.; Rehm, J.; Burd, L.; Popova, S. Estimation of National, Regional, and Global Prevalence of Fetal Alcohol Spectrum Disorder among Children and Youth: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2017, 171, 948–956. [Google Scholar] [CrossRef]
- Chasnoff, I.J.; Wells, A.M.; King, L. Misdiagnosis and Missed Diagnoses in Foster and Adopted Children with Prenatal Alcohol Exposure. Pediatrics 2015, 135, 264–270. [Google Scholar] [CrossRef]
- Berger, S.L.; Kouzarides, T.; Shiekhattar, R.; Shilatifard, A. An Operational Definition of Epigenetics. Genes Dev. 2009, 23, 781–783. [Google Scholar] [CrossRef] [PubMed]
- Waddington, C.H. Canalization of Development and the Inheritance of Acquired Characters. Nature 1942, 150, 563–565. [Google Scholar] [CrossRef]
- Deichmann, U. Epigenetics: The Origins and Evolution of a Fashionable Topic. Dev. Biol. 2016, 416, 249–254. [Google Scholar] [CrossRef]
- Peschansky, V.J.; Wahlestedt, C. Non-Coding RNAs as Direct and Indirect Modulators of Epigenetic Regulation. Epigenetics 2014, 9, 3–12. [Google Scholar] [CrossRef]
- Deichmann, U. The Social Construction of the Social Epigenome and the Larger Biological Context. Epigenet. Chromatin 2020, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pickersgill, M.; Niewöhner, J.; Müller, R.; Martin, P.; Cunningham-Burley, S. Mapping the New Molecular Landscape: Social Dimensions of Epigenetics. New Genet. Soc. 2013, 32, 429–447. [Google Scholar] [CrossRef] [PubMed]
- Norouzitallab, P.; Baruah, K.; Vanrompay, D.; Bossier, P. Can Epigenetics Translate Environmental Cues into Phenotypes? Sci. Total Environ. 2019, 647, 1281–1293. [Google Scholar] [CrossRef]
- Van Otterdijk, S.D.; Michels, K.B. Transgenerational Epigenetic Inheritance in Mammals: How Good Is the Evidence? FASEB J. 2016, 30, 2457–2465. [Google Scholar] [CrossRef]
- Annunziato, A. DNA Packaging: Nucleosomes and Chromatin|Learn Science at Scitable. Nat. Educ. 2008, 1, 26. [Google Scholar]
- Edwards, J.R.; Yarychkivska, O.; Boulard, M.; Bestor, T.H. DNA Methylation and DNA Methyltransferases. Epigenet. Chromatin 2017, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Horsthemke, B. A Critical View on Transgenerational Epigenetic Inheritance in Humans. Nat. Commun. 2018, 9, 1–4. [Google Scholar] [CrossRef]
- Perez, M.F.; Lehner, B. Intergenerational and Transgenerational Epigenetic Inheritance in Animals. Nat. Cell Biol. 2019, 21, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Cedar, H.; Bergman, Y. Linking DNA Methylation and Histone Modification: Patterns and Paradigms. Nat. Rev. Genet. 2009, 10, 295–304. [Google Scholar] [CrossRef]
- Seisenberger, S.; Peat, J.R.; Hore, T.A.; Santos, F.; Dean, W.; Reik, W. Reprogramming DNA Methylation in the Mammalian Life Cycle: Building and Breaking Epigenetic Barriers. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20110330. [Google Scholar] [CrossRef] [PubMed]
- Neidhart, M. DNA Methylation and Complex Human Disease; Academic Press: Cambridge, MA, USA, 2015; ISBN 978-0-12-420194-1. [Google Scholar]
- Dolinoy, D.C.; Huang, D.; Jirtle, R.L. Maternal Nutrient Supplementation Counteracts Bisphenol A-Induced DNA Hypomethylation in Early Development. Proc. Natl. Acad. Sci. USA 2007, 104, 13056–13061. [Google Scholar] [CrossRef]
- Kubsad, D.; Nilsson, E.E.; King, S.E.; Sadler-Riggleman, I.; Beck, D.; Skinner, M.K. Assessment of Glyphosate Induced Epigenetic Transgenerational Inheritance of Pathologies and Sperm Epimutations: Generational Toxicology. Sci. Rep. 2019, 9, 6372. [Google Scholar] [CrossRef]
- Meaney, M.J. Maternal Care, Gene Expression, and the Transmission of Individual Differences in Stress Reactivity across Generations. Annu. Rev. Neurosci. 2001, 24, 1161–1192. [Google Scholar] [CrossRef] [PubMed]
- Weaver, I.C.G.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epigenetic Programming by Maternal Behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov (accessed on 23 June 2023).
