Abstract
(1) Background: A substantial proportion of COVID-19 patients continue to experience long-lasting effects that hamper their quality of life. The objectives of this study were (1) to report the prevalence of persistent clinical symptoms 6–12 months after the onset of COVID-19 and (2) to identify potential factors at admission associated with the occurrence of long COVID. (2) Methods: A prospective study was conducted among COVID-19 adult patients, hospitalized in four French university hospitals. Patients were invited to two ambulatory follow-up medical visits, 6–8 months (visit #1) and one year (visit #2) after the onset of their COVID-19. A multivariate logistic regression was performed to assess factors associated with long COVID. (3) Results: In total, 189 patients participated in this study (mean age of 63.4 years). BMI > 30 kg/m2 (aOR 3.52), AST levels between 31 and 42 U/L (aOR 8.68), and AST levels > 42 U/L (aOR 3.69) were associated with persistent clinical symptoms at visit #1. Anosmia (aOR 13.34), AST levels between 31 and 42 U/L (aOR 10.27), stay in ICU (aOR 5.43), pain (aOR 4.31), and longer time before hospitalization (aOR 1.14) were significantly associated with persistent clinical symptoms at visit #2. Patients with ageusia (aOR 0.17) had a lower risk of long COVID. (4) Conclusions: This study showed that some patients experienced persistent clinical symptoms one year after COVID-19 onset that were associated with some determinants at the acute phase/stage.
1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and can manifest a wide spectrum of disease severity, from asymptomatic to fatal forms [1]. More than two years after the start of COVID-19, a growing proportion of the population who have recovered from acute infection have suffered from persistent symptomatology that has lasted from weeks to years. This status is defined as ”long COVID” or ”post-acute sequelae of SARS-CoV-2 infection” [2]. Long COVID consists of a set of symptoms related to the respiratory, cardiovascular, neurological, endocrine, urinary, and immune systems. Patients suffering from long COVID display persistent or relapsing or new symptoms that include fatigue, dyspnea, cough, myalgia, cardiac or skin anomalies, sleep trouble, post-traumatic stress disorder, and cognitive impairment [3].
The risk factors and the extent of the severity of clinical symptoms of long COVID are subject to debate. The persistence and severity of long COVID appear to be associated with the severity at the acute phase onset (such as the need for mechanical ventilation, admission to the intensive care unit (ICU), the presence of comorbidities, older age, and others. However, these findings have not been confirmed in different populations [1,4,5]. This may be explained by the heterogeneity of the included populations, collected data, variables included in multivariate models, severity of illness, and the follow-up period [6,7,8]. There have been many attempts to organize and systematize post-COVID symptoms. In a recent systematic review and meta-analysis, more than 50 symptoms were reported to be associated with sequelae of COVID-19. Furthermore, 80% of patients with COVID-19 reported having one or more symptoms for more than two and up to 16 weeks after recovery [9]. A large cross-sectional population-based cohort in France found that persistent physical symptoms, 10–12 months after the first wave of the COVID-19 pandemic, were more associated with a patient’s belief of having SARS-CoV-2 infection rather than laboratory-confirmed COVID-19 [10]. The objectives of this study were (1) to report the prevalence of persistent clinical symptoms 6–12 months after the first episode of COVID-19 and (2) to identify potential factors at admission associated with the occurrence of long COVID in patients hospitalized in four French university hospitals.
2. Materials and Methods
2.1. Study Design
A prospective study was conducted among laboratory-confirmed COVID-19 patients hospitalized in four university-affiliated hospitals (Hospices Civils de Lyon, Lyon, France) to evaluate the prevalence of persistent clinical symptoms 6–12 months after the first episode of COVID-19. Then, a case-control study was completed to evaluate the factors associated with long COVID. Cases were patients who presented at least one persistent clinical symptom, and controls were asymptomatic patients.
2.2. Study Population
Laboratory-confirmed COVID-19 patients who had been previously enrolled in the NOSO-COR cohort study [11] were eligible to participate. This study was approved by the National Ethical Committee (Comité de Protection des Personnes, Ile de France V, 14 October 2020), registered in ClinicalTrials (NCT04637867), and conducted in accordance with Good Clinical Practices of the European General Data Protection Regulation dated as of 25 May 2018 (“GDPR”), (collectively, “Data Protection Legislation”).
