Circadian Modulation of the Antioxidant Effect of Grape Consumption: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Study Design
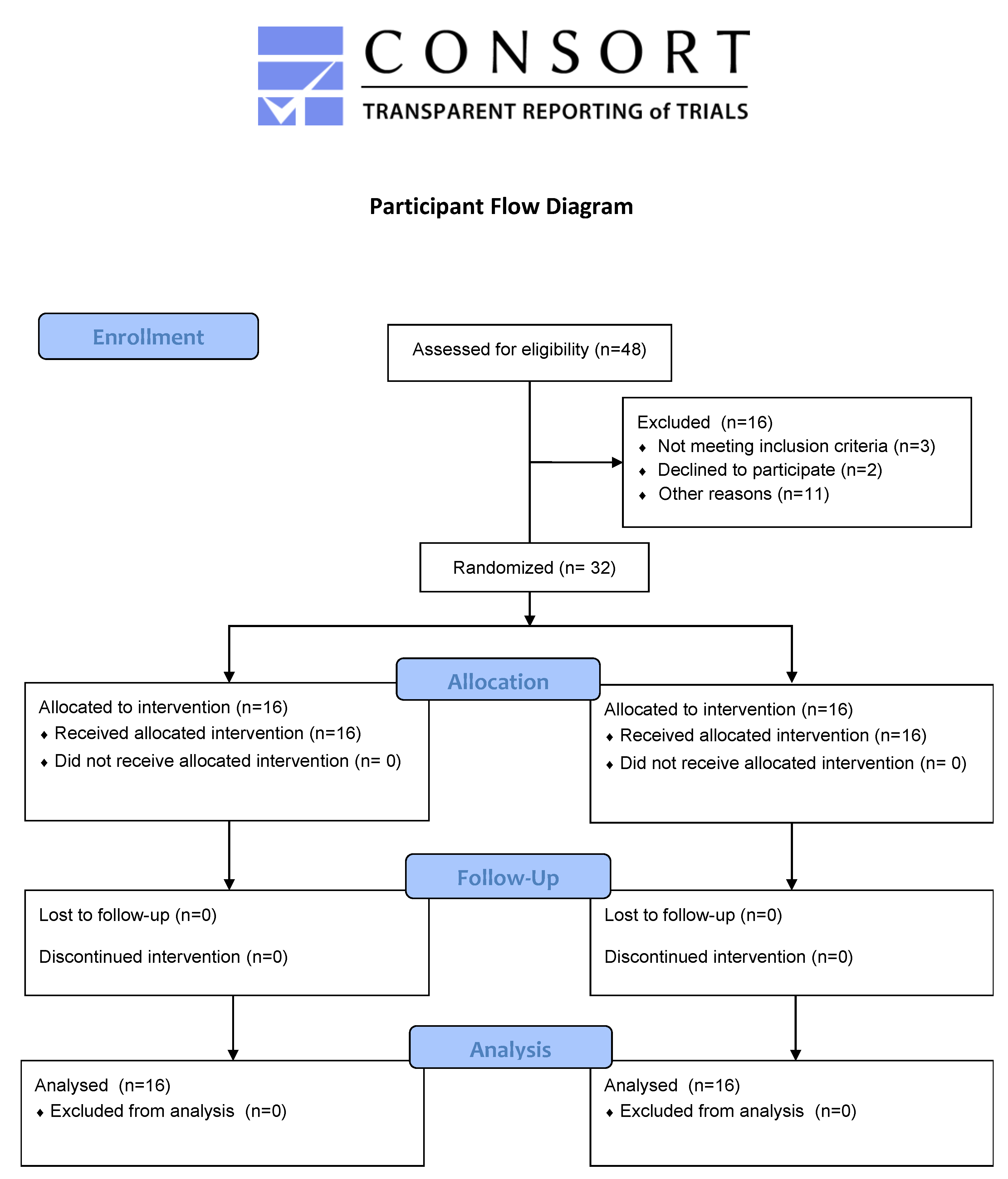
2.3. Participants
2.4. Dietary Assessment
2.5. Intervention
2.6. Anthropometric Measurements, Urine Collection and Biomarker/Metabolite Analysis
2.7. Sample Preparation
2.8. LC-MS/MS Conditions
2.9. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Biomarker Response to Treatment
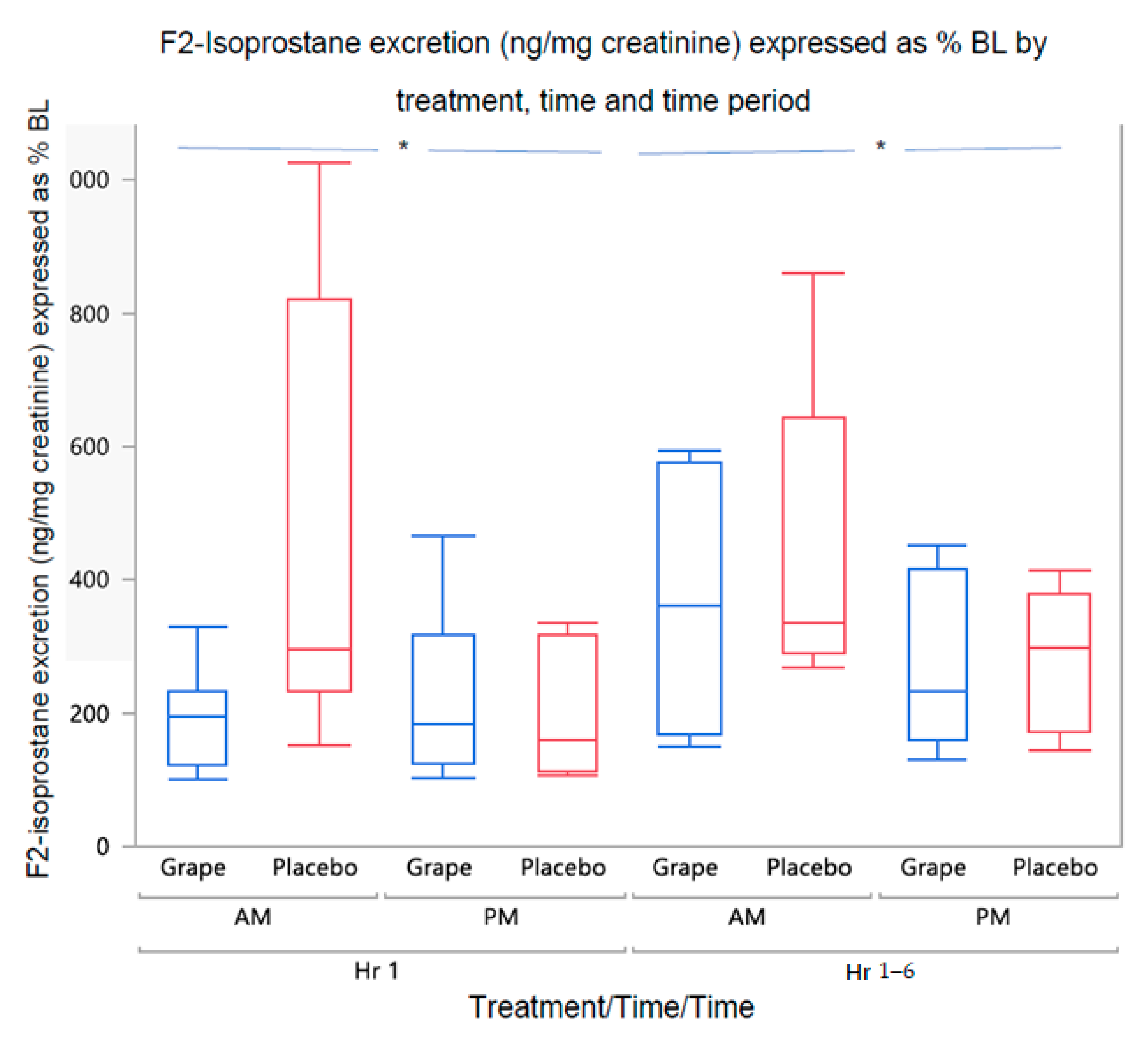
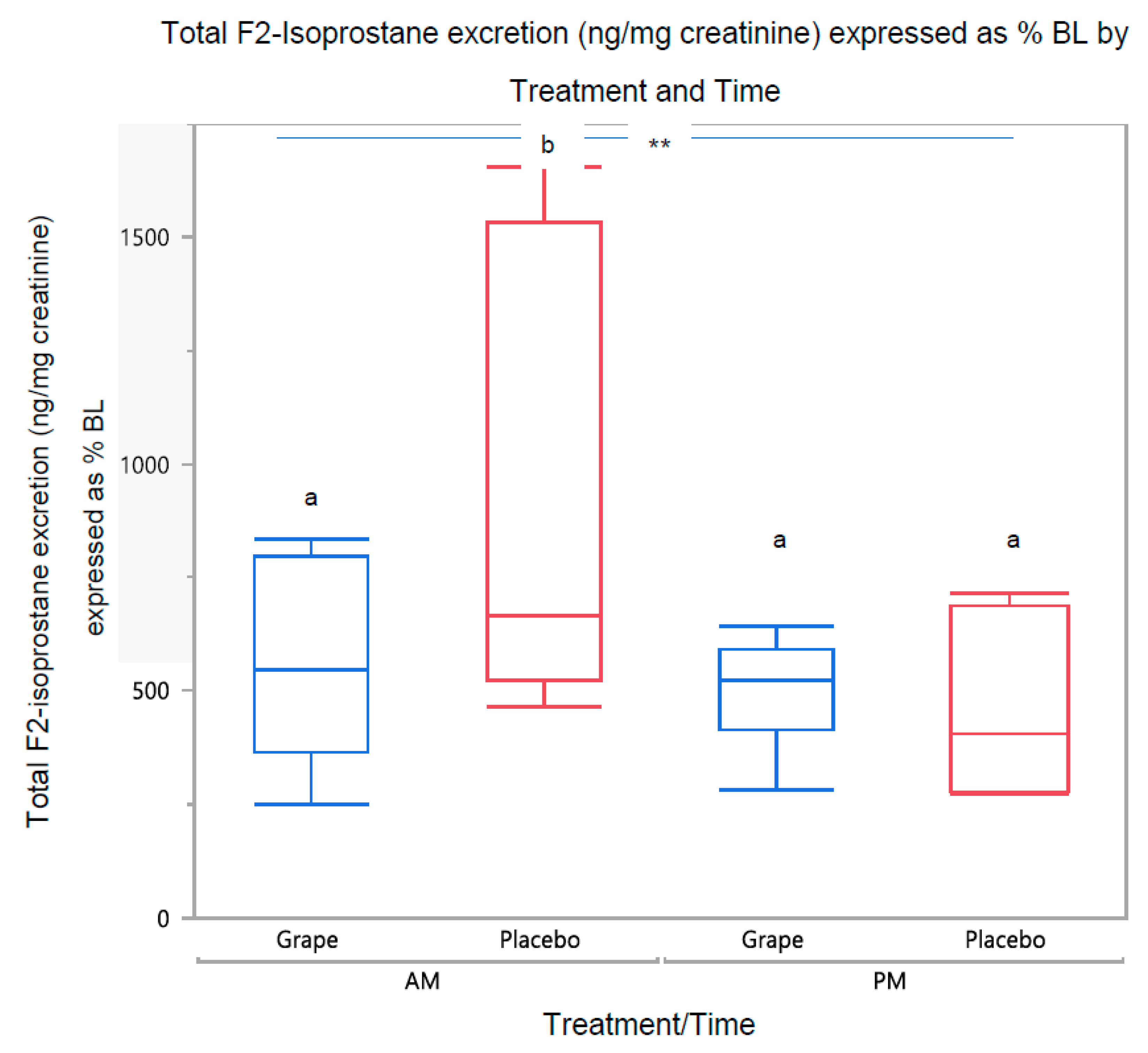
3.2.1. Urine F2-Isoprostane Concentration
3.2.2. Urine Grape Metabolite Concentrations
Grape Metabolite Excretion by Treatment and Time
Relationship of F2-Isoprostane to Grape Metabolites
3.3. Dietary Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Curtis, A.M.; Bellet, M.M.; Sassone-Corsi, P.; O’Neill, L.A. Circadian Clock Proteins and Immunity. Immunity 2014, 40, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Stenvers, D.; Scheer, F.; Schrauwen, P.; la Fleur, S.E.; Kalsbeek, A. Circadian Clocks and Insulin Resistance. Nat. Rev. Endocrinol. 2019, 15, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Manoogian, E.; Panda, S. Circadian Clock, Nutrient Quality, and Eating Pattern Tune Diurnal Rhythms in the Mitochondrial Proteome. Proc. Natl. Acad. Sci. USA 2016, 113, 3127–3129. [Google Scholar] [CrossRef]
- Panda, S. Circadian Physiology of Metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef]
- Adafer, R.; Messaadi, W.; Meddahi, M.; Patey, A.; Haderbache, A.; Bayen, S.; Messaadi, N. Food Timing, Circadian Rhythm and Chrononutrition: A Systematic Review of Time-Restricted Eating’s Effects on Human Health. Nutrients 2020, 12, 3770. [Google Scholar] [CrossRef]
- Katsi, V.; Papakonstantinou, I.P.; Soulaidopoulos, S.; Katsiki, N.; Tsioufis, K. Chrononutrition in Cardiometabolic Health. J. Clin. Med. 2022, 11, 296. [Google Scholar] [CrossRef]
- Praticò, D. The Neurobiology of Isoprostanes and Alzheimer’s Disease. Biochim. Biophys. Acta 2010, 1801, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.J.; Morrow, J.D. Measurement of F(2)-Isoprostanes as an Index of Oxidative Stress in Vivo. Free Radic. Biol. Med. 2000, 28, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Basu, S. F2-Isoprostanes in Human Health and Diseases: From Molecular Mechanisms to Clinical Implications. Antioxid. Redox Signal. 2008, 10, 1405–1434. [Google Scholar] [CrossRef] [PubMed]
