Abstract
Emotional dysregulation symptoms following a concussion are associated with an increased risk for emotional dysregulation disorders (e.g., depression and anxiety), especially in adolescents. However, predicting the emergence or worsening of emotional dysregulation symptoms after concussion and the extent to which this predates the onset of subsequent psychiatric morbidity after injury remains challenging. Although advanced neuroimaging techniques, such as functional magnetic resonance imaging and diffusion magnetic resonance imaging, have been used to detect and monitor concussion-related brain abnormalities in research settings, their clinical utility remains limited. In this narrative review, we have performed a comprehensive search of the available literature regarding emotional regulation, adolescent concussion, and advanced neuroimaging techniques in electronic databases (PubMed, Scopus, and Google Scholar). We highlight clinical evidence showing the heightened susceptibility of adolescents to experiencing emotional dysregulation symptoms following a concussion. Furthermore, we describe and provide empirical support for widely used magnetic resonance imaging modalities (i.e., functional and diffusion imaging), which are utilized to detect abnormalities in circuits responsible for emotional regulation. Additionally, we assess how these abnormalities relate to the emotional dysregulation symptoms often reported by adolescents post-injury. Yet, it remains to be determined if a progression of concussion-related abnormalities exists, especially in brain regions that undergo significant developmental changes during adolescence. We conclude that neuroimaging techniques hold potential as clinically useful tools for predicting and, ultimately, monitoring the treatment response to emotional dysregulation in adolescents following a concussion.
1. Introduction
Concussion, also known as mild traumatic brain injury (mTBI), is defined as a transitory disturbance in brain functioning due to complex pathophysiological processes induced by a traumatic event [1,2]. Sport- and recreation-related concussions represent the majority of these injuries in children and adolescents, affecting 1.9 million youth in the U.S. each year [3]. Diagnosing concussions and determining clinical recovery depend on an individual-centered approach. In this approach, identifying an ‘injury state’ depends on the presence of concussion symptoms that began immediately following the traumatic event. Common concussion symptoms include headache, fatigue, dizziness, nausea, memory difficulties, sleep disturbances, and changes in mood and personality [4]. Thus, the self-reporting (or caregiver-reporting) of these symptoms is a fundamental part of this diagnosis. This is complicated by the subjective nature of the symptom reporting (and, thus, by the possibility of patients over- or under-reporting their symptoms) and by the absence of objective diagnostic tests and/or biological markers of concussion [5]. As a result, a high percentage of concussions may go undiagnosed. In contrast, an equally high portion of those with new or preexisting psychiatric symptoms may be erroneously diagnosed with a concussion [6]. This becomes even more complicated in adolescents, where an undiagnosed or unresolved concussion might increase the risk of downstream consequences (e.g., prolonged symptoms, academic difficulties, and, less commonly, second impact syndrome or negative outcomes) [4,7] in developing brains.
On average, concussion symptoms in youth resolve within the first two weeks of injury [3,8,9]. However, in some instances, symptoms persist for longer, leading to Persistent Post-Concussion Symptoms (PPCS). Adolescents typically take longer (i.e., around four weeks) to recover from a concussion than children and adults [10,11,12], with adolescent girls taking the longest [13]. The longer recovery time can increase the impact of concussion on this age group. This may lead to persistent symptomatology, with approximately 21–35% of concussed adolescents developing PPCS [14,15,16]. PPCS in adolescents can adversely affect social functioning and academic performance [4] and are often associated with emotional dysregulation [17,18].
It is important to note that due to the rapid development of limbic regions prior to adolescence and the more gradual development of the prefrontal cortex throughout adolescence, this age period is considered a window of heightened vulnerability for psychiatric morbidity. The imbalance in maturity between limbic regions and the prefrontal cortex is thought to contribute to the typical difficulties in regulating emotions and behaviors that occur during adolescence (see Figure 1) [19]. Therefore, sustaining a concussion during adolescence may increase susceptibility to emotional dysregulation in this age group. Despite substantial progress in identifying the clinical outcomes of concussion [20,21,22], the complex pathophysiologic mechanisms underlying emotional dysregulation symptoms are poorly understood in both pediatric and adult samples.

Figure 1.