- Morgan, H.D.; Sutherland, H.G.E.; Martin, D.I.K.; Whitelaw, E. Epigenetic Inheritance at the Agouti Locus in the Mouse. Nat. Genet. 1999, 23, 314–318. [Google Scholar] [CrossRef]
- Lu, D.; Willard, D.; Patel, I.R.; Kadwell, S.; Overton, L.; Kost, T.; Luther, M. Agouti Protein Is an Antagonist of the Melanocyte-Stimulating-Hormone Receptor. Nature 1994, 371, 799–802. [Google Scholar] [CrossRef] [PubMed]
- Pang, T.Y.; Short, A.K.; Bredy, T.W.; Hannan, A.J. Transgenerational Paternal Transmission of Acquired Traits: Stress-Induced Modification of the Sperm Regulatory Transcriptome and Offspring Phenotypes. Curr. Opin. Behav. Sci. 2017, 14, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Heard, E.; Martienssen, R.A. Transgenerational Epigenetic Inheritance: Myths and Mechanisms. Cell 2014, 157, 95–109. [Google Scholar] [CrossRef]
- Lehrner, A.; Yehuda, R. Cultural Trauma and Epigenetic Inheritance. Dev. Psychopathol. 2018, 30, 1763–1777. [Google Scholar] [CrossRef]
- Brink, R.A. A Genetic Change Associated with the R Locus in Maize Which Is Directed and Potentially Reversible. Genetics 1956, 41, 872–889. [Google Scholar] [CrossRef] [PubMed]
- Coe, E.H. A Regular and Continuing Conversion-Type Phenomenon at the B Locus in Maize. Proc. Natl. Acad. Sci. USA 1959, 45, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Lussier, A.A.; Weinberg, J.; Kobor, M.S. Epigenetics Studies of Fetal Alcohol Spectrum Disorder: Where Are We Now? Epigenomics 2017, 9, 291–311. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.A.; Gluckman, P.D. Developmental Origins of Health and Disease: New Insights. Basic Clin. Pharmacol. Toxicol. 2008, 102, 90–93. [Google Scholar] [CrossRef]
- Herringa, R.J. Trauma, PTSD, and the Developing Brain. Curr. Psychiatry Rep. 2017, 19, 69. [Google Scholar] [CrossRef] [PubMed]
- Lossie, A.C.; Muir, W.M.; Lo, C.-L.; Timm, F.; Liu, Y.; Gray, W.; Zhou, F.C. Implications of Genomic Signatures in the Differential Vulnerability to Fetal Alcohol Exposure in C57BL/6 and DBA/2 Mice. Front. Genet. 2014, 5, 173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mason, S.; Zhou, F.C. Genetics and Epigenetics of Fetal Alcohol Spectrum Disorders. Front. Genet. 2015, 6, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Hoyme, E.H.; Kalberg, W.O.; Elliott, A.J.; Blankenship, J.; Buckley, D.; Marais, A.-S.; Manning, M.A. Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics 2016, 138, e20154256. [Google Scholar] [CrossRef]
- Kleiber, M.L.; Mantha, K.; Stringer, R.L.; Singh, S.M. Neurodevelopmental Alcohol Exposure Elicits Long-Term Changes to Gene Expression That Alter Distinct Molecular Pathways Dependent on Timing of Exposure. J. Neurodev. Disord. 2013, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Hard, M.L.; Abdolell, M.; Robinson, B.H.; Koren, G. Gene-Expression Analysis after Alcohol Exposure in the Developing Mouse. J. Lab. Clin. Med. 2005, 145, 47–54. [Google Scholar] [CrossRef]
- Hashimoto-Torii, K.; Imamura Kawasawa, Y.; Kuhn, A.; Rakic, P. Combined Transcriptome Analysis of Fetal Human and Mouse Cerebral Cortex Exposed to Alcohol. Proc. Natl. Acad. Sci. USA 2011, 108, 4212–4217. [Google Scholar] [CrossRef] [PubMed]
- Lussier, A.A.; Stepien, K.A.; Neumann, S.M.; Pavlidis, P.; Kobor, M.S.; Weinberg, J. Prenatal Alcohol Exposure Alters Steady-State and Activated Gene Expression in the Adult Rat Brain. Alcohol. Clin. Exp. Res. 2015, 39, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Resendiz, M.; Chen, Y.; Oztürk, N.C.; Zhou, F.C. Epigenetic Medicine and Fetal Alcohol Spectrum Disorders. Epigenomics 2013, 5, 73–86. [Google Scholar] [CrossRef]
- Lu, S.C.; Huang, Z.Z.; Yang, H.; Mato, J.M.; Avila, M.A.; Tsukamoto, H. Changes in Methionine Adenosyltransferase and S-Adenosylmethionine Homeostasis in Alcoholic Rat Liver. Am. J. Physiol.-Gastrointest. Liver Physiol. 2000, 279, 178–185. [Google Scholar] [CrossRef]
