Characterizing the Contribution of Indoor Residential Phthalate and Phthalate Alternative Dust Concentrations to Internal Dose in the US General Population: An Updated Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Study Inclusion and Exclusion Criteria
2.2. Systematic Literature Search
2.3. Screening of Studies
2.4. Strength of Evidence Assessment
2.5. Meta-Analysis of Phthalate and Phthalate Alternative Dust Concentrations
2.6. Sensitivity Analysis Exploring the Impact of Studies with a High Risk of Bias
2.7. Daily Intake Rate Assessment from Dust Concentrations
2.8. Total Daily Intake-Rate Estimated from Metabolite Concentrations in Urine
2.9. Comparing Daily Intake Rates
2.10. Hazard Identification
3. Results and Discussion
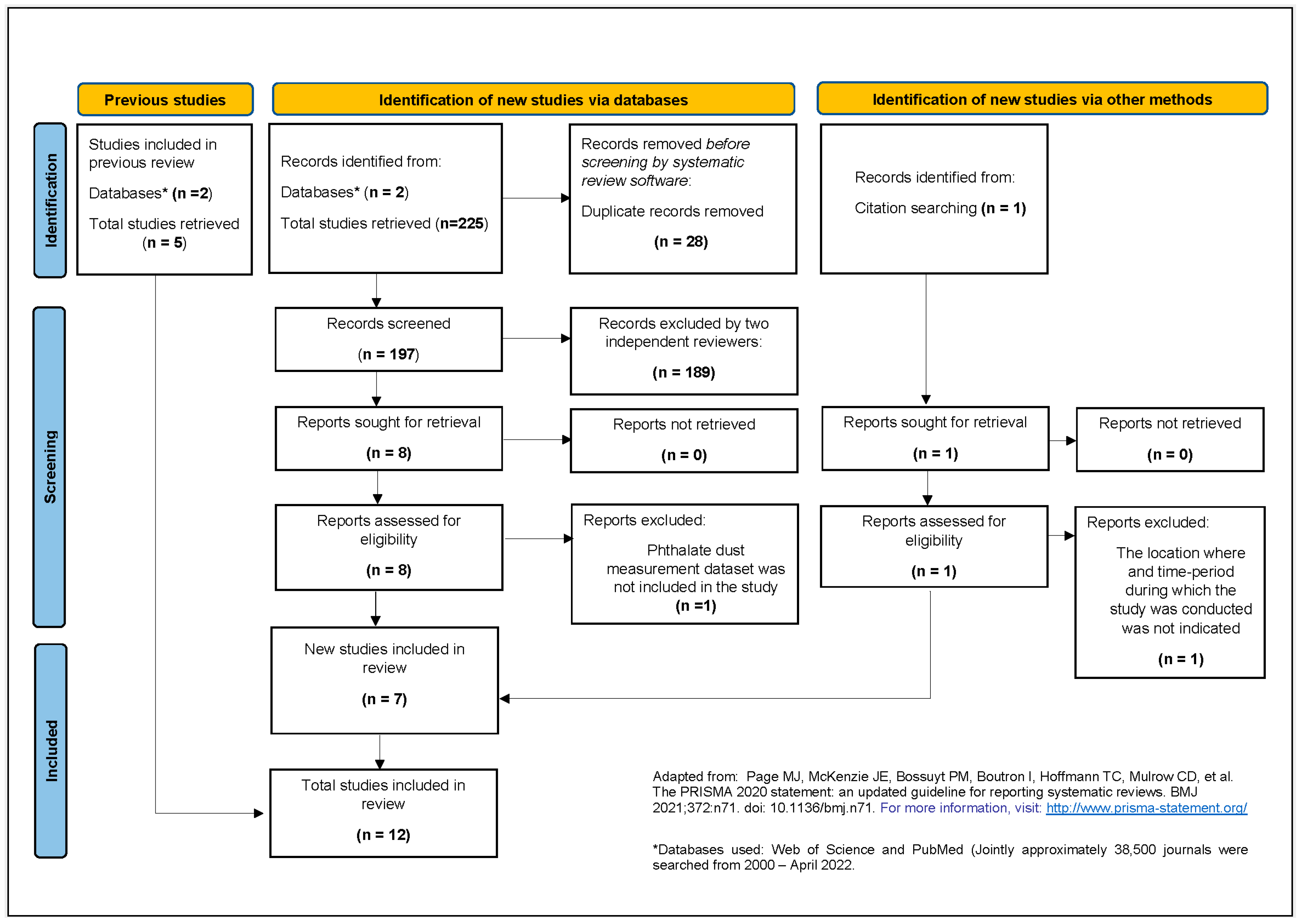
3.1. Systematic Literature Search
3.2. Meta-Summary of Included Studies/Strength of Evidence Assessment
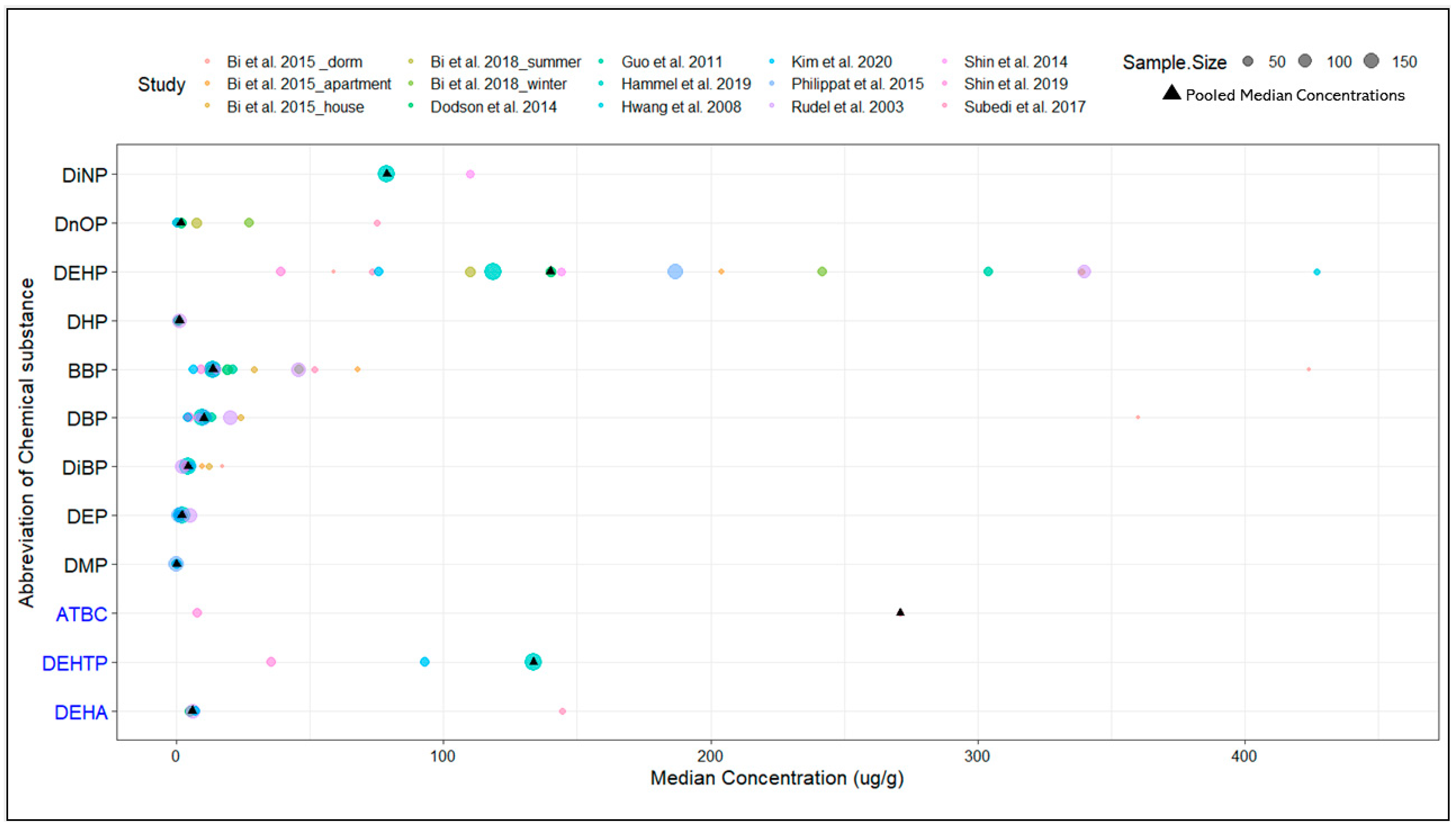
3.3. Meta-Analysis of Phthalate and Phthalate Alternative Dust Concentrations
3.4. Sensitivity Analysis Exploring the Impact of High-Risk Bias Studies