- Seet, R.C.S.; Lee, C.-Y.J.; Lim, E.C.H.; Tan, J.J.H.; Quek, A.M.L.; Chong, W.-L.; Looi, W.-F.; Huang, S.-H.; Wang, H.; Chan, Y.-H.; et al. Oxidative Damage in Parkinson Disease: Measurement Using Accurate Biomarkers. Free Radic. Biol. Med. 2010, 48, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Minghetti, L.; Levi, G. Isoprostanes, Novel Markers of Oxidative Injury, Help Understanding the Pathogenesis of Neurodegenerative Diseases. Neurochem. Res. 2000, 25, 1357–1364. [Google Scholar] [CrossRef]
- Miller, S.A.; White, J.A.; Chowdhury, R.; Gales, D.N.; Tameru, B.; Tiwari, A.K.; Samuel, T. Effects of Consumption of Whole Grape Powder on Basal NF-ΚB Signaling and Inflammatory Cytokine Secretion in a Mouse Model of Inflammation. J. Nutr. Intermed. Metab. 2018, 11, 1–8. [Google Scholar] [CrossRef]
- Chuang, C.C.; McIntosh, M.K. Potential Mechanisms by Which Polyphenol-Rich Grapes Prevent Obesity-Mediated Inflammation and Metabolic Diseases. Annu. Rev. Nutr. 2011, 31, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Stamer, D.; Nizami, S. Whole Grape Alleviates Inflammatory Arthritis through Inhibition of Tumor Necrosis Factor. J. Funct. Foods 2017, 35, 458–465. [Google Scholar] [CrossRef]
- Seymour, E.M.; Singer, A.A.; Bennink, M.R.; Parikh, R.V.; Kirakosyan, A.; Kaufman, P.B.; Bolling, S.F. Chronic Intake of a Phytochemical-Enriched Diet Reduces Cardiac Fibrosis and Diastolic Dysfunction Caused by Prolonged Salt-Sensitive Hypertension. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Zern, T.L.; Wood, R.J.; Greene, C.; West, K.L.; Liu, Y.; Aggarwal, D.; Shachter, N.S.; Fernandez, M.L. Grape Polyphenols Exert a Cardioprotective Effect in Pre- and Postmenopausal Women by Lowering Plasma Lipids and Reducing Oxidative Stress. J. Nutr. 2005, 135, 1911–1917. [Google Scholar] [CrossRef]
- Das, S.; Das, D.K. Resveratrol: A Therapeutic Promise for Cardiovascular Diseases. Recent Pat. Cardiovasc. Drug Discov. 2007, 2, 133–138. [Google Scholar] [CrossRef]