2.3. Patients and Follow-up Visits
After the exclusion of deceased NOSO-COR patients and those who had several hospitalizations (to reduce confounding issues, such as disability related to other illnesses), the remaining 762 patients who had been previously hospitalized in one of the four Lyon University affiliated hospitals were informed about the study by a letter sent to their domicile. A total of 189 patients agreed to participate in the study. These patients were invited to two ambulatory follow-ups, at 6–8 months and at one year after the initial COVID-19 onset.
Written consent was obtained at the first medical visit. At each visit, the patients were interviewed by the study physician about persistent or newly occurring symptoms, any medical event during the window time, close contact with confirmed COVID-19 patients, and influenza and/or COVID-19 vaccination since their initial hospital discharge. Blood, nasopharyngeal, and sputum samples were collected at each visit.
Data regarding the initial episode were previously described in the NOSO-COR cohort study [11].
2.4. Definitions
Long COVID was defined as the presence of at least one persisting clinical symptom of COVID-19 since hospital discharge [12]. We did not investigate cognitive and psychological disorders.
2.5. Outcomes
The primary outcome was to estimate the prevalence of long COVID, defined as the persistence of at least one clinical symptom (i.e., fatigue, dyspnea, cough, headache, pain, anosmia, ageusia, diarrhea), six months to one year after COVID-19 onset.
The secondary endpoint was the identification of potential factors associated with long COVID based on the clinical, laboratorial, and X-ray features of their first episode of acute COVID-19.
2.6. Statistical Analysis
A descriptive analysis was performed, and the frequency of long COVID symptoms was determined. Continuous variables are summarized by mean (min–max) and categorical variables are described by frequency and percentage. A Shapiro–Wilk test was used to assess the normality of data. The comparison of categorical variables was performed by using Fisher or chi-square tests and Student or Mann–Whitney tests for continuous variables. A multivariate logistic regression analysis with stepwise forward selection was performed to assess factors associated with long COVID. A p-value of ≤0.20 in the univariate analysis was defined as the criteria for model inclusion. At the second visit, univariate and multivariate analyses were limited to patients who suffered from persistent clinical symptoms at the first and second visits. These patients were compared to asymptomatic patients for both visits. A stepwise regression was designed to find the most parsimonious set of predictors that are most effective in predicting the dependent variable (i.e., long COVID, yes or no according to our definition). Variables are added to the logistic regression equation one at a time, using the statistical criterion of reducing the likelihood error for the included variables. The Hosmer–Lemeshow goodness-of-fit test was used to assess the model-to-data fit. The results were expressed as adjusted odds ratios (aORs) and their 95% confidence intervals (CI 95%). All p-values were two-tailed and p < 0.05 was considered to be statistically significant. Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 21.0. IBM Corp: Armonk, NY, USA.
3. Results
3.1. Patient Characteristics
Between November 2020 and May 2021, 189 COVID-19 patients, who had been hospitalized during the first wave of the pandemic in France (26 February to 19 June 2020), agreed to participate in this study. The demographic characteristics of these patients were similar to those informed about this study. Table 1 summarizes the demographic and clinical characteristics of the study population at hospital admission. The mean age was 63.4 years (min–max, 25–93) and 51% of the participants were over 65 years old. Males represented 59.8% of the study participants, and 57% of the participants had never smoked. The mean body mass index (BMI) was 27.4 kg/m2 (16.4–44.4). In total, 151 patients (79.9%) had underlying comorbidities, mainly cardiovascular diseases (44.4%), hypertension (36%), and diabetes (19%). The mean number of symptoms during the acute COVID-19, was 5 (min–max, 1–9) with a mean duration of 24.5 days (min–max, 2–1260; they included cough (79.8%), fatigue (73.9%), shortness of breath (66%), pain (37.8%), and diarrhea (33.5%). Among the 189 participants, 64 (34.0%) participants required hospitalization in intensive care units (ICU) with a mean length of stay of 20.0 days (min–max, 1–1840. The biological results were in the mean range except for aspartate transaminase (AST), C-reactive protein (CRP), lactate dehydrogenase (LDH), and urea levels.