3.5. Daily Intake Rate Calculated from Dust Concentrations
3.6. Estimated Urine Metabolite Concentration of Phthalates and Phthalate Alternatives
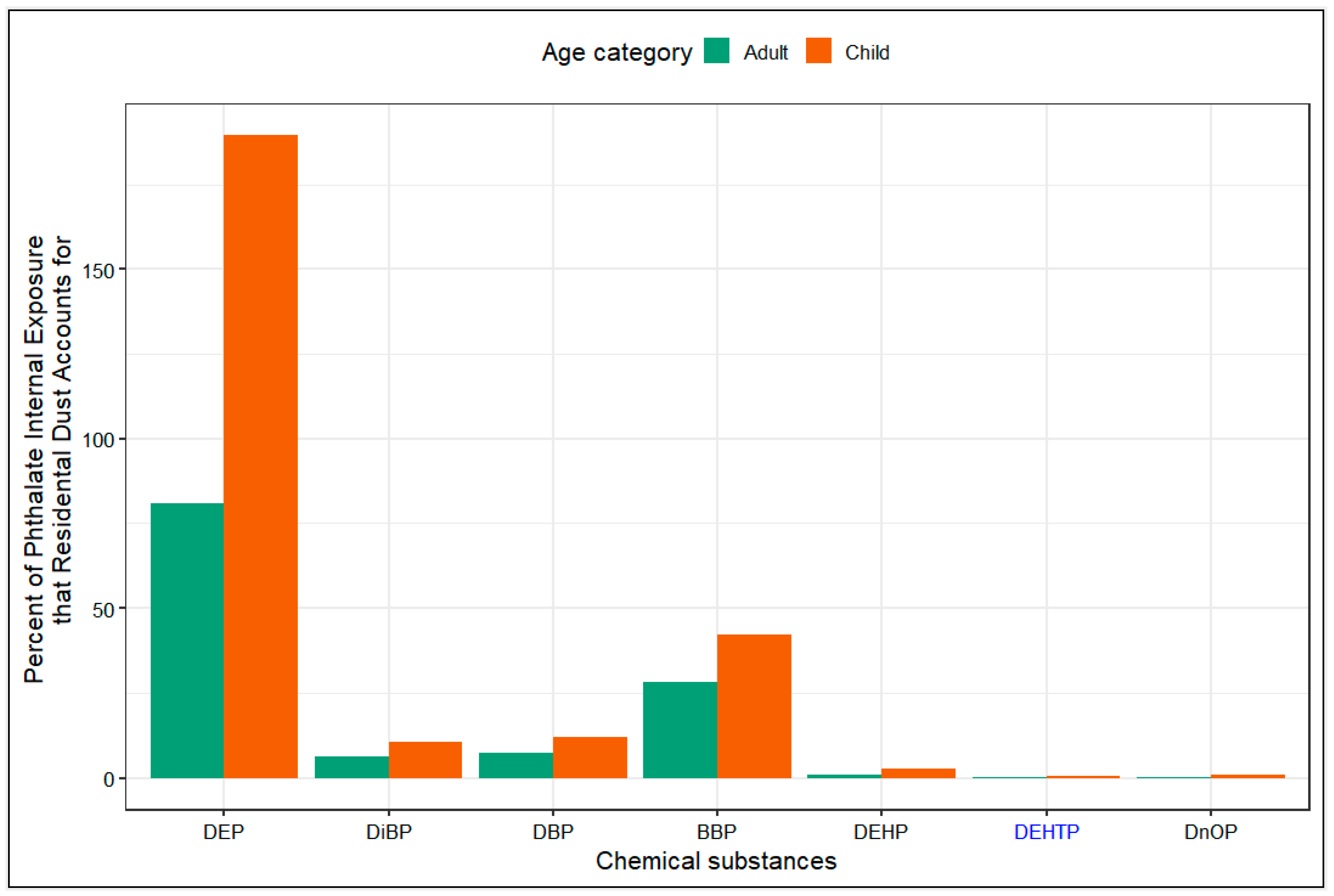
3.7. Daily Intake Rates from Dust Compared to Daily Intake Rates from NHANES
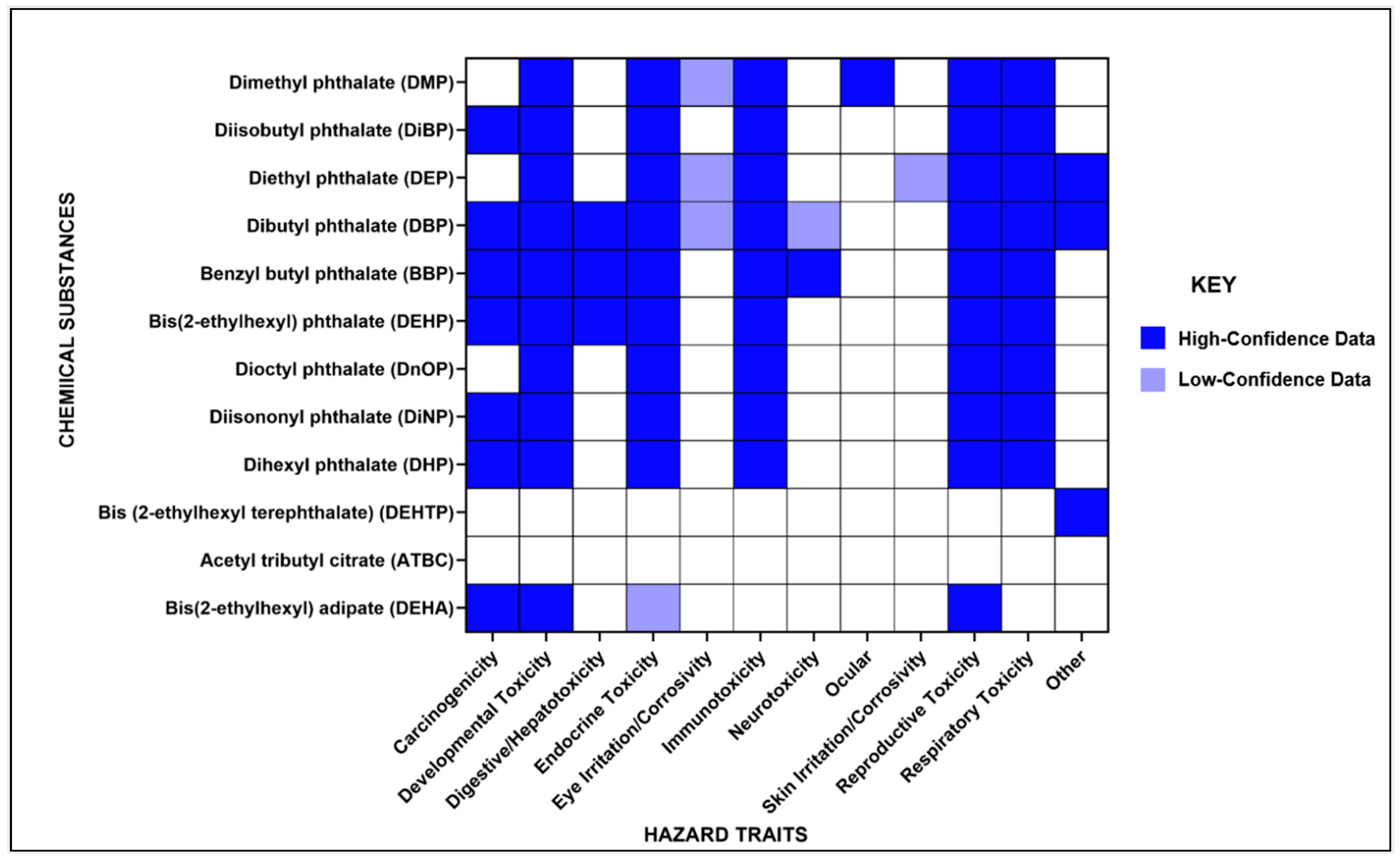
3.8. Overview of Hazard Identification
4. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Phthalates Factsheet|National Biomonitoring Program|CDC. Available online: https://www.cdc.gov/biomonitoring/Phthalates_FactSheet.html (accessed on 3 May 2022).