Developmental curves of neural circuitries. This figure displays the differences between developmental trajectories of limbic systems and prefrontal control regions over time (Figure reproduced with permission from Ref [19]. Copyright 2007 by Elsevier Inc.).
Emotional regulation refers to the ability to monitor, evaluate, and adapt behaviors in response to emotional stimuli [23]. Emotions are patterns of perceptions, experiences, and reactions to challenging situations, and the dynamic cycle of perception, evaluation, and response changes continuously [24]. This adaptive function may help or harm someone, depending on how well emotions are regulated. Controversial among researchers is whether concussed patients develop emotional dysregulation symptoms as (1) a direct result of injury to brain regions involved in emotional regulation circuits, (2) a secondary effect of frustrations and/or uncertainty associated with their injury, or withdrawal from sports participation, and/or (3) because of a preexisting vulnerability.
Neuroimaging techniques, including computed tomography and magnetic resonance imaging, have been widely used to objectively characterize neurodevelopmental changes as they occur throughout the lifespan [25,26,27,28]. Neuroimaging has also been extensively used to detect brain abnormalities in concussed samples [29,30]. An emerging line of research further indicates that detecting abnormalities in emotional regulation regions may help explain anxiety symptoms in adolescents following concussion [29]. However, using these techniques is currently clinically indicated as neither a diagnostic nor a prognostic tool for concussion [30,31]. There are practical challenges to including advanced neuroimaging techniques in clinical practice [31], including (1) high costs for implementation and maintenance, (2) prolonged time to collect neuroimaging data that does not match current workflows, and (3) specialized training needed to properly interpret findings. Their use is also often inaccessible in clinical practice, contributing to their limited use in clinical settings. Nonetheless, neuroimaging techniques offer an objective way to identify neural correlates of concussion and/or predict outcomes in adolescents [30] and hold promise in further helping to disentangle the contribution of concussion-related brain abnormalities from changes associated with normative adolescent development, typical of this period. Demonstrating the utility of neuroimaging to identify abnormalities associated with emotional dysregulation in adolescent concussions may promote the use of neuroimaging in clinical practice.
The goal of this narrative review is to highlight current evidence on the utility of advanced neuroimaging techniques in investigating emotional dysregulation in adolescents following concussions. To this end, we provide a comprehensive narrative review of the available literature in electronic databases (PubMed, Scopus, and Google Scholar) using keywords such as concussion, post-concussion symptoms, emotional dysregulation, psychological symptoms, psychiatric symptoms, mood symptoms, depression, anxiety, neuroimaging, functional magnetic resonance imaging, and diffusion magnetic resonance imaging. An additional search was conducted using keywords such as children, adolescent, adolescence, youth, and adult to identify studies reporting findings in different age ranges between 2010 and 2023. To provide the reader with a conceptual framework, we will (1) review emotional dysregulation following concussion from a neurodevelopmental perspective; (2) provide an overview of how the employment of different neuroimaging modalities could enhance our understanding of the neuropathophysiological mechanisms driving emotional dysregulation in adolescent concussion; and (3) highlight neuroimaging findings linking concussion and emotional dysregulation from the existing literature. With this work, we seek to convey the breadth and depth of available research on the utility of advanced neuroimaging techniques in investigating emotional dysregulation in adolescent concussions. Additionally, we aim to identify research gaps and provide recommendations for future research in this area.
2. Emotional Dysregulation following Concussion
In the early—acute (<72 h) and sub-acute (72 h to 1 week)—stages of concussion, symptoms tend to be global in nature and reflect overall injury severity [32]. After the first week, symptoms typically cluster into more distinct clinical profiles—i.e., cognitive/fatigue, vestibular, ocular-motor, headache/migraine, cervical, and anxiety/mood [33]—with emotional dysregulation symptoms among the most challenging for clinicians and practitioners to recognize [34,35] and address [36]. With estimates ranging from 7% to 36%, emotional dysregulation symptoms in concussion are highly non-specific. These symptoms include anxiety, depression, affect lability, irritability, hostility, impulsivity, and personality changes. Prominent in mood and anxiety disorders, these symptoms are typically transient in concussion, resolving within one to two weeks of injury [32]. However, in some adolescents, these symptoms persist for longer (more than four weeks), with a significant minority experiencing emotional dysregulation symptoms up to one year post-injury [17,18,37,38]. For example, in a large (N = 14,765) sample of high school students in the United States, 36% of students with a concussion history reported persistent depressive symptoms for up to 12 months [35].