Table 1.
Characteristics of included patients in a prospective study in 4 French university hospitals.
3.2. Prevalence of Clinical Symptoms at Follow-up Visits
The prevalence of persistent clinical symptoms at follow-up visits are summarized in Table 2. Among the 178 patients who completed the two follow-up visits, 86 (48.3%) patients had reported persistent clinical symptoms since the beginning of their infection. The prevalence of clinical symptoms at the acute phase, i.e., visit #1, and visit #2, are shown in Figure 1.

Table 2.
Prevalence of persistent clinical symptoms at the 2 follow-up visits in included patients.
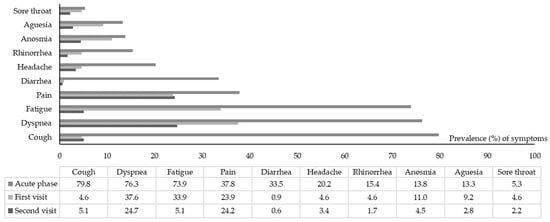
Figure 1.
Prevalence of clinical symptoms at the acute phase, visit #1 and visit #2 in included patients.
Overall, 109 (57.7%) and 103 (57.9%) patients declared the persistence of at least one symptom at visit #1 and visit #2, respectively. New clinical symptoms were reported by 27 (14.3%) patients at visit #1 and 9 (5.1%) patients at visit #2.
The most persistent clinical symptoms at visit #1 (mean 8.8 months) were dyspnea (37.6%), fatigue (33.9%), and pain (23.9%). The mean number of symptoms decreased significantly from five symptoms (min–max, 1–9) at the onset to 1.5 (min–max, 0–10) at visit #1 (p < 0.001). The scatter plot does not depict a correlation between the number of clinical symptoms at the onset of COVID-19 infection and those self-reported at visit #1 (p = 0.10).
The most reported symptoms at visit #2 (mean 12 months) were dyspnea (24.7%) and pain (24.2%). New-onset clinical symptoms, mainly fatigue, were reported in nine patients (5.1%). The number of persistent clinical symptoms was significantly lower than described at the onset of COVID-19 (p < 0.001); they showed no correlation (p = 0.21).
3.3. Factors Associated with Persistent Clinical Symptoms at Follow-up
Table 3 and Table 4 summarize the results of the univariate and multivariate regression analyses. The univariate logistic regression showed that a body mass index (BMI) >30 kg/m2, ICU admission or complications during initial hospitalization, duration of initial symptoms, and aspartate transaminase (AST) levels between 31 and 42 U/L were significantly associated with persistent clinical symptoms at the two visits.

Table 3.
Factors associated with persistent clinical symptom(s) at first and second follow-up visits in included patients’ results of univariate analysis.

Table 4.
Factors independently associated with persistent clinical symptom(s) at first and second follow-up visits in included patients’ results of the multivariate logistic regression.
In the multivariate analysis, BMI > 30 kg/m2 (aOR 3.52, 95% CI 1.25–9.91), AST level between 31 and 42 U/L (aOR 8.68, 95% CI 2.41–31.28), and AST level >42 U/L (aOR 3.69, 95% CI 1.32–10.31) were associated with persistent clinical symptoms at visit #1.
Anosmia (aOR 13.34, 95% CI 2.07–86.21), AST levels between 31 and 42 U/L (aOR 10.27, 95% CI 1.88–56.29), stay in ICU (aOR 5.43, 95% CI 1.39–21.25), pain (aOR 4.31, 95% CI 1.23–15.05), and longer time between the onset of clinical symptoms and hospitalization (aOR 1.14, 95% CI 1.01–1.29) were independently associated with persistent symptoms 12 months after acute COVID-19. While patients who suffered from ageusia (aOR 0.17, 95% CI 0.03–0.92) had a lower risk of long COVID, patients with rheumatic diseases (aOR 5.25, 95% CI 0.75–36.84) or a BMI > 30 kg/m2 (aOR 3.80, 95% CI 0.97–14.91) showed a higher risk of long COVID; the latter association was not statistically significant.