- Kashyap, D.; Agarwal, T. Concentration and Factors Affecting the Distribution of Phthalates in the Air and Dust: A Global Scenario. Sci. Total Environ. 2018, 635, 817–827. [Google Scholar] [CrossRef]
- Langer, S.; Fredricsson, M.; Weschler, C.; Beko, G.; Strandberg, B.; Remberger, M.; Toftum, J.; Clausen, G. Organophosphate Esters in Dust Samples Collected from Danish Homes and Daycare Centers. Chemosphere 2016, 154, 559–566. [Google Scholar] [CrossRef] [PubMed]
- EPA. America’s Children and the Environment Phthalates: Biomonitoring, Third Edition. 2017. Available online: https://www.epa.gov/sites/default/files/2017-08/documents/phthalates_updates_live_file_508_0.pdf (accessed on 21 August 2022).
- Mitro, S.; Dodson, R.; Singla, V.; Adarnkiewicz, G.; Elmi, A.; Tilly, M.; Zota, A. Consumer Product Chemicals in Indoor Dust: A Quantitative Meta-Analysis of US Studies. Environ. Sci. Technol. 2016, 50, 10661–10672. [Google Scholar] [CrossRef] [PubMed]
- Zota, A.R.; Singla, V.; Adamkiewicz, G.; Mitro, S.D.; Dodson, R.E. Reducing Chemical Exposures at Home: Opportunities for Action. J. Epidemiol. Community Health 2017, 71, 937–940. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, J.; Hanke, W. Exposure to Phthalates: Reproductive Outcome and Children Health. A Review of Epidemiological Studies. Int. J. Occup. Med. Environ. Health 2011, 24, 115–141. [Google Scholar] [CrossRef]
- Engel, S.; Patisaul, H.; Brody, C.; Hauser, R.; Zota, A.; Bennet, D.; Swanson, M.; Whyatt, R. Neurotoxicity of Ortho-Phthalates: Recommendations for Critical Policy Reforms to Protect Brain Development in Children. Am. J. Public. Health 2021, 111, 687–695. [Google Scholar] [CrossRef]
- CPSC Prohibits Certain Phthalates in Children’s Toys and Child Care Products. Available online: https://www.cpsc.gov/Newsroom/News-Releases/2018/CPSC-Prohibits-Certain-Phthalates-in-Childrens-Toys-and-Child-Care-Products (accessed on 15 April 2022).
- Qadeer, A.; Kirsten, K.L.; Ajmal, Z.; Jiang, X.; Zhao, X. Alternative Plasticizers as Emerging Global Environmental and Health Threat: Another Regrettable Substitution? Environ. Sci. Technol. 2022, 56, 1482–1488. [Google Scholar] [CrossRef]
- Bui, T.T.; Giovanoulis, G.; Cousins, A.P.; Magnér, J.; Cousins, I.T.; de Wit, C.A. Human Exposure, Hazard and Risk of Alternative Plasticizers to Phthalate Esters. Sci. Total Environ. 2016, 541, 451–467. [Google Scholar] [CrossRef]
- Hammel, S.C.; Levasseur, J.L.; Hoffman, K.; Phillips, A.L.; Lorenzo, A.M.; Calafat, A.M.; Webster, T.F.; Stapleton, H.M. Children’s Exposure to Phthalates and Non-Phthalate Plasticizers in the Home: The TESIE Study. Environ. Int. 2019, 132, 105061. [Google Scholar] [CrossRef]
- Wormuth, M.; Scheringer, M.; Vollenweider, M.; Hungerbühler, K. What Are the Sources of Exposure to Eight Frequently Used Phthalic Acid Esters in Europeans? Risk Anal. 2006, 26, 803–824. [Google Scholar] [CrossRef]
- Fromme, H.; Bolte, G.; Koch, H.M.; Angerer, J.; Boehmer, S.; Drexler, H.; Mayer, R.; Liebl, B. Occurrence and Daily Variation of Phthalate Metabolites in the Urine of an Adult Population. Int. J. Hyg. Environ. Health 2007, 210, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Adibi, J.J.; Perera, F.P.; Jedrychowski, W.; Camann, D.E.; Barr, D.; Jacek, R.; Whyatt, R.M. Prenatal Exposures to Phthalates among Women in New York City and Krakow, Poland. Environ. Health Perspect. 2003, 111, 1719. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Prevedouros, K.; Alli, R.; Cousins, I.T. Comparison and Analysis of Different Approaches for Estimating the Human Exposure to Phthalate Esters. Environ. Int. 2007, 33, 283–291. [Google Scholar] [CrossRef] [PubMed]
- US EPA, O. Chemicals Undergoing Risk Evaluation under TSCA. Available online: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/chemicals-undergoing-risk-evaluation-under-tsca (accessed on 21 August 2022).