- Choy, K.W.; Murugan, D.; Leong, X.-F.; Abas, R.; Alias, A.; Mustafa, M.R. Flavonoids as Natural Anti-Inflammatory Agents Targeting Nuclear Factor-Kappa B (NFκB) Signaling in Cardiovascular Diseases: A Mini Review. Front. Pharmacol. 2019, 10, 1295. [Google Scholar]
- Costa, E.; Cosme, F.; Jordão, A.M.; Mendes-Faia, A. Anthocyanin Profile and Antioxidant Activity from 24 Grape Varieties Cultivated in Two Portuguese Wine Regions. OENO One 2014, 48, 51–62. [Google Scholar] [CrossRef]
- Silva, M.M.; Lidon, F.C. An Overview on Applications and Side Effects of Antioxidant Food Additives. Emir. J. Food Agric. 2016, 28, 823–832. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Zhao, H.; Wang, X.; Pang, J.; Li, Q.; Yang, Y.; Ling, W. Anthocyanin Supplementation Improves Anti-Oxidative and Anti-Inflammatory Capacity in a Dose-Response Manner in Subjects with Dyslipidemia. Redox Biol. 2020, 32, 101474. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified Anthocyanin Supplementation Reduces Dyslipidemia, Enhances Antioxidant Capacity, and Prevents Insulin Resistance in Diabetic Patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Herieka, M.; Erridge, C. High-Fat Meal Induced Postprandial Inflammation. Mol. Nutr. Food Res. 2014, 58, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Roehrs, M.; Conte, L.; da Silva, D.T.; Duarte, T.; Maurer, L.H.; de Carvalho, J.A.M.; Moresco, R.N.; Somacal, S.; Emanuelli, T. Annatto Carotenoids Attenuate Oxidative Stress and Inflammatory Response after High-Calorie Meal in Healthy Subjects. Food Res. Int. 2017, 100, 771–779. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Meng, X.; Maliakal, P.; Lu, H.; Lee, M.J.; Yang, C.S. Urinary and Plasma Levels of Resveratrol and Quercetin in Humans, Mice, and Rats after Ingestion of Pure Compounds and Grape Juice. J. Agric. Food Chem. 2004, 52, 935–942. [Google Scholar] [CrossRef]
- Garcia-Perez, I.; Posma, J.M.; Chambers, E.S.; Nicholson, J.K.; Mathers, J.C.; Beckmann, M.; Draper, J.; Holmes, E.; Frost, G. An Analytical Pipeline for Quantitative Characterization of Dietary Intake: Application To Assess Grape Intake. J. Agric. Food Chem. 2016, 64, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Ulaszewska, M.; Garcia-Aloy, M.; Vázquez-Manjarrez, N.; Soria-Florido, M.T.; Llorach, R.; Mattivi, F.; Manach, C. Food Intake Biomarkers for Berries and Grapes. Genes Nutr. 2020, 15, 17. [Google Scholar] [CrossRef]