4. Discussion
After recovery from acute COVID-19, some patients continue to experience symptoms and disabilities weeks to months after the initial episode of COVD-19; a diagnosis of long COVID syndrome should be considered if other alternative diagnoses have been excluded [13]. The pathophysiological mechanisms underlying post-acute COVID-19 symptoms are likely to be multifactorial. In this study, long COVID was reported in 48% of patients one year after the initial hospitalization.
Previous studies on the prevalence of long COVID, summarized in a systematic review, found that the median proportion of patients with at least one persistent clinical symptom ≥60 days after diagnosis or at least 30 days after recovery was 72.5% [7]; 50–70% hospitalized patients did not fully recover [14]. The prevalence of persistent COVID-19 was high in the herein study, as well as in the previous systematic review. Lower prevalence of persistent clinical symptoms has been reported in a community-based study in the Netherlands [15]. The estimation of prevalence depends on the length of follow-up, the included population, and the definition of long COVID, which can explain the heterogenic results in the literature [7].
The most reported long COVID symptoms during both visits were pain and dyspnea, while fatigue was mainly reported during the first visit. Other studies have reported similar rates and patterns of long COVID symptoms [6,16,17]. In the WHO Delphi procedure, fatigue and dyspnea were considered to be the most important symptoms of the post-COVID-19 condition [12]; however, in our patients, other distinctive symptoms were reported, including pain (mainly myalgia), ageusia, or anosmia. These symptoms were recently described in a study of the general population [15].
Our results showed that ICU admission was significantly associated with an increased risk of long COVID. Previous studies have described a high burden of persistent clinical symptoms in patients admitted to the ICU for severe and critical COVID-19, but the follow-up was only 2 to 6 months following ICU discharge [18,19,20]. Before the pandemic, post-intensive care syndrome was described in patients admitted to the ICU [21], which highlighted the need to find a management plan through both pharmacologic and non-pharmacologic modalities to be used for this group of patients.
Our results showed consistency with previous reports that anosmia in the acute phase was associated with a higher risk of long COVID. Respiratory viruses are known to cause qualitative olfactory disorders [22,23,24]. The incidence of olfactory disturbances after COVID-19 is very high and its persistence increases patient anxiety and reduces quality of life [25]. In addition to olfactory impairment, the detection of both SARS-CoV-2 RNA and protein in brain tissue is considered to be an important diagnostic and prognostic indicator for long-term neurological complications associated with COVID-19 [26].
Our study showed that AST levels between 31 and 42 U/L are independently associated with long COVID. Abnormal liver function and liver injury related to COVID-19 during hospitalization have been reported, and they can persist as long as one year after hospital discharge. [27]. Alterations in liver function are associated with fatigue and muscle pain, prompting the screening of liver function tests on admission and during the follow-up period.
At admission, 38% of patients reported pain (muscle pain, joint pain, chest pain, or abdominal pain). The multivariate analysis showed that this symptom was associated with long COVID one year after the initial episode of COVID-19. Indeed, fatigue is thought to be a long-lasting symptom of post-COVID with a slow recovery curve during the following years after the infection, regardless of the SARS-CoV-2 variant [28]. There is increasing evidence that individuals with long COVID share common symptoms with myalgic encephalomyelitis/chronic fatigue syndrome. A better understanding of the mechanisms behind post-COVID-19 fatigue is needed to improve the management of patients suffering from long COVID [29].
The main strength of this study was its prospective design in which the data were collected starting from the first hospital admission in addition to a scheduled assessment of persistent or newly occurring clinical symptoms after hospital discharge. Furthermore, the included patients were followed as long as 12 months after the acute COVID-19. Currently, the most frequently used description for long COVID refers to symptoms that last more than three months after the onset [30]. RT-PCR tests were performed for both visits, which permitted us to decrease the likelihood that persistent clinical symptoms could be related to a new episode of COVID-19. Finally, face-to-face interviews with physicians reduced information bias and allowed an accurate data collection.