- Diamond, M.L.; Okeme, J.O.; Melymuk, L. Hands as Agents of Chemical Transport in the Indoor Environment. Environ. Sci. Technol. Lett. 2021, 8, 326–332. [Google Scholar] [CrossRef]
- Bekö, G.; Weschler, C.J.; Langer, S.; Callesen, M.; Toftum, J.; Clausen, G. Children’s Phthalate Intakes and Resultant Cumulative Exposures Estimated from Urine Compared with Estimates from Dust Ingestion, Inhalation and Dermal Absorption in Their Homes and Daycare Centers. PLoS ONE 2013, 8, e62442. [Google Scholar] [CrossRef]
- Branch, F.; Woodruff, T.J.; Mitro, S.D.; Zota, A.R. Vaginal Douching and Racial/Ethnic Disparities in Phthalates Exposures among Reproductive-Aged Women: National Health and Nutrition Examination Survey 2001–2004. Environ. Health 2015, 14, 57. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on the Health Risks of Phthalates. Phthalates and Cumulative Risk Assessment: The Tasks Ahead; National Academies Press (US): Washington, DC, USA, 2008; ISBN 978-0-309-12841-4. [Google Scholar]
- Bi, X.; Yuan, S.; Pan, X.; Winstead, C.; Wang, Q. Comparison, Association, and Risk Assessment of Phthalates in Floor Dust at Different Indoor Environments in Delaware, USA. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2015, 50, 1428–1439. [Google Scholar] [CrossRef]
- Not Just Dirt: Toxic Chemicals in Indoor Dust. Available online: https://www.nrdc.org/resources/not-just-dirt-toxic-chemicals-indoor-dust (accessed on 21 August 2022).
- Butte, W.; Heinzow, B. Pollutants in House Dust as Indicators of Indoor Contamination. Rev. Environ. Contam. Toxicol. 2002, 175, 1–46. [Google Scholar]
- Weschler, C.J.; Nazaroff, W.W. SVOC Partitioning between the Gas Phase and Settled Dust Indoors. Atmos. Environ. 2010, 44, 3609–3620. [Google Scholar] [CrossRef]
- Dodson, R.E.; Camann, D.E.; Morello-Frosch, R.; Brody, J.G.; Rudel, R.A. Semivolatile Organic Compounds in Homes: Strategies for Efficient and Systematic Exposure Measurement Based on Empirical and Theoretical Factors. Environ. Sci. Technol. 2015, 49, 113–122. [Google Scholar] [CrossRef]
- Little, J.C.; Weschler, C.J.; Nazaroff, W.W.; Liu, Z.; Cohen Hubal, E.A. Rapid Methods to Estimate Potential Exposure to Semivolatile Organic Compounds in the Indoor Environment. Environ. Sci. Technol. 2012, 46, 11171–11178. [Google Scholar] [CrossRef] [PubMed]
- Weschler, C.J.; Nazaroff, W.W. SVOC Exposure Indoors: Fresh Look at Dermal Pathways. Indoor Air 2012, 22, 356–377. [Google Scholar] [CrossRef]
- US EPA, O. Understanding Exposures in Children’s Environments. Available online: https://www.epa.gov/healthresearch/understanding-exposures-childrens-environments (accessed on 21 August 2022).
- Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia. Available online: www.Covidence.org (accessed on 5 May 2022).
- DeLuca, N.M.; Angrish, M.; Wilkins, A.; Thayer, K.; Cohen Hubal, E.A. Human Exposure Pathways to Poly- and Perfluoroalkyl Substances (PFAS) from Indoor Media: A Systematic Review Protocol. Environ. Int. 2021, 146, 106308. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Maestre, J.; Li, H.; Zhang, G.; Givehchi, R.; Mahdavi, A.; Kinney, K.; Siegel, J.; Horner, S.; Xu, Y. Phthalates and Organophosphates in Settled Dust and HVAC Filter Dust of US Low-Income Homes: Association with Season, Building Characteristics, and Childhood Asthma. Environ. Int. 2018, 121, 916–930. [Google Scholar] [CrossRef]
- McGrath, S.; Zhao, X.; Qin, Z.Z.; Steele, R.; Benedetti, A. One-Sample Aggregate Data Meta-Analysis of Medians. Stat. Med. 2019, 38, 969–984. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.A.-E.; Covaci, A. Organophosphate Flame Retardants in Indoor Dust from Egypt: Implications for Human Exposure. Environ. Sci. Technol. 2014, 48, 4782–4789. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Kang, L.; Wu, S.; Zhou, X.; Wang, X. Effect of Dust Formation on the Fate of Indoor Phthalates: Model Analysis. Build. Environ. 2023, 229, 109957. [Google Scholar] [CrossRef]
- Schossler, P.; Schripp, T.; Salthammer, T.; Bahadir, M. Beyond Phthalates: Gas Phase Concentrations and Modeled Gas/Particle Distribution of Modern Plasticizers. Sci. Total Environ. 2011, 409, 4031–4038. [Google Scholar] [CrossRef]
- Williams, A.J.; Grulke, C.M.; Edwards, J.; McEachran, A.D.; Mansouri, K.; Baker, N.C.; Patlewicz, G.; Shah, I.; Wambaugh, J.F.; Judson, R.S.; et al. The CompTox Chemistry Dashboard: A Community Data Resource for Environmental Chemistry. J. Cheminform. 2017, 9, 61. [Google Scholar] [CrossRef]
- European Chemical Agency. European Chemical Agency Assessment of Regulatory Needs: Ortho-Phthalates 2021. Available online: https://echa.europa.eu/documents/10162/7adf54e4-22de-fbbf-8b79-a709681d6f4a (accessed on 20 August 2022).
- European Chemical Agency. European Chemical Agency Assessment of Regulatory Needs: Isophthalates, Terephthalates and Trimellitates. 2021. Available online: https://echa.europa.eu/documents/10162/c2bd91c0-1593-1ff3-1551-009204638501 (accessed on 20 August 2022).
- NHANES Questionnaires, Datasets, and Related Documentation. Available online: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx (accessed on 29 December 2022).