- Lord, R.S.; Burdette, C.K.; Bralley, J.A. Significance of Urinary Tartaric Acid. Clin. Chem. 2005, 51, 672–673. [Google Scholar] [CrossRef]
- Donovan, J.L.; Bell, J.R.; Kasim-Karakas, S.; German, J.B.; Walzem, R.L.; Hansen, R.J.; Waterhouse, A.L. Catechin Is Present as Metabolites in Human Plasma after Consumption of Red Wine. J. Nutr. 1999, 129, 1662–1668. [Google Scholar] [CrossRef]
- Tsang, C.; Auger, C.; Mullen, W.; Bornet, A.; Rouanet, J.M.; Crozier, A.; Teissedre, P.L. The Absorption, Metabolism and Excretion of Flavan-3-Ols and Procyanidins Following the Ingestion of a Grape Seed Extract by Rats. Br. J. Nutr. 2005, 94, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.; Lombardi, D.; Marucci-Wellman, H.; Roenneberg, T. Chronotypes in the US—Influence of Age and Sex. PLoS ONE 2017, 12, e0178782. [Google Scholar] [CrossRef]
- Turco, M.; Corrias, F.; Chiaromanni, M.; Bano, M.; Salamanca, M. The Self-Morningness/Eveningness (Self-ME): An Extremely Concise and Totally Subjective Assessment of Diurnal Preference. Chronobiol. Int. 2015, 32, 1192–1200. [Google Scholar]
- Hurtado-Barroso, S.; Quifer-Rada, P.; Rinaldi de Alvarenga, J.F.; Pérez-Fernández, S.; Tresserra-Rimbau, A.; Lamuela-Raventos, R.M. Changing to a Low-Polyphenol Diet Alters Vascular Biomarkers in Healthy Men after Only Two Weeks. Nutrients 2018, 10, 1766. [Google Scholar] [CrossRef] [PubMed]
- Chun, O.K.; Chung, S.J.; Song, W.O. Estimated Dietary Flavonoid Intake and Major Food Sources of U.S. Adults. J. Nutr. 2007, 137, 1244–1252. [Google Scholar] [CrossRef]
- Chun, O.K.; Floegel, A.; Chung, S.J.; Chung, C.E.; Song, W.O.; Koo, S.I. Estimation of Antioxidant Intakes from Diet and Supplements in U.S. Adults. J. Nutr. 2010, 140, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Vance, T.M.; Chun, O.K. Estimated Intake and Major Food Sources of Flavonoids among US Adults: Changes between 1999-2002 and 2007-2010 in NHANES. Eur. J. Nutr. 2016, 55, 833–843. [Google Scholar] [CrossRef]
- National Cancer Institute, Surveillance Research Branch. Available online: http://riskfactor.cancer.gov/DHQ/index.html (accessed on 1 December 2020).