However, the relatively small sample size limited the statistical power for detecting differences, and the study population was limited to those previously hospitalized in Lyon University hospitals, which biased the representativeness of our sample. Second, the symptoms we studied herein have already been reported in previous reports on long COVID. Third, the prevalence of persistent clinical symptoms was based on what was subjectively reported by the included patients, which could have been influenced by psychological and cognitive factors, especially in the elderly. In fact, cognitive and psychological disorders were not investigated in this study. Davies et al. suggested that a pre-existing mental health condition may be associated with vulnerabilities in executive and cognitive function revealed by SARS-CoV-2 infection [31]. Prior history of mental illness may mitigate the psychological burden of long-term symptomatology of COVID-19 [32]. Currently, no agreement exists on a label for post-COVID-19 cognitive/mental disorders. The time of symptom onset after acute infection and the duration is still debated. Memory and attention-executive complaints and deficits, together with fatigue, anxiety, and depression symptoms are constantly described, but the objective assessment of these symptoms is still not standardized [33]. Lastly, because of all the limitations in our research, we are unable to conclude that the results given are applicable for all populations.
5. Conclusions
The long-term sequelae of COVID-19 is now considered to be a public health issue with great challenges for the community and health economic system. Multiple studies have confirmed the long-lasting multisystemic effects of COVID-19 [30]. Despite primary guidance from different studies, there is not yet a standardized approach to identify clinical manifestations and risk factors of post-COVID-19 symptoms. This issue is further complicated by the lack of a widely accepted definition, timeline, classification and diagnostic criteria, and treatment recommendations. What the global medical community needs at this time is a consensus definition of this long COVID syndrome [34]. Clinical characteristics of the disease will help in appropriate diagnosis, care, public health interventions and policy, and resource planning. Empirical data on the scale and scope of the problem are urgently needed to support the development of appropriate public health responses [15,35].
Author Contributions
Conceptualization, M.S.-E. and P.V.; methodology, M.S.-E., L.H. and P.V.; data collection, S.B., A.T., M.P., R.C., C.M., C.P., N.T.-M. and F.D.; virologic investigation, V.E.; validation, N.K., L.H., M.S.-E. and P.V.; formal analysis, N.K.; writing—original draft preparation, N.K.; writing—review and editing, N.K., M.S.-E., S.B., L.H. and P.V.; visualization, N.K.; supervision, P.V. All authors have read and agreed to the published version of the manuscript.
Funding
This study has benefited funding from the Agence Nationale de Recherches sur le Sida et les Hépatites Virales (ANRS-0154 and ANRS-0174).
Institutional Review Board Statement
The study was approved by the National ethical committee (Comité de Protection des Personnes, Ile de France V, 14 October 2020) and registered in ClinicalTrials (NCT04637867) and conducted in accordance with Good Clinical Practices.
Informed Consent Statement
Written consent was obtained at the first visit presentation.
Data Availability Statement
Data can be available on specific demand to be addressed to the corresponding author.