Research over the past decade has helped inform practitioners that depression [12,39,40], anxiety [40,41], posttraumatic stress disorder (PTSD) [42], substance use [43], and suicidal ideation or attempts [44,45,46] are prevalent in adolescents with a history of concussion. Nonetheless, estimates of psychiatric morbidity following concussion continue to vary (for recent reviews, see [47,48]), partly reflecting the challenges of identifying psychiatric symptoms in individuals without a preexisting psychiatric condition [49]. The heterogeneity of screening tools is another likely contributor to this variability, as these tools (e.g., Patient Health Questionnaire-9 and General Anxiety Disorder-7) [50,51] tend to be used in place of more rigorous structural clinical interviews or psychiatric rating scales in routine assessments of concussion [49]. Thus, while there has been substantial progress in understanding the clinical outcomes of concussion, it remains unclear whether we can identify patients at high risk for psychiatric morbidity following a concussion, especially adolescents.
3. Neural Correlates of Emotional Regulation
In the brain, ventro-limbic and dorso-limbic systems are involved in processing and regulating emotions (see Figure 2) [52]. The ventral system is primarily involved in coordinating information from the cortex, evaluating the salience of a stimulus, and eliciting an emotional state in response to the stimulus [53,54]. The amygdala plays a central role in the ventral system by evaluating emotional valence stimuli based on previously learned experiences [55,56,57]. The dorsal system is primarily involved in regulating emotional states and their associated behaviors [58,59]. The dorsal anterior cingulate gyrus is a hub within the executive functioning network [60], while the ventral striatum plays a key role in reward and reinforcement learning [61,62].
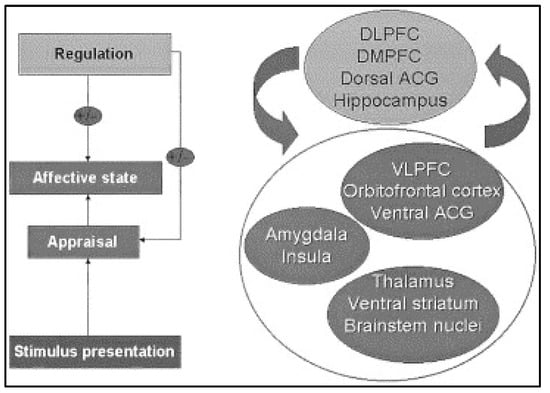
Figure 2.
Schematic diagram of emotion perception. This figure displays the brain regions important for the processes underlying emotion perception. Abbreviations: DLPFC, dorsolateral prefrontal cortex; DMPFC, dorsomedial prefrontal cortex; ACG, anterior cingulate gyrus; VLPFC, ventrolateral prefrontal cortex (Figure reproduced with permission from Ref. [52]. Copyright 2003 by Society of Biological Psychiatry).
Within and between these systems, the subregions of the prefrontal cortex are directly and indirectly connected to other cortical and subcortical regions by major white matter tracts [59]. The inferior longitudinal fasciculus connects the occipital lobe with the medial temporal regions to attribute emotional salience to visual stimuli [63]. The uncinate fasciculus connects subcortical limbic regions with different subregions of the prefrontal cortex to regulate emotions and plan complex behaviors [64]. The cingulum bundle connects subcortical limbic regions with the cingulate cortex and is associated with emotional processing, emotional regulation, and executive functioning [64,65,66,67,68,69,70,71].
During adolescence, prefrontal cortical regions undergo significant structural and functional changes [72]. As adolescents transition from childhood to adulthood, the combination of pubertal and environmental factors (e.g., the rise of sex hormones, exposure to new experiences, social interactions, and acquisition of new skills) promote the strengthening or weakening of the connections within and between these systems [73]. The ability of the brain to reorganize its structure and function in response to environmental demand is referred to as brain plasticity [73]. The plastic changes that, during adolescence, occur in the prefrontal cortex support higher-order cognitive functions such as emotion regulation, decision-making, and social behavior [74]. The learning of new skills and the acquisition of new competence during this developmental period are key to adaptation [75]. While plasticity is beneficial for positive adaptation, it also increases the vulnerability of these systems to neurological injuries, such as concussions [76].