- Automated Self-Administered 24-Hour (ASA24®®) Dietary Assessment Tool. Available online: https://epi.grants.cancer.gov/asa24/ (accessed on 1 December 2020).
- Agricultural Research Service, Food Surveys Research Group. USDA Food and Nutrient Database for Dietary Studies, 1.0 2004. Available online: https://data.nal.usda.gov/dataset/food-and-nutrient-database-dietary-studies-fndds (accessed on 1 December 2020).
- Miglio, C.; Peluso, I.; Raguzzini, A.; Villan, D.; Cesqui, E.; Catasta, G.; Toti, E.; Serafini, M. Antioxidant and Inflammatory Response Following High-Fat Meal Consumption in Overweight Subjects. Eur. J. Nutr. 2013, 52, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Wang-Polagruto, J.; Polagruto, J.; Keen, C.L.; Jialal, I. High-Fat, Energy-Dense, Fast-Food-Style Breakfast Results in an Increase in Oxidative Stress in Metabolic Syndrome. Metabolism 2008, 57, 867–870. [Google Scholar] [CrossRef]
- Zaheer, K. Hen Egg Carotenoids (Lutein and Zeaxanthin) and Nutritional Impacts on Human Health: A Review. CyTA—J. Food 2017, 15, 474–487. [Google Scholar] [CrossRef]
- Andersen, C.J. Bioactive Egg Components and Inflammation. Nutrients 2015, 7, 7889–7913. [Google Scholar] [CrossRef]
- Dorjgochoo, T.; Gao, Y.T.; Chow, W.H.; Shu, X.O.; Yang, G.; Cai, Q.; Rothman, N.; Cai, H.; Li, H.; Deng, X.; et al. Major Metabolite of F2-Isoprostane in Urine May Be a More Sensitive Biomarker of Oxidative Stress than Isoprostane Itself. Am. J. Clin. Nutr. 2012, 96, 405–414. [Google Scholar] [CrossRef]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-Restricted Feeding Is a Preventative and Therapeutic Intervention against Diverse Nutritional Challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef]
- Flanagan, A.; Bechtold, D.A.; Pot, G.K.; Johnston, J.D. Chrono-Nutrition: From Molecular and Neuronal Mechanisms to Human Epidemiology and Timed Feeding Patterns. J. Neurochem. 2020, 157, 53–72. [Google Scholar] [CrossRef]
- St-Onge, M.-P.; Ard, J.; Baskin, M.L.; Chiuve, S.E.; Johnson, H.M.; Kris-Etherton, P.; Varady, K.; on behalf of the American Heart Association Obesity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; et al. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation 2017, 135, e96–e121. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Allison, D.B.; Fontana, L.; Harvie, M.; Longo, V.D.; Malaisse, W.J.; Mosley, M.; Notterpek, L.; Ravussin, E.; Scheer, F.A.; et al. Meal Frequency and Timing in Health and Disease. Proc. Natl. Acad. Sci. USA 2014, 111, 16647–16653. [Google Scholar] [CrossRef] [PubMed]
- Blanton, C.; Gordon, B. Effect of Morning vs. Evening Turmeric Consumption on Urine Oxidative Stress Biomarkers in Obese, Middle-Aged Adults: A Feasibility Study. Int. J. Environ. Res. Public Health 2020, 17, 4088. [Google Scholar] [CrossRef]
- Li, Z.; Henning, S.M.; Zhang, Y.; Zerlin, A.; Li, L.; Gao, K.; Lee, R.P.; Karp, H.; Thames, G.; Bowerman, S.; et al. Antioxidant-Rich Spice Added to Hamburger Meat during Cooking Results in Reduced Meat, Plasma, and Urine Malondialdehyde Concentrations. Am. J. Clin. Nutr. 2010, 91, 1180–1184. [Google Scholar] [CrossRef]
- Regueiro, J.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Estruch, R.; Lamuela-Raventós, R. Development of a LC-ESI-MS/MS Approach for the Rapid Quantification of Main Wine Organic Acids in Human Urine. J. Agric. Food Chem. 2013, 61, 6763–6768. [Google Scholar] [CrossRef]
- Baba, S.; Osakabe, N.; Natsume, M.; Muto, Y.; Takizawa, T.; Terao, J. In Vivo Comparison of the Bioavailability of (+)-Catechin, (-)-Epicatechin and Their Mixture in Orally Administered Rats. J. Nutr. 2001, 131, 2885–2891. [Google Scholar] [CrossRef]
- Brose, S.A.; Thuen, B.T.; Golovko, M.Y. LC/MS/MS Method for Analysis of E₂ Series Prostaglandins and Isoprostanes. J. Lipid Res. 2011, 52, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Morris, M.E. Liquid Chromatography-Tandem Mass Spectroscopy Assay for Quercetin and Conjugated Quercetin Metabolites in Human Plasma and Urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2005, 821, 194–201. [Google Scholar] [CrossRef] [PubMed]
- National Center for Health Statistics; U.S. Department of Agriculture, Center for Nutrition Policy and Promotion. What We Eat in America/National Health and Nutrition Examination Survey, 2015–2016. Healthy Eating Index—2015 Scores; National Center for Health Statistics; U.S. Department of Agriculture, Center for Nutrition Policy and Promotion: Washington, DC, USA, 2016.