Acknowledgments
Julie Haesebaert, Florence Morphin-Sherpa, Nathalie Paquin, Vinca Icard, Benjamin Leveau, Sarah Vautier, Magali Herve, Morgan Taillardet and Jacques Cussey. The authors wish to thank Martin Moussy for editing this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Frere, J.J.; tenOever, B.R. Cardiometabolic syndrome-an emergent feature of Long COVID? Nat. Rev. Immunol. 2022, 22, 399–400. [Google Scholar] [CrossRef]
- Sapkota, H.R.; Nune, A. Long COVID from rheumatology perspective-a narrative review. Clin. Rheumatol. 2021, 41, 337–348. [Google Scholar] [CrossRef]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef]
- Sugiyama, A.; Miwata, K.; Kitahara, Y.; Okimoto, M.; Abe, K.; Bunthen, E.; Ouoba, S.; Akita, T.; Tanimine, N.; Ohdan, H.; et al. Long COVID occurrence in COVID-19 survivors. Sci. Rep. 2022, 12, 6039. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Nasserie, T.; Hittle, M.; Goodman, S.N. Assessment of the Frequency and Variety of Persistent Symptoms Among Patients with COVID-19: A Systematic Review. JAMA Netw. Open 2021, 4, e2111417. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Hoang, V.T.; Dao, T.L.; Dudouet, P.; Eldin, C.; Gautret, P. Clinical patterns of somatic symptoms in patients suffering from post-acute long COVID: A systematic review. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 515–545. [Google Scholar] [CrossRef]
- Haider, H.F.; Szczepek, A.J. Editorial: Neurotological consequences of long COVID. Front. Neurol. 2022, 13, 1087896. [Google Scholar] [CrossRef]
- Matta, J.; Wiernik, E.; Robineau, O.; Carrat, F.; Touvier, M.; Severi, G.; de Lamballerie, X.; Blanché, H.; Deleuze, J.F.; Gouraud, C.; et al. Association of Self-reported COVID-19 Infection and SARS-CoV-2 Serology Test Results With Persistent Physical Symptoms Among French Adults during the COVID-19 Pandemic. JAMA Intern. Med. 2021, 182, 19–25. [Google Scholar] [CrossRef]
- Saadatian-Elahi, M.; Picot, V.; Henaff, L.; Pradel, F.K.; Escuret, V.; Dananche, C.; Elias, C.; Endtz, H.P.; Vanhems, P. Protocol for a prospective, observational, hospital-based multicentre study of nosocomial SARS-CoV-2 transmission: NOSO-COR Project. BMJ Open 2020, 10, e039088. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V.; on behalf of the WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Scherlinger, M.; Felten, R.; Gallais, F.; Nazon, C.; Chatelus, E.; Pijnenburg, L.; Mengin, A.; Gras, A.; Vidailhet, P.; Arnould-Michel, R.; et al. Refining “Long-COVID” by a Prospective Multimodal Evaluation of Patients with Long-Term Symptoms Attributed to SARS-CoV-2 Infection. Infect. Dis. Ther. 2021, 10, 1747–1763. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Ballering, A.V.; van Zon, S.K.R.; Olde Hartman, T.C.; Rosmalen, J.G.M.; Lifelines Corona Research Initiative. Persistence of somatic symptoms after COVID-19 in the Netherlands: An observational cohort study. Lancet 2022, 400, 452–461. [Google Scholar] [CrossRef]
- Carvalho-Schneider, C.; Laurent, E.; Lemaignen, A.; Beaufils, E.; Bourbao-Tournois, C.; Laribi, S.; Flament, T.; Ferreira-Maldent, N.; Bruyere, F.; Stefic, K.; et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin. Microbiol. Infect. 2021, 27, 258–263. [Google Scholar] [CrossRef]
- Arnold, D.T.; Hamilton, F.W.; Milne, A.; Morley, A.J.; Viner, J.; Attwood, M.; Noel, A.; Gunning, S.; Hatrick, J.; Hamilton, S.; et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: Results from a prospective UK cohort. Thorax 2021, 76, 399–401. [Google Scholar] [CrossRef]
- D’Cruz, R.F.; Waller, M.D.; Perrin, F.; Periselneris, J.; Norton, S.; Smith, L.J.; Patrick, T.; Walder, D.; Heitmann, A.; Lee, K.; et al. Chest radiography is a poor predictor of respiratory symptoms and functional impairment in survivors of severe COVID-19 pneumonia. ERJ Open Res. 2021, 7, 00655–02020. [Google Scholar] [CrossRef]