4. Role of Neuroimaging in Concussion
Concussions can damage the brain by either direct collision or the acceleration/deceleration forces associated with impact [77]. Direct collisions can cause diffuse damage due to the propagation of waves through the brain [78]. Biochemically, the effect of these forces on the brain is thought to activate a ‘neurometabolic cascade’ of events that, following the sheering/disruption of neuronal membranes, involves non-specific depolarization, the release of neurotransmitters (particularly excitatory amino acids), and the influx/efflux of ions such as calcium, magnesium, and potassium across the neuronal cytoplasmic membrane [78,79,80]. Concomitantly, there is a physiological decrease in cerebral blood flow [81]. The rapid and dynamic forces associated with the impact can cause profound geometric distortions and microscopic damage to the axon cytoskeleton of white matter fibers (diffuse axonal injury). This type of injury is usually associated with axonal dysfunction [82]. Frontal, temporal, and occipital midline brain regions are particularly vulnerable to acceleration and deceleration forces [83].
Neuroimaging procedures offer a way to examine the pathophysiologic processes of concussion in vivo. Quick and capable of detecting a broad spectrum of pathologies, computed tomography is the current standard neuroimaging procedure for brain injuries on the day of injury. However, patients with concussion typically undergo neither computed tomography nor ‘conventional’ magnetic resonance imaging as the diagnostic capacity of these procedures for mild forms of brain injury is limited [84]. In fact, computed tomography is not suitable for the detection of developmental changes occurring in the adolescent brain or pathophysiological processes characteristic of acute, subacute, or chronic stages of concussion [31,85]. These limitations significantly restrict the clinical utility of this technique in concussion. Current guidelines for adults and pediatrics (e.g., the American Medical Society of Sports Medicine) recommend clinical neuroimaging only when there is concern about intracranial hemorrhage [31]. Magnetic resonance imaging is also widely used in clinical settings as an essential tool for diagnosing and monitoring a wide range of medical conditions. Technological advances in magnetic resonance imaging have led to improvements in image quality through the use of higher magnetic fields (3 Tesla and above) and a decrease in scanning time. Techniques such as GeneRalized Autocalibrating Partially Parallel Acquisitions—GRAPPA, SENsitivity Encoding—SENSE, and/or Simultaneous Multi-Slice Imaging—SMS enable the acquisition of high-quality images in a relatively short period of time. Of the magnetic resonance imaging modalities, functional and diffusion magnetic resonance imaging have been widely used to characterize different properties of the brain functioning and structure, ranging from normative brain development to medical conditions [86,87,88,89].
Over the past fifteen years, the employment of advanced magnetic resonance imaging methods has promoted a better understanding of the pathophysiologic mechanisms of concussion. Their use in longitudinal designs is likely to shed light on important mechanisms underpinning the acute, sub-acute, and chronic stages of concussion. For example, in adolescence, these approaches are well-suited to determine if a progression of concussion-related abnormalities exists in chronic stages of concussion after disentangling the contribution of the neurodevelopmental changes typical of these ages.
4.1. Functional Magnetic Resonance Imaging
Broadly, functional magnetic resonance imaging measures the hemodynamic response, the Blood-oxygen-level-dependent (BOLD) signal, as a proxy of neural activity. This activity corresponds to the oxygen consumption of firing neurons, and it can be assessed either during rest (resting state magnetic resonance imaging) or during task performance (task magnetic resonance imaging) [90,91]. In addition, these methods allow for identifying the temporal correlation between brain regions and quantifying their functional integration or connectivity [90,91].