- Buttgereit, F.; Doering, G.; Schaeffler, A.; Witte, S.; Sierakowski, S.; Gromnica-Ihle, E.; Jeka, S.; Krueger, K.; Szechinski, J.; Alten, R. Targeting Pathophysiological Rhythms: Prednisone Chronotherapy Shows Sustained Efficacy in Rheumatoid Arthritis. Ann. Rheum. Dis. 2010, 69, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Buttgereit, F.; Mehta, D.; Kirwan, J.; Szechinski, J.; Boers, M.; Alten, R.E.; Supronik, J.; Szombati, I.; Romer, U.; Witte, S.; et al. Low-Dose Prednisone Chronotherapy for Rheumatoid Arthritis: A Randomised Clinical Trial (CAPRA-2). Ann. Rheum. Dis. 2013, 72, 204–210. [Google Scholar] [CrossRef]
- Cutolo, M. Glucocorticoids and Chronotherapy in Rheumatoid Arthritis. RMD Open 2016, 2, e000203. [Google Scholar] [CrossRef]
- Bonten, T.N.; Snoep, J.D.; Assendelft, W.J.J.; Zwaginga, J.J.; Eikenboom, J.; Huisman, M.V.; Rosendaal, F.R.; van der Bom, J.G. Time-Dependent Effects of Aspirin on Blood Pressure and Morning Platelet Reactivity: A Randomized Cross-over Trial. Hypertension 2015, 65, 743–750. [Google Scholar] [CrossRef]
- Hermida, R.C.; Ayala, D.E.; Fernández, J.R.; Mojón, A.; Smolensky, M.H.; Fabbian, F.; Portaluppi, F. Administration-Time Differences in Effects of Hypertension Medications on Ambulatory Blood Pressure Regulation. Chronobiol. Int. 2013, 30, 280–314. [Google Scholar] [CrossRef]
- Ince, L.M.; Barnoud, C.; Lutes, L.K.; Pick, R.; Wang, C.; Sinturel, F.; Chen, C.-S.; de Juan, A.; Weber, J.; Holtkamp, S.J.; et al. Influence of Circadian Clocks on Adaptive Immunity and Vaccination Responses. Nat. Commun. 2023, 14, 476. [Google Scholar] [CrossRef]
- Vassalle, C.; Bianchi, S.; Battaglia, D.; Landi, P.; Bianchi, F.; Carpeggiani, C. Elevated Levels of Oxidative Stress as a Prognostic Predictor of Major Adverse Cardiovascular Events in Patients with Coronary Artery Disease. J. Atheroscler. Thromb. 2012, 19, 712–717. [Google Scholar] [CrossRef]
- Pigazzani, F.; Gorni, D.; Dyar, K.A.; Pedrelli, M.; Kennedy, G.; Costantino, G.; Bruno, A.; Mackenzie, I.; MacDonald, T.M.; Tietge, U.J.F.; et al. The Prognostic Value of Derivatives-Reactive Oxygen Metabolites (d-ROMs) for Cardiovascular Disease Events and Mortality: A Review. Antioxidants 2022, 11, 1514. [Google Scholar] [CrossRef]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Ding, Y.; Li, H.; Li, Y.; Liu, D.; Zhang, L.; Wang, T.; Liu, T.; Ma, L. Protective Effects of Grape Seed Proanthocyanidins on the Kidneys of Diabetic Rats through the Nrf2 Signalling Pathway. Evid. Based Complement. Altern. Med. 2020, 2020, 5205903. [Google Scholar] [CrossRef]
- Kode, A.; Rajendrasozhan, S.; Caito, S.; Yang, S.-R.; Megson, I.L.; Rahman, I. Resveratrol Induces Glutathione Synthesis by Activation of Nrf2 and Protects against Cigarette Smoke-Mediated Oxidative Stress in Human Lung Epithelial Cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L478–L488. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M. Circadian Rhythms and Rheumatoid Arthritis. Jt. Bone Spine 2018, 86, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; De Giorgi, A.; D’Onghia, M.; De Giorgio, R.; Fabbian, F.; Manfredini, R. Chronobiology and Chronotherapy in Inflammatory Joint Diseases. Pharmaceutics 2021, 13, 1832. [Google Scholar] [CrossRef] [PubMed]
- Bowles, N.P.; Thosar, S.S.; Herzig, M.X.; Shea, S.A. Correction to: Chronotherapy for Hypertension. Curr. Hypertens. Rep. 2018, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, M.H.; Hermida, R.C.; Geng, Y.J. Chronotherapy of Cardiac and Vascular Disease: Timing Medications to Circadian Rhythms to Optimize Treatment Effects and Outcomes. Curr. Opin. Pharmacol. 2020, 57, 41–48. [Google Scholar] [CrossRef]