- Taboada, M.; Rodriguez, N.; Diaz-Vieito, M.; Dominguez, M.J.; Casal, A.; Riveiro, V.; Carinena, A.; Moreno, E.; Pose, A.; Valdes, L.; et al. Quality of life and persistent symptoms after hospitalization for COVID-19. A prospective observational study comparing ICU with non-ICU patients. Rev. Esp. Anestesiol. Reanim. 2022, 69, 326–335. [Google Scholar] [CrossRef]
- Peghin, M.; Palese, A.; Venturini, M.; De Martino, M.; Gerussi, V.; Graziano, E.; Bontempo, G.; Marrella, F.; Tommasini, A.; Fabris, M.; et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin. Microbiol. Infect. 2021, 27, 1507–1513. [Google Scholar] [CrossRef]
- Needham, D.M.; Davidson, J.; Cohen, H.; Hopkins, R.O.; Weinert, C.; Wunsch, H.; Zawistowski, C.; Bemis-Dougherty, A.; Berney, S.C.; Bienvenu, O.J.; et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit. Care Med. 2012, 40, 502–509. [Google Scholar] [CrossRef]
- Wang, J.H.; Kwon, H.J.; Jang, Y.J. Detection of parainfluenza virus 3 in turbinate epithelial cells of postviral olfactory dysfunction patients. Laryngoscope 2007, 117, 1445–1449. [Google Scholar] [CrossRef]
- Suzuki, M.; Saito, K.; Min, W.P.; Vladau, C.; Toida, K.; Itoh, H.; Murakami, S. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope 2007, 117, 272–277. [Google Scholar] [CrossRef]
- Konstantinidis, I.; Haehner, A.; Frasnelli, J.; Reden, J.; Quante, G.; Damm, M.; Hummel, T. Post-infectious olfactory dysfunction exhibits a seasonal pattern. Rhinology 2006, 44, 135–139. [Google Scholar]
- Gary, J.B.; Gallagher, L.; Joseph, P.V.; Reed, D.; Gudis, D.A.; Overdevest, J.B. Qualitative Olfactory Dysfunction and COVID-19: An Evidence-Based Review with Recommendations for the Clinician. Am. J. Rhinol. Allergy 2022, 37, 95–101. [Google Scholar] [CrossRef]
- Park, J.W.; Wang, X.; Xu, R.H. Revealing the mystery of persistent smell loss in Long COVID patients. Int. J. Biol. Sci. 2022, 18, 4795–4808. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Du, J.; Chen, S.; Chen, S.; Li, J.; Shen, B. Changes in Serum Liver Function for Patients with COVID-19: A 1-Year Follow-Up Study. Infect. Drug Resist. 2022, 15, 1857–1870. [Google Scholar] [CrossRef]
- Fernandez-de-Las-Penas, C.; Notarte, K.I.; Peligro, P.J.; Velasco, J.V.; Ocampo, M.J.; Henry, B.M.; Arendt-Nielsen, L.; Torres-Macho, J.; Plaza-Manzano, G. Long-COVID Symptoms in Individuals Infected with Different SARS-CoV-2 Variants of Concern: A Systematic Review of the Literature. Viruses 2022, 14, 2629. [Google Scholar] [CrossRef]
- Mackay, A. A Paradigm for Post-COVID-19 Fatigue Syndrome Analogous to ME/CFS. Front. Neurol. 2021, 12, 701419. [Google Scholar] [CrossRef]
- Guziejko, K.; Talalaj, J.; Czupryna, P.; Moniuszko-Malinowska, A. Long COVID. Przegl. Epidemiol. 2022, 76, 287–295. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Rastogi, R.; Cerda, I.H.; ElTohamy, A.; Chen, J.A.; Stevens, C.; Liu, C.H. Long COVID and psychological distress in young adults: Potential protective effect of a prior mental health diagnosis. J. Affect. Disord. 2023, 340, 639–648. [Google Scholar] [CrossRef]
- Nicotra, A.; Masserini, F.; Calcaterra, F.; Di Vito, C.; Doneddu, P.E.; Pomati, S.; Nobile-Orazio, E.; Riva, A.; Mavilio, D.; Pantoni, L. What do we mean by long-COVID? A scoping review of the cognitive sequelae of SARS-CoV-2 infection. Eur. J. Neurol. 2023, 0, 1–11. [Google Scholar] [CrossRef]
- Baimukhamedov, C.; Mirakhmedova, K.; Dossybayeva, G. Long COVID: The time has come for globally acceptable definitions. Rheumatol. Int. 2023. [Google Scholar] [CrossRef]
- Brightling, C.E.; Evans, R.A. Long COVID: Which symptoms can be attributed to SARS-CoV-2 infection? Lancet 2022, 400, 411–413. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).