- Garro, A.J.; McBeth, D.L.; Lima, V.; Lieber, C.S. Ethanol Consumption Inhibits Fetal DNA Methylation in Mice: Implications for the Fetal Alcohol Syndrome. Alcohol Clin. Exp. Res. 1991, 15, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Bonsch, D.; Lenz, B.; Fiszer, R.; Frieling, H.; Kornhuber, J.; Bleich, S. Lowered DNA Methyltransferase (DNMT-3b) MRNA Expression Is Associated with Genomic DNA Hypermethylation in Patients with Chronic Alcoholism. J. Neural Transm. 2006, 113, 1299–1304. [Google Scholar] [CrossRef]
- Streissguth, A.P.; Aase, J.M.; Clarren, S.K.; Randels, S.P.; LaDue, R.A.; Smith, D.F. Fetal Alcohol Syndrome in Adolescents and Adults. JAMA-J. Am. Med. Assoc. 1991, 265, 1961–1967. [Google Scholar] [CrossRef]
- Halsted, C.H.; Medici, V. Aberrant Hepatic Methionine Metabolism and Gene Methylation in the Pathogenesis and Treatment of Alcoholic Steatohepatitis. Int. J. Hepatol. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nagre, N.N.; Subbanna, S.; Shivakumar, M.; Psychoyos, D.; Basavarajappa, B.S. CB1-Receptor Knockout Neonatal Mice Are Protected Against Ethanol-Induced Impairments of DNMT1, DNMT3A and DNA Methylation. J. Neurochem. 2015, 132, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Otero, N.K.H.; Thomas, J.D.; Saski, C.A.; Xia, X.; Kelly, S.J. Choline supplementation and dna methylation in the hippocampus and prefrontal cortex of rats exposed to alcohol during development. Alcohol Clin. Exp. Res. 2012, 36, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Perkins, A.; Lehmann, C.; Lawrence, C.R.; Kelly, S.J. Alcohol Exposure during Development: Impact on the Epigenome. Int. J. Dev. Neurosci. 2013, 31, 391–397. [Google Scholar] [CrossRef]
- Liu, Y.; Balaraman, Y.; Wang, G.; Nephew, K.P.; Zhou, F.C. Alcohol Exposure Alters DNA Methylation Profiles in Mouse Embryos at Early Neurulation. Epigenetics 2009, 4, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Raffin-Sanson, M.L.; de Keyzer, Y.; Bertagna, X. Proopiomelanocortin, a Polypeptide Precursor with Multiple Functions: From Physiology to Pathological Conditions. Eur. J. Endocrinol. 2003, 149, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Bekdash, R.A.; Zhang, C.; Sarkar, D.K. Gestational Choline Supplementation Normalized Fetal Alcohol-Induced Alterations in Histone Modifications, DNA Methylation, and Proopiomelanocortin (POMC) Gene Expression in β-Endorphin-Producing POMC Neurons of the Hypothalamus. Alcohol. Clin. Exp. Res. 2013, 23, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Gangisetty, O.; Sinha, R.; Sarkar, D.K. Hypermethylation of Proopiomelanocortin and Period 2 Genes in Blood Are Associated with Greater Subjective and Behavioral Motivation for Alcohol in Humans. Alcohol Clin. Exp. Res. 2019, 43, 212–220. [Google Scholar] [CrossRef]
- Govorko, D.; Bekdash, R.A.; Zhang, C.; Sarkar, D.K. Male Germline Transmits Fetal Alcohol Adverse Effect on Hypothalamic Proopiomelanocortin Gene across Generations Dmitry. Biol. Psychiatry 2012, 72, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.K.; Gangisetty, O.; Wozniak, J.R.; Eckerle, J.K.; Georgieff, M.K.; Foroud, T.M.; Wetherill, L.; Wertelecki, W.; Chambers, C.D.; Riley, E.; et al. Persistent Changes in Stress-Regulatory Genes in Pregnant Women or Children Exposed Prenatally to Alcohol. Alcohol. Clin. Exp. Res. 2019, 43, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Gangisetty, O.; Chaudhary, S.; Palagani, A.; Sarkar, D.K. Transgenerational Inheritance of Fetal Alcohol Effects on Proopiomelanocortin Gene Expression and Methylation, Cortisol Response to Stress, and Anxiety-like Behaviors in Offspring for Three Generations in Rats: Evidence for Male Germline Transmission. PLoS ONE 2022, 17, e0263340. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. The Diagnostic and Statistical Manual of Mental Disorders: DSM-5TM, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- The National Child Traumatic Stress Network. Available online: https://www.nctsn.org (accessed on 12 December 2020).