- Lee, S.H.; Wan, Q.; Wentworth, A.; Ballinger, I.; Ishida, K.; Collins, J.E.; Tamang, S.; Huang, H.-W.; Li, C.; Hess, K.; et al. Implantable System for Chronotherapy. Sci. Adv. 2021, 7, eabj4624. [Google Scholar] [CrossRef]
- Gadacha, W.; Ben-Attia, M.; Bonnefont-Rousselot, D.; Aouani, E.; Ghanem-Boughanmi, N.; Touitou, Y. Resveratrol Opposite Effects on Rat Tissue Lipoperoxidation: Pro-Oxidant during Day-Time and Antioxidant at Night. Redox Rep. 2009, 14, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Helmersson, J.; Basu, S. F2-Isoprostane Excretion Rate and Diurnal Variation in Human Urine. Prostaglandins Leukot. Essent. Fat. Acids 1999, 61, 203–205. [Google Scholar] [CrossRef]
- Savage, K.; Gogarty, L.; Lea, A.; Deleuil, S.; Nolidin, K.; Croft, K.; Stough, C. The Relationship between F(2)-Isoprostanes Plasma Levels and Depression Symptoms in Healthy Older Adults. Antioxidants 2022, 11, 822. [Google Scholar] [CrossRef] [PubMed]
- Wiener, C.; Rassier, G.T.; Kaster, M.P.; Jansen, K.; Pinheiro, R.T.; Klamt, F.; Magalhães, P.V.; Kapczinski, F.; Ghisleni, G.; da Silva, R.A. Gender-Based Differences in Oxidative Stress Parameters Do Not Underlie the Differences in Mood Disorders Susceptibility between Sexes. Eur. Psychiatry 2014, 29, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, E.; Domanico, F.; La Russa, D.; Pellegrino, D. Sex Differences in Oxidative Stress Biomarkers. Curr. Drug Targets 2014, 15, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Klawitter, J.; Haschke, M.; Shokati, T.; Klawitter, J.; Christians, U. Quantification of 15-F2t-Isoprostane in Human Plasma and Urine: Results from Enzyme-Linked Immunoassay and Liquid Chromatography/Tandem Mass Spectrometry Cannot Be Compared. Rapid Commun. Mass Spectrom. 2011, 25, 463–468. [Google Scholar] [CrossRef] [PubMed]

| Activity | 2 Days before the Lab Visit | 12 h before the Lab Visit | Lab Visit | 1–6 h Period Post-Meal |
|---|---|---|---|---|
| Low-antioxidant diet | x | |||
| Food records | x | |||
| Water-only fast | x | x | ||
| Urine sample * | x | x | ||
| Treatment administered | x | |||
| Diet assessment | x |
| Ingredient | kJ | Fat (g) | Carbohydrate (g) | Protein (g) | (% Total Energy) | ||
|---|---|---|---|---|---|---|---|
| Fat | Carb | Protein | |||||
| Biscuit (150 g) | 2196 | 30 | 50 | 12 | |||
| Egg White (150 g) | 347 | 0 | 3 | 15 | |||
| Cheddar Cheese (50 g) | 845 | 18 | 1 | 11 | |||
| Butter (20 g) | 598 | 17 | 0 | 0 | |||
| Total | 3987 | 65 | 54 | 38 | 61% | 23% | 16% |
| Analyte | Q1 | Q3 | DP | CE | CXP |
|---|---|---|---|---|---|
| Catechin | 289.0 | 108.9 | −90 | −90 | −5 |
| Catechin | 289.0 | 205.0 | −90 | −90 | −15 |
| Quercetin | 300.9 | 150.8 | −90 | −90 | −13 |
| Quercetin | 301.1 | 106.8 | −90 | −90 | −13 |
| Resveratrol | 227.0 | 143.0 | −90 | −90 | −13 |
| Resveratrol | 226.9 | 185.1 | −75 | −75 | −13 |
| PGF2α | 353.0 | 193.0 | −90 | −90 | −19 |
| PGF2α | 353.4 | 309.1 | −50 | −50 | −13 |
| Fisetin | 285 | 135 | −90 | −90 | −13 |
| Fisetin | 285 | 121 | −90 | −90 | −13 |
| PGF2α-d4 | 357.0 | 197.0 | −90 | −90 | −19 |
| PGF2α-d4 | 357.0 | 295.0 | −80 | −80 | −13 |
| Tartaric acid | 149 | 87 | −25 | −20 | −10 |
| Tartaric acid | 149 | 73 | −25 | −25 | −10 |
| Tartaric acid-d2 | 151 | 88 | −25 | −20 | −10 |
| Tartaric acid-d2 | 151 | 74 | −25 | −25 | −10 |