- Saravanabhavan, G.; Walker, M.; Guay, M.; Aylward, L. Urinary Excretion and Daily Intake Rates of Diethyl Phthalate in the General Canadian Population. Sci. Total Environ. 2014, 500–501, 191–198. [Google Scholar] [CrossRef]
- Huang, P.-C.; Cheng, P.-K.; Chen, H.-C.; Shiue, I.; Chang, W.-T.; Huang, H.-I.; Chang, J.-W.; Wang, I.-J. Are Phthalate Exposure Related to Oxidative Stress in Children and Adolescents with Asthma? A Cumulative Risk Assessment Approach. Antioxidants 2022, 11, 1315. [Google Scholar] [CrossRef]
- David, R.M. Exposure to Phthalate Esters. Environ. Health Perspect. 2000, 108, A440. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.M.; Drexler, H.; Angerer, J. An Estimation of the Daily Intake of Di(2-Ethylhexyl)Phthalate (DEHP) and Other Phthalates in the General Population. Int. J. Hyg. Environ. Health 2003, 206, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-W.; Lee, C.-C.; Pan, W.-H.; Chou, W.-C.; Huang, H.-B.; Chiang, H.-C.; Huang, P.-C. Estimated Daily Intake and Cumulative Risk Assessment of Phthalates in the General Taiwanese after the 2011 DEHP Food Scandal. Sci. Rep. 2017, 7, 45009. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, H.; Zhou, W.; Chen, Y.; Zhou, Y.; Jiang, Q. Urinary Excretion of Phthalate Metabolites in School Children of China: Implication for Cumulative Risk Assessment of Phthalate Exposure. Environ. Sci. Technol. 2015, 49, 1120–1129. [Google Scholar] [CrossRef]
- Lumley, T. Analysis of Complex Survey Samples. J. Stat. Softw. 2004, 9, 1–19. [Google Scholar] [CrossRef]
- Mage, D.T.; Allen, R.H.; Gondy, G.; Smith, W.; Barr, D.B.; Needham, L.L. Estimating Pesticide Dose from Urinary Pesticide Concentration Data by Creatinine Correction in the Third National Health and Nutrition Examination Survey (NHANES-III). J. Expo. Anal. Environ. Epidemiol. 2004, 14, 457–465. [Google Scholar] [CrossRef]
- Mage, D.T.; Allen, R.H.; Kodali, A. Creatinine Corrections for Estimating Children’s and Adult’s Pesticide Intake Doses in Equilibrium with Urinary Pesticide and Creatinine Concentrations. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 360–368. [Google Scholar] [CrossRef]
- Organophosphate Metabolites in Urine Samples from Danish Children and Women. Available online: https://mst.dk/service/publikationer/publikationsarkiv/2016/sep/democrophes/ (accessed on 3 February 2023).
- Lee, I.; Pälmke, C.; Ringbeck, B.; Ihn, Y.; Gotthardt, A.; Lee, G.; Alakeel, R.; Alrashed, M.; Tosepu, R.; Jayadipraja, E.A.; et al. Urinary Concentrations of Major Phthalate and Alternative Plasticizer Metabolites in Children of Thailand, Indonesia, and Saudi Arabia, and Associated Risks. Environ. Sci. Technol. 2021, 55, 16526–16537. [Google Scholar] [CrossRef]
- Anderson, W.A.C.; Castle, L.; Hird, S.; Jeffery, J.; Scotter, M.J. A Twenty-Volunteer Study Using Deuterium Labelling to Determine the Kinetics and Fractional Excretion of Primary and Secondary Urinary Metabolites of Di-2-Ethylhexylphthalate and Di-Iso-Nonylphthalate. Food Chem. Toxicol. 2011, 49, 2022–2029. [Google Scholar] [CrossRef]
- Anderson, W.A.; Castle, L.; Scotter, M.J.; Massey, R.C.; Springall, C. A Biomarker Approach to Measuring Human Dietary Exposure to Certain Phthalate Diesters. Food Addit. Contam. 2001, 18, 1068–1074. [Google Scholar] [CrossRef]
- Itoh, H.; Yoshida, K.; Masunaga, S. Quantitative Identification of Unknown Exposure Pathways of Phthalates Based on Measuring Their Metabolites in Human Urine. Environ. Sci. Technol. 2007, 41, 4542–4547. [Google Scholar] [CrossRef]
- Koch, H.M.; Wittassek, M.; Brüning, T.; Angerer, J.; Heudorf, U. Exposure to Phthalates in 5-6 Years Old Primary School Starters in Germany--a Human Biomonitoring Study and a Cumulative Risk Assessment. Int. J. Hyg. Environ. Health 2011, 214, 188–195. [Google Scholar] [CrossRef]
- Lessmann, F.; Correia-Sá, L.; Calhau, C.; Domingues, V.F.; Weiss, T.; Brüning, T.; Koch, H.M. Exposure to the Plasticizer Di(2-Ethylhexyl) Terephthalate (DEHTP) in Portuguese Children—Urinary Metabolite Levels and Estimated Daily Intakes. Environ. Int. 2017, 104, 25–32. [Google Scholar] [CrossRef]
- Stanfield, Z.; Setzer, R.W.; Hull, V.; Sayre, R.R.; Isaacs, K.K.; Wambaugh, J.F. Bayesian Inference of Chemical Exposures from NHANES Urine Biomonitoring Data. J. Expo. Sci. Environ. Epidemiol. 2022, 32, 833–846. [Google Scholar] [CrossRef]
- Home: CalSAFER. Available online: https://calsafer.dtsc.ca.gov/ (accessed on 16 January 2023).