- Heim, C.; Newport, D.J.; Mletzko, T.; Miller, A.H.; Nemeroff, C.B. The Link between Childhood Trauma and Depression: Insights from HPA Axis Studies in Humans. Psychoneuroendocrinology 2008, 33, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Saunders, B.E.; Adams, Z.W. Epidemiology of Traumatic Experiences in Childhood. Child Adolesc. Psychiatr. Clin. N. Am. 2014, 23, 167–184. [Google Scholar] [CrossRef] [PubMed]
- McGowan, P.O.; Sasaki, A.; D’Alessio, A.C.; Dymov, S.; Labonté, B.; Szyf, M.; Turecki, G.; Meaney, M.J. Epigenetic Regulation of the Glucocorticoid Receptor in Human Brain Associates with Childhood Abuse. Nat. Neurosci. 2009, 12, 342–348. [Google Scholar] [CrossRef]
- Painter, R.C.; Roseboom, T.J.; Bleker, O.P. Prenatal Exposure to the Dutch Famine and Disease in Later Life: An Overview. Reprod. Toxicol. 2005, 20, 345–352. [Google Scholar] [CrossRef]
- Yehuda, R.; Daskalakis, N.P.; Bierer, L.M.; Bader, H.N.; Klengel, T.; Holsboer, F.; Binder, E.B. Holocaust Exposure Induced Intergenerational Effects on FKBP5 Methylation. Biol. Psychiatry 2016, 80, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Hompes, T.; Izzi, B.; Gellens, E.; Morreels, M.; Fieuws, S.; Pexsters, A.; Schops, G.; Dom, M.; Van Bree, R.; Freson, K.; et al. Investigating the Influence of Maternal Cortisol and Emotional State during Pregnancy on the DNA Methylation Status of the Glucocorticoid Receptor Gene (NR3C1) Promoter Region in Cord Blood. J. Psychiatr. Res. 2014, 56, 165–167. [Google Scholar] [CrossRef]
- Mulligan, C.J.; D’Errico, N.; Stees, J.; Hughes, D.A. Methylation Changes AtNR3C1in Newborns Associate with Maternal Prenatal Stress Exposure and Newborn Birth Weight. Epigenetics 2012, 7, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Oberlander, T.F.; Weinberg, J.; Papsdorf, M.; Grunau, R.; Misri, S.; Devlin, A.M. Prenatal Exposure to Maternal Depression, Neonatal Methylation of Human Glucocorticoid Receptor Gene (NR3C1) and Infant Cortisol Stress Responses. Epigenetics 2008, 3, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Radtke, K.M.; Ruf, M.; Gunter, H.M.; Dohrmann, K.; Schauer, M.; Meyer, A.; Elbert, T. Transgenerational Impact of Intimate Partner Violence on Methylation in the Promoter of the Glucocorticoid Receptor. Transl. Psychiatry 2011, 1, e21. [Google Scholar] [CrossRef]
- Yang, H.H.; Hu, N.; Wang, C.; Ding, T.; Dunn, B.K.; Goldstein, A.M.; Taylor, P.R.; Lee, M.P. Influence of Genetic Background and Tissue Types on Global DNA Methylation Patterns. PLoS ONE 2010, 5, e0009355. [Google Scholar] [CrossRef]
- Waterland, R.A.; Kellermayer, R.; Laritsky, E.; Rayco-Solon, P.; Harris, R.A.; Travisano, M.; Zhang, W.; Torskaya, M.S.; Zhang, J.; Shen, L.; et al. Season of Conception in Rural Gambia Affects DNA Methylation at Putative Human Metastable Epialleles. PLoS Genet. 2010, 6, e1001252. [Google Scholar] [CrossRef]