When analyzing resting-state magnetic resonance imaging, researchers generally focus on functional segregation or functional integration across networks [92,93,94,95,96]. Different statistical approaches have been proposed to investigate these aspects of brain activity. Functional segregation examines regional characteristics of the brain and divides these regions based on their functionality [92,93,94,95,96]. Proposed approaches involve identifying (1) the Amplitude of Low Frequency Fluctuations (ALFF) for individual regions or (2) the Regional Homogeneity (ReHo) or synchronization between regions and their nearest neighbors [92,93,94,95,96]. Functional integration measures the connectivity and synchrony between different regions of the brain [92,93,94,95,96]. This connectivity can be direct (a structural pathway connecting two regions) or indirect (a connection between two regions is mediated by another region) [92,93,94,95,96]. Common approaches to assessing functional integration are Independent Component Analysis (ICA) and Graph theory analysis [92,93,94,95,96]. ICA involves separating the functional signal into independent functional networks based on synchronized BOLD activity and measuring the connectivity within such networks [92,93,94,95,96]. Graph theory analysis models the brain as a network of nodes and edges [92,93,94,95,96]. Nodes indicate the brain regions and the edges indicate the connection between these brain regions [92,93,94,95,96]. Within these models, several features are measured, including path length (number of edges), clustering (local neighborhood connectivity), and small-world (connectivity between nodes when most of them are not directly connected but can be reached through a few connections) [92,93,94,95,96]. In concussion, these methods are suitable for identifying region-specific or network-based changes following injury, how brain networks adapt to the functional changes associated with concussion, and whether changes in connectivity patterns are implicated in the presence or persistency of symptoms, including emotional dysregulation symptoms. Resting state studies in adolescents who sustained a concussion have shown hyperconnectivity and hypoconnectivity compared to controls, depending on the regions studied [97,98,99,100]. Hyperconnectivity has been found within posterior regions (e.g., precuneus and cerebellum) [99] and between the salience network and cerebellum [98]. Hypoconnectivity has been found within anterior regions (inferior and middle frontal gyrus) [99], between the dorsal attention network and inferior frontal gyrus [100], within the default mode network (involved with episodic memory and emotional processing) [97], and between the salience network and the thalamus [98]. However, the correlation between clinical measures and resting state abnormalities has yet to be extensively studied.
Task magnetic resonance imaging maps the response of the brain to tasks administered during imaging collection [101]. Different tasks have been used in humans to engage functionally distinct brain regions while performing a given task. These tasks include the stop signal, which assesses response inhibition by measuring the reaction time to stop and go trials [102]. The monetary incentive evaluates the anticipation and feedback processing of rewards that depend on task performance [103]. Additionally, the emotional face N-back task examines attentional control during a memory task while presenting emotional distractors such as happy, fearful, and neutral faces [104]. In essence, the increased bold signal change elicited by a task (hyperactivation) is thought to reflect neural activity associated with the recruitment of greater brain resources or a compensatory response required to maintain task performance. On the other hand, decreased bold signal change elicited by the same task (hypoactivation) is thought to reflect difficulties in allocating resources to perform this task [101,105]. In concussion, this approach allows us to understand how the brain functionally responds to the direct or indirect effects (e.g., neurometabolic changes) of injury and which patterns of brain activity may help explain the cognitive and emotional challenges adolescents face following injury. Task magnetic resonance imaging findings suggest that those with concussion need to recruit more brain resources to sustain working memory [106,107,108,109,110,111,112,113,114] and attention [115,116] when compared to controls. In adolescents with concussion, poorer working memory is associated with reduced cortical activation, especially in the dorsolateral prefrontal cortex [117,118,119].
4.2. Diffusion Magnetic Resonance Imaging
On the other hand, diffusion magnetic resonance imaging provides indices of tissue microarchitecture by measuring the diffusion imaging properties of the water molecules in the brain tissue [120,121,122]. This technique enables the study of structural connectivity between brain regions. Fractional anisotropy is the most common metric used in diffusion magnetic resonance imaging, indexing the structural collinearity of the fibers and/or their integrity—e.g., damage to axon membranes or myelin sheaths [121,122]. It reflects the preponderance of the water motility along the principal diffusion direction (axial diffusivity) over secondary diffusion directions that are transverse to the axial diffusivity (i.e., radial diffusivity) within a voxel.