- US EPA, O. Basic Information about the Integrated Risk Information System. Available online: https://www.epa.gov/iris/basic-information-about-integrated-risk-information-system (accessed on 16 January 2023).
- Pharos—About. Available online: https://pharos.healthybuilding.net/about?_gl=1*rqv3wh*_ga*MTQ1NDEzNzcyNy4xNjczOTAyMTA1*_ga_9HBMPKD00P*MTY3MzkwMjEwNC4xLjEuMTY3MzkwMjMwOS4wLjAuMA (accessed on 16 January 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.E.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brenna, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kim, K.; Shin, H.; Wong, L.; Young, T.; Bennett, D. Temporal Variability of Indoor Dust Concentrations of Semivolatile Organic Compounds. Indoor Air 2021, 31, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-M.; McKone, T.E.; Nishioka, M.G.; Fallin, M.D.; Croen, L.A.; Hertz-Picciotto, I.; Newschaffer, C.J.; Bennett, D.H. Determining Source Strength of Semivolatile Organic Compounds Using Measured Concentrations in Indoor Dust. Indoor Air 2014, 24, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Moschet, C.; Young, T.; Bennett, D. Measured Concentrations of Consumer Product Chemicals in California House Dust: Implications for Sources, Exposure, and Toxicity Potential. Indoor Air 2020, 30, 60–75. [Google Scholar] [CrossRef]
- Hwang, H.-M.; Park, E.-K.; Young, T.M.; Hammock, B.D. Occurrence of Endocrine-Disrupting Chemicals in Indoor Dust. Sci. Total Environ. 2008, 404, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Rudel, R.A.; Camann, D.E.; Spengler, J.D.; Korn, L.R.; Brody, J.G. Phthalates, Alkylphenols, Pesticides, Polybrominated Diphenyl Ethers, and Other Endocrine-Disrupting Compounds in Indoor Air and Dust. Environ. Sci. Technol. 2003, 37, 4543–4553. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kannan, K. Comparative Assessment of Human Exposure to Phthalate Esters from House Dust in China and the United States. Environ. Sci. Technol. 2011, 45, 3788–3794. [Google Scholar] [CrossRef] [PubMed]
- Subedi, B.; Sullivan, K.D.; Dhungana, B. Phthalate and Non-Phthalate Plasticizers in Indoor Dust from Childcare Facilities, Salons, and Homes across the USA. Environ. Pollut. 2017, 230, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Philippat, C.; Bennett, D.; Krakowiak, P.; Rose, M.; Hwang, H.; Hertz-Picciotto, I. Phthalate Concentrations in House Dust in Relation to Autism Spectrum Disorder and Developmental Delay in the CHildhood Autism Risks from Genetics and the Environment (CHARGE) Study. Environ. Health 2015, 14, 56. [Google Scholar] [CrossRef]
- Mitro, S.D.; Chu, M.T.; Dodson, R.E.; Adamkiewicz, G.; Chie, L.; Brown, F.M.; James-Todd, T.M. Phthalate Metabolite Exposures among Immigrants Living in the United States: Findings from NHANES, 1999–2014. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 71–82. [Google Scholar] [CrossRef] [PubMed]
- James-Todd, T.; Meeker, J.; Huang, T.; Hauser, R.; Seely, E.; Ferguson, K.; Rich-Edwards, J.; McElrath, T. Racial and Ethnic Variations in Phthalate Metabolite Concentrations across Pregnancy. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 160–166. [Google Scholar] [CrossRef]
- Phthalates Business Guidance & Small Entity Compliance Guide. Available online: https://www.cpsc.gov/Business--Manufacturing/Business-Education/Business-Guidance/Phthalates-Information (accessed on 29 December 2022).
- Silva, M.J.; Samandar, E.; Calafat, A.M.; Ye, X. Identification of Di-2-Ethylhexyl Terephthalate (DEHTP) Metabolites Using Human Liver Microsomes for Biomonitoring Applications. Toxicol. Vitr. 2015, 29, 716–721. [Google Scholar] [CrossRef]
- Nagorka, R.; Conrad, A.; Scheller, C.; Süssenbach, B.; Moriske, H.-J. Diisononyl 1,2-Cyclohexanedicarboxylic Acid (DINCH) and Di(2-Ethylhexyl) Terephthalate (DEHT) in Indoor Dust Samples: Concentration and Analytical Problems. Int. J. Hyg. Environ. Health 2011, 214, 26–35. [Google Scholar] [CrossRef]
- Phthalates and Plasticizers Metabolites-Urine (PHTHTE_J). Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/PHTHTE_J.htm#Description_of_Laboratory_Methodology (accessed on 17 February 2023).