- Cao-Lei, L.; Massart, R.; Suderman, M.J.; Machnes, Z.; Elgbeili, G.; Laplante, D.P.; Szyf, M.; King, S. DNA Methylation Signatures Triggered by Prenatal Maternal Stress Exposure to a Natural Disaster: Project Ice Storm. PLoS ONE 2014, 9, e0107653. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Bierer, L.M.; Schmeidler, J.; Aferiat, D.H.; Breslau, I.; Dolan, S. Low Cortisol and Risk for PTSD in Adult Offspring of Holocaust Survivors. Am. J. Psychiatry 2000, 157, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Bierer, L.M.; Bader, H.N.; Daskalakis, N.P.; Lehrner, A.; Provençal, N.; Wiechmann, T.; Klengel, T.; Makotkine, I.; Binder, E.B.; Yehuda, R. Intergenerational Effects of Maternal Holocaust Exposure on FKBP5 Methylation. Am. J. Psychiatry 2020, 177, 744–753. [Google Scholar] [CrossRef]
- Yehuda, R.; Daskalakis, N.P.; Lehrner, A.; Desarnaud, F.; Bader, H.N.; Makotkine, I.; Flory, J.D.; Bierer, L.M.; Meaney, M.J. Influences of Maternal and Paternal PTSD on Epigenetic Regulation of the Glucocorticoid Receptor Gene in Holocaust Survivor Offspring. Am. J. Psychiatry 2014, 171, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Burger, G.C.E.; Drummond, J.C.; Sandstead, H.R.; Staatsuitgeverij’s, G. Malnutrition and Starvation in Western Netherlands September 1944–July 1945. Part I. Part II: Appendices. J. Am. Med. Assoc. 1950, 142, 857–858. [Google Scholar] [CrossRef]
- De Rooij, S.R.; Bleker, L.S.; Painter, R.C.; Ravelli, A.C.; Roseboom, T.J. Lessons Learned from 25 Years of Research into Long Term Consequences of Prenatal Exposure to the Dutch Famine 1944–45: The Dutch Famine Birth Cohort. Int. J. Environ. Health Res. 2021, 32, 1432–1446. [Google Scholar] [CrossRef] [PubMed]
- Bleker, L.S.; De Rooij, S.R.; Painter, R.C.; Ravelli, A.C.; Roseboom, T.J. Cohort Profile: The Dutch Famine Birth Cohort (DFBC)—A Prospective Birth Cohort Study in the Netherlands. BMJ Open 2021, 11, e042078. [Google Scholar] [CrossRef] [PubMed]
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent Epigenetic Differences Associated with Prenatal Exposure to Famine in Humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef] [PubMed]
- Tobi, E.W.; Lumey, L.H.; Talens, R.P.; Kremer, D.; Putter, H.; Stein, A.D.; Slagboom, P.E.; Heijmans, B.T. DNA Methylation Differences after Exposure to Prenatal Famine Are Common and Timing- and Sex-Specific. Hum. Mol. Genet. 2009, 18, 4046–4053. [Google Scholar] [CrossRef]
- Veenendaal, M.V.; Costello, P.M.; Lillycrop, K.A.; de Rooij, S.R.; van der Post, J.A.; Bossuyt, P.M.; Hanson, M.A.; Painter, R.C.; Roseboom, T.J. Prenatal Famine Exposure, Health in Later Life and Promoter Methylation of Four Candidate Genes. J. Dev. Orig. Health Dis. 2012, 3, 450–457. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harris, A.; Seckl, J. Glucocorticoids, Prenatal Stress and the Programming of Disease. Horm. Behav. 2011, 59, 279–289. [Google Scholar] [CrossRef]
- Coelho, R.; Viola, T.W.; Walss-Bass, C.; Brietzke, E.; Grassi-Oliveira, R. Childhood Maltreatment and Inflammatory Markers: A Systematic Review. Acta Psychiatr. Scand. 2014, 129, 180–192. [Google Scholar] [CrossRef]
- Juengst, E.T.; Fishman, J.R.; McGowan, M.L.; Settersten, R.A. Serving Epigenetics before Its Time. Trends Genet. 2014, 30, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Lehrner, A.; Bierer, L.M. The Public Reception of Putative Epigenetic Mechanisms in the Transgenerational Effects of Trauma. Environ. Epigenet. 2018, 4, dvy018. [Google Scholar] [CrossRef]
- Carey, B. Can We Really Inherit Trauma? Available online: https://www.nytimes.com/2018/12/10/health/mind-epigenetics-genes.html (accessed on 10 December 2022).