| Characteristic | Mean (SD) or Number (%) | |
|---|---|---|
| Females | Males | |
| Sex | 19 (59%) | 13 (41%) |
| Age, y | 31.42 (9.24) | 30.08 (9.57) |
| Height, cm | 164.23 (6.27) | 177.41 (9.04) |
| Weight, kg | 72.42 (18.39) | 78.76 (11.39) |
| BMI, kg/m2 | 26.79 (6.28) | 25.12 (3.93) |
| Time Period | Grape, a.m. | Grape, p.m. | ||
|---|---|---|---|---|
| Mean (SD) ng/mg Cr | Median (IQR) ng/mg Cr | Mean (SD) ng/mg Cr | Median (IQR) ng/mg Cr | |
| 0 h (baseline) | 3.49 (2.39) | 2.73 (3.93) | 2.84 (1.58) | 2.56 (1.41) |
| 0–1 h | 6.28 (4.62) * | 5.11 (4.86) | 6.15 (3.84) | 5.98 (5.27) |
| 1–6 h | 9.25 (1.86) # | 9.27 (1.69) | 7.01 (3.71) | 5.32 (6.57) |
| Placebo, a.m. | Placebo, p.m. | |||
| 0 h (baseline) | 2.61 (1.30) | 2.90 (2.46) | 3.10 (1.95) | 2.42 (2.26) |
| 0–1 h | 9.08 (2.58) ## | 9.03 (4.08) | 5.43 (3.27) | 4.49 (5.73) |
| 1–6 h | 9.92 (2.62) | 9.78.(4.12) | 7.73 (3.89) | 6.77 (4.10) |
| HEI-2015 Output | Mean (SD) | Median (IQR) |
|---|---|---|
| Total HEI-2015 score (maximum = 100) | 64.77 (11.79) | 63.43 (13.51) |
| Food Group (Component score maximum) | ||
| Total Vegetables (5) | 3.84 (1.05) | 4.13 (2.15) |
| Greens and Beans (5) | 4.10 (1.33) | 5.00 (1.69) |
| Total Fruits (5) | 3.49 (1.49) | 3.83 (2.83) |
| Whole Fruits (5) | 4.11 (1.39) | 5.00 (1.69) |
| Whole Grains (10) | 3.88 (2.77) | 2.97 (3.00) |
| Dairy (10) | 7.51 (2.33) | 7.34 (4.61) |
| Total Protein Foods (5) | 4.74 (0.59) | 5.00 (0) |
| Seafood and Plant Proteins (5) | 4.54 (1.07) | 5.00 (0) |
| Fatty Acids (10) | 4.38 (2.29) | 4.37 (3.01) |
| Sodium (10) | 3.29 (2.11) | 3.24 (2.41) |
| Refined Grains (10) | 7.69 (2.69) | 9.08 (4.83) |
| Saturated Fats (10) | 4.93 (2.69) | 4.8 (3.88) |
| Added Sugars (10) | 8.24 (2.04) | 8.57 (2.28) |
| Low-Antioxidant Days | Usual Diet | p Value | RDA *, Age 19–50 Years | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | |||
| Total vitamin A activity (RAE), mcg | 362.57 (280.43) | 321.61 (338.89) | 1243.26 (787.36) | 1015.70 (651.27) | <0.001 | 900 (males), 700 (females) |
| Vitamin C, mg | 32.84 (35.92) | 20.09 (35.06) | 98.90 (67.62) | 95.50 (73.19) | <0.001 | 90 (males), 75 (females) |
| Vitamin E (alpha-tocopherol), mg | 5.99 (3.89) | 5.04 (4.52) | 14.62 (10.96) | 10.96 (7.7) | <0.001 | 15 (males and females) |
| Selenium, mcg | 86.61 (47.24) | 85.49 (56.24) | 116.99 (88.70) | 81.25 (68.75) | 0.31 | 55 (males and females) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanton, C.; Ghimire, B.; Khajeh Pour, S.; Aghazadeh-Habashi, A. Circadian Modulation of the Antioxidant Effect of Grape Consumption: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2023, 20, 6502. https://doi.org/10.3390/ijerph20156502
Blanton C, Ghimire B, Khajeh Pour S, Aghazadeh-Habashi A. Circadian Modulation of the Antioxidant Effect of Grape Consumption: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2023; 20(15):6502. https://doi.org/10.3390/ijerph20156502
Chicago/Turabian StyleBlanton, Cynthia, Biwash Ghimire, Sana Khajeh Pour, and Ali Aghazadeh-Habashi. 2023. "Circadian Modulation of the Antioxidant Effect of Grape Consumption: A Randomized Controlled Trial" International Journal of Environmental Research and Public Health 20, no. 15: 6502. https://doi.org/10.3390/ijerph20156502
APA StyleBlanton, C., Ghimire, B., Khajeh Pour, S., & Aghazadeh-Habashi, A. (2023). Circadian Modulation of the Antioxidant Effect of Grape Consumption: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 20(15), 6502. https://doi.org/10.3390/ijerph20156502