- Rodríguez-Carmona, Y.; Ashrap, P.; Calafat, A.M.; Ye, X.; Rosario, Z.; Bedrosian, L.D.; Huerta-Montanez, G.; Vélez-Vega, C.M.; Alshawabkeh, A.; Cordero, J.F.; et al. Determinants and Characterization of Exposure to Phthalates, DEHTP and DINCH among Pregnant Women in the PROTECT Birth Cohort in Puerto Rico. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 56–69. [Google Scholar] [CrossRef]
- Schripp, T. Distribution of Phthalates in the Indoor Environment: Application and Evaluation of Indoor Air Models. Ph.D. Thesis, Technical University of Carolo-Wilhelmina, Braunschweig, Germany, 2009. [Google Scholar] [CrossRef]
- Cherrie, J.W.; Fransman, W.; Heussen, G.A.H.; Koppisch, D.; Jensen, K.A. Exposure Models for REACH and Occupational Safety and Health Regulations. Int. J. Environ. Res. Public Health 2020, 17, 383. [Google Scholar] [CrossRef]
- Adamkiewicz, G.; Zota, A.R.; Fabian, M.P.; Chahine, T.; Julien, R.; Spengler, J.D.; Levy, J.I. Moving Environmental Justice Indoors: Understanding Structural Influences on Residential Exposure Patterns in Low-Income Communities. Am. J. Public. Health 2011, 101, S238–S245. [Google Scholar] [CrossRef] [PubMed]
- Gochfeld, M.; Burger, J. Disproportionate Exposures in Environmental Justice and Other Populations: The Importance of Outliers. Am. J. Public. Health 2011, 101, S53–S63. [Google Scholar] [CrossRef] [PubMed]


| Dust Ingestion Intake, ug/kg-day = (Dust Conc. xIR × ED)/BW | ||
|---|---|---|
| Exposure Factors | Value | Source |
| Dust Concentration data (Conc.): ug/g dust | Study data (see Table S11) | - |
| Ingestion Rate (IR): g dust/day | Child (2 to <6 years) 2: 0.030 (central tendency) 0.100 (95th percentile) | US EPA Exposure Factors Handbook Table 5-1 (2017 update) |
| Adult: 0.020 (central tendency) 0.060 (95th percentile) | US EPA Exposure Factors Handbook Table 5-1 (2017 update) | |
| Exposure duration at home (fraction of time per day spent at home) | Child: 0.861 | Abdallah et al. (2014) [34] |
| Adult: 0.638 | Abdallah et al. (2014) [34] | |
| Body Weight (BW): kg | Child (3 to <6 years) 2: 17.8 (central tendency) | US EPA Exposure Factors Handbook Table 8.3 (2011) |
| Adult: 76.9 (central tendency) | US EPA Exposure Factors Handbook Table 8.3 (2011) | |
| Air Inhalation Intake, ug/day-kg = (Total Air Conc. (ug/m3) × Vol. of Air Inhaled × ED)/BW | ||
| Total Air Concentration (ug/m3) | Varies by chemical 1 | - |
| Volume of Air Inhaled (m3/day) | Child (3 to <6 years) 2: 12.64 (central tendency) 15.41 (95th percentile) | US EPA Exposure Factors Handbook Table 6-16 (2011) |
| Adult: 18.63 (central tendency) 25.16 (95th percentile) | US EPA Exposure Factors Handbook Table 6-16 (2011) | |
| Dermal Exposure through Air Intake, ug/kg-day = (Gaseous Air Conc. (ug/m3) × (Indoor Air Transdermal Permeability /100) × Body SA × ET)/BW | ||
| Gaseous Air Concentration (ug/m3) | Varies by chemical 1 | - |
| Indoor Air Transdermal Permeability (cm) | Varies by chemical 1 | - |
| Body Surface Area, m2 | Child (3 to <6 years): 0.76 (central tendency) 0.95 (95th percentile) | US EPA Exposure Factors Handbook Table 7-1 (2011) |
| Adult (male) 3: 2.07 (central tendency) 2.47 (95th percentile) | US EPA Exposure Factors Handbook Table 7-1 (2011) | |
| Exposure in hours (daily) | Child: 21 | Based on assumed exposure duration at home, observed above |
| Adult: 15 | Based on assumed exposure duration at home, observed above | |
| CASRN | Abbreviation | Substance Name |
|---|---|---|
| 131-11-3 | DMP | Dimethyl phthalate |
| 84-69-5 | DiBP | Diisobutyl phthalate |
| 84-66-2 | DEP | Diethyl phthalate |
| 84-74-2 | DBP | Dibutyl phthalate |
| 85-68-7 | BBP | Benzyl butyl phthalate |
| 117-81-7 | DEHP | Bis(2-ethylhexyl) phthalate |
| 84-75-3 | DHP | Dihexyl phthalate |
| 117-84-0 | DnOP | Dioctyl phthalate |
| 28553-12-0 | DiNP | Diisononyl phthalate |
| 4654-26-6; 6422-86-2 | DOTP, DEHT or DEHTP | Dioctyl terephthalate or bis (2-ethylhexyl terephthalate) |
| 77-90-7 | ATBC | Acetyl tributyl citrate |
| 103-23-1 | DEHA | Bis(2-ethylhexyl) adipate or di (2-ethylhexyl) adipate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milton, S.G.; Tejiram, R.A.; Joglekar, R.; Hoffman, K. Characterizing the Contribution of Indoor Residential Phthalate and Phthalate Alternative Dust Concentrations to Internal Dose in the US General Population: An Updated Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 6589. https://doi.org/10.3390/ijerph20166589
Milton SG, Tejiram RA, Joglekar R, Hoffman K. Characterizing the Contribution of Indoor Residential Phthalate and Phthalate Alternative Dust Concentrations to Internal Dose in the US General Population: An Updated Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2023; 20(16):6589. https://doi.org/10.3390/ijerph20166589
Chicago/Turabian StyleMilton, Sashoy G., Rachel A. Tejiram, Rashmi Joglekar, and Kate Hoffman. 2023. "Characterizing the Contribution of Indoor Residential Phthalate and Phthalate Alternative Dust Concentrations to Internal Dose in the US General Population: An Updated Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 20, no. 16: 6589. https://doi.org/10.3390/ijerph20166589
APA StyleMilton, S. G., Tejiram, R. A., Joglekar, R., & Hoffman, K. (2023). Characterizing the Contribution of Indoor Residential Phthalate and Phthalate Alternative Dust Concentrations to Internal Dose in the US General Population: An Updated Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 20(16), 6589. https://doi.org/10.3390/ijerph20166589