- Fitzgerald, M.; London-Johnson, A.; Gallus, K.L. Intergenerational Transmission of Trauma and Family Systems Theory: An Empirical Investigation. J. Fam. Ther. 2020, 42, 406–424. [Google Scholar] [CrossRef]
- Harding, K.D.; Turner, K.; Howe, S.J.; Bagshawe, M.J.; Flannigan, K.; Mela, M.; McMorris, C.A.; Badry, D. Caregivers’ Experiences and Perceptions of Suicidality among Their Children and Youth with Fetal Alcohol Spectrum Disorder. Front. Psychiatry 2022, 13, 931528. [Google Scholar] [CrossRef] [PubMed]
- Dickson, J.; Stewart, M. Risk, Rights and Deservedness: Navigating the Tensions of Gladue, Fetal Alcohol Spectrum Disorder and Settler Colonialism in Canadian Courts. Behav. Sci. Law 2021, 40, 14–30. [Google Scholar] [CrossRef]
- King, B.; Fallon, B.; Boyd, R.; Black, T.; Antwi-Boasiako, K.; O’Conner, C. Factors Associated with Racial Differences in Child Welfare Investigative Decision-Making in Ontario, Canada. Child Abuse Negl. 2017, 73, 89–105. [Google Scholar] [CrossRef]
- Pei, J.; Carlson, E.; Tremblay, M.; Poth, C. Exploring the Contributions and Suitability of Relational and Community-Centered Fetal Alcohol Spectrum Disorder (FASD) Prevention Work in First Nation Communities. Birth Defects Res. 2019, 111, 835–847. [Google Scholar] [CrossRef]
- Felitti, V.J.; Anda, R.F.; Nordenberg, D.; Williamson, D.F.; Spitz, A.M.; Edwards, V.; Koss, M.P.; Marks, J.S. Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults. Am. J. Prev. Med. 1998, 14, 245–258. [Google Scholar] [CrossRef]
- Andersson, S.-O.; Annerback, E.-M.; Sondergaard, H.P.; Hallqvist, J.; Kristiansson, P. Adverse Childhood Experiences Are Associated with Choice of Partner, Both Partners’ Relationship and Psychosocial Health as Reported One Year after Birth of a Common Child. A Cross-Sectional Study. PLoS ONE 2021, 16, e0244696. [Google Scholar] [CrossRef]
- Kambeitz, C.; Klug, M.G.; Greenmyer, J.; Popova, S.; Burd, L. Association of Adverse Childhood Experiences and Neurodevelopmental Disorders in People with Fetal Alcohol Spectrum Disorders (FASD) and Non-FASD Controls. BMC Pediatr. 2019, 19, 498. [Google Scholar] [CrossRef] [PubMed]
- Griffin, G. Defining Trauma and a Trauma-Informed COVID-19 Response. Psychol. Trauma Theory Res. Pract. Policy 2020, 12, S279–S280. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada. Canada’s Residential Schools: The Final Report of the Truth and Reconciliation Commission of Canada; (v.1, pt.1: PDF); McGill-Queen’s University Press: Montreal, GC, Canada, 2015; ISBN 978-0-7735-9817. [Google Scholar]
- Buller, M.; Audette, M.; Robinson, Q.; Evolfson, B. Reclaiming Power and Place. The Final Report of the National Inquiry into Missing and Murdered Indigenous Women and Girls Reclaiming Power and Place Volume 1a; CP32-163/2-1-2019E-PDF; National Inquirty into Missing and Murdered Indigenous Women and Girls: Gatineau, QC, Canada, 2019. [Google Scholar]
- Burczycka, M. Section 1: Profile of Canadian Adults Who Experienced Childhood Maltreatment. Available online: https://www150.statcan.gc.ca/n1/pub/85-002-x/2017001/article/14698/01-eng.htm (accessed on 15 November 2020).
- Greenwood, M.; de Leeuw, S.; Lindsay, N.M. Determinants of Indigenous Peoples’ Health: Beyond the Social; Canadian Scholars: Toronto, ON, Canada, 2015; ISBN 978-1-55130-732-9. [Google Scholar]
- Ryan, J.; Chaudieu, I.; Ancelin, M.L.; Saffery, R. Biological Underpinnings of Trauma and Post-Traumatic Stress Disorder: Focusing on Genetics and Epigenetics. Epigenomics 2016, 8, 1553–1569. [Google Scholar] [CrossRef] [PubMed]
- Youssef, N.A.; Lockwood, L.; Su, S.; Hao, G.; Rutten, B.P.F. The Effects of Trauma, with or without PTSD, on the Transgenerational DNA Methylation Alterations in Human Offsprings. Brain Sci. 2018, 8, 83. [Google Scholar] [CrossRef]
- Matthews, S.G.; Phillips, D.I. Transgenerational Inheritance of Stress Pathology. Exp. Neurol. 2012, 233, 95–101. [Google Scholar] [CrossRef]
- Maylea, C. The End of Social Work. Br. J. Soc. Work 2020, 51, 772–789. [Google Scholar] [CrossRef]
- Combs-Orme, T. Epigenetics and the Social Work Imperative. Soc. Work 2013, 58, 23–30. [Google Scholar] [CrossRef] [PubMed]
- White, S.J.; Wastell, D.G. Epigenetics Prematurely Born(e): Social Work and the Malleable Gene. Br. J. Soc. Work 2016, 47, 2256–2272. [Google Scholar] [CrossRef]
- Wastell, D.; White, S. Blinded by Science. The Social Implications of Epigenetics and Neuroscience; Policy Press: Bristol, UK, 2017; ISBN 978-1447322344. [Google Scholar]
- Lappé, M. Epigenetics, Media Coverage, and Parent Responsibilities in the Post-Genomic Era. Curr. Genet. Med. Rep. 2016, 4, 92–97. [Google Scholar] [CrossRef]
- Liberman, N.; Wang, S.Y.; Greer, E.L. Transgenerational Epigenetic Inheritance: From Phenomena to Molecular Mechanisms. Curr. Opin. Neurobiol. 2019, 59, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Gladkova, M.G.; Leidmaa, E.; Anderzhanova, E.A. Epidrugs in the Therapy of Central Nervous System Disorders: A Way to Drive On? Cells 2023, 12, 1464. [Google Scholar] [CrossRef]
- Motz, M.; Andrews, N.C.Z.; Bondi, B.C.; Leslie, M.; Pepler, D.J. Addressing the Impact of Interpersonal Violence in Women Who Struggle with Substance Use through Developmental-Relational Strategies in a Community Program. Int. J. Environ. Res. Public Health 2019, 16, 4197. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orton, S.M.; Millis, K.; Choate, P. Epigenetics of Trauma Transmission and Fetal Alcohol Spectrum Disorder: What Does the Evidence Support? Int. J. Environ. Res. Public Health 2023, 20, 6706. https://doi.org/10.3390/ijerph20176706
Orton SM, Millis K, Choate P. Epigenetics of Trauma Transmission and Fetal Alcohol Spectrum Disorder: What Does the Evidence Support? International Journal of Environmental Research and Public Health. 2023; 20(17):6706. https://doi.org/10.3390/ijerph20176706
Chicago/Turabian StyleOrton, Sarah M., Kimberly Millis, and Peter Choate. 2023. "Epigenetics of Trauma Transmission and Fetal Alcohol Spectrum Disorder: What Does the Evidence Support?" International Journal of Environmental Research and Public Health 20, no. 17: 6706. https://doi.org/10.3390/ijerph20176706
APA StyleOrton, S. M., Millis, K., & Choate, P. (2023). Epigenetics of Trauma Transmission and Fetal Alcohol Spectrum Disorder: What Does the Evidence Support? International Journal of Environmental Research and Public Health, 20(17), 6706. https://doi.org/10.3390/ijerph20176706