Advances in diffusion magnetic resonance imaging have increased our ability to characterize micro- and macro-structural properties of different brain tissues and detect abnormalities across different medical conditions (e.g., diffuse axonal injury, transitory ischemic attack, stroke) [123,124]. State-of-the-art diffusion imaging protocols collect a high number of diffusion gradient directions (i.e., High Angular Resolution Diffusion Imaging; HARDI) that allow resolving the crossing fiber issue, for which the tensor model remains agnostic. Recent recommendations suggest including multiple b-values (i.e., multishell diffusion imaging [86,125] and Diffusional Kurtosis Imaging; DKI) that, by using multiple concentric shells, can either inform on the fine-grained micro-structural properties of the brain tissues or quantify the deviation of diffusion imaging properties from Gaussian behavior. Specifically, by using different b values, it is possible to better account for the effect of noise in the diffusion imaging data [87,126,127]. These advanced protocols allow for the employment of multi-tensor models and cutting-edge mathematical algorithms, such as Multishell Multi-Tissue Constrained Spherical Deconvolution (MSMT-CSD) and Neurite Density Dispersion Imaging (NODDI). MSMT-CSD is a diffusion imaging model that maximizes the precision of the fiber orientation distribution function in white matter voxels and minimizes partial volume bias in voxels containing gray matter and/or corticospinal fluid [128,129,130]. NODDI allows for the compartmentalization of the diffusion imaging signal in intra- (i.e., axons and dendrites) and extra- (i.e., glia, cerebrospinal fluid) neuritic spaces, leading to estimates of the microstructural complexity of different tissues in the brain [131]. Notably, NODDI measures have been shown to be more sensitive than standard diffusion tensor imaging measures (e.g., fractional anisotropy) to structural-developmental changes in normative [25,132,133] and clinical [87,88,89,134,135,136,137] samples. Specific NODDI metrics include the free-water isotropic volume fraction (FISO), reflecting the free water volume fraction; the Neurite Density Index (NDI), reflecting the intra-neurite density; and the Orientation Dispersion Index (ODI), reflecting the angular dispersion of neurites.
Studies using diffusion magnetic resonance imaging have found concussion-related microstructural abnormalities in several brain regions [138,139,140], including the corpus callosum [141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163], internal capsule/anterior thalamic radiation [147,149,153,156,158,159,162,163,164,165,166], and centrum semiovalis/superior longitudinal fasciculus [138,147,158,160,162]. Lower fractional anisotropy in the inferior longitudinal fasciculus and the inferior fronto-occipital fasciculus has been associated with longer recovery time in adolescent concussion [167]. This is likely due to a lack of long associative tracts and their integrative functions. In adolescents, diffusion magnetic resonance imaging abnormalities have been detected up to a month post-injury despite improvements in cognitive ability, suggesting that these abnormalities may persist even after full clinical recovery [168]. Yet, the long-term clinical implications of these findings remain unclear. Few studies have applied NODDI to study concussion in adolescents. One study has shown an association between lower NDI in the corpus callosum and increased brain-injury blood markers (e.g., tau) in adolescent concussions [169]. Another NODDI study has shown that concussion, when followed by poor quality sleep, is associated with decreased NDI in several white matter tracts such as the cingulum bundle, optic radiation, striato-fronto-orbital tract, and superior longitudinal fasciculus I. Furthermore, the study found that decreased NDI in these tracts is associated with increased postconcussive symptom severity in adolescents [170].
4.3. Other Neuroimaging Modalities
While functional and diffusion imaging have been mostly used to document brain abnormalities in concussion, other modalities (e.g., susceptibility-weighted imaging, magnetic resonance spectroscopy, and dynamic susceptibility contrast) have also been used.
Susceptibility-weighted imaging is susceptible to the parametric properties of different blood products of micro- and macro-hemorrhage (e.g., deoxyhemoglobin and hemosiderin). In concussion, employing this technique allows the detection of micro-hemorrhagic lesions that would otherwise go unnoticed (so-called ‘non-hemorrhagic shearing injury’) [171]. Some studies have shown a relationship between the number and volume of acute hemorrhages and clinical outcomes in moderate/severe forms of traumatic brain injury. However, susceptibility-weighted imaging results are inconclusive for mild traumatic brain injury/concussion (for recent reviews, see [30,171,172,173,174]).
Magnetic resonance spectroscopy is a metabolic imaging method that detects and quantifies metabolites (e.g., N-Acetyl Aspartate, Choline, and Creatine) in the brain tissue [175]. In concussion, magnetic resonance spectroscopy allows for studying neurometabolic changes associated with concussion-related lesions [172,176]. Low levels of n-acetyl aspartate and high levels of choline have been interpreted as neuronal damage/stress and glia abnormalities, respectively, in concussion. The magnitude of the changes has also been associated with the severity of symptoms and history of previous concussions. In contrast, the persistency of abnormalities after acute/subacute periods has been associated with persistent post-concussion symptoms [172].
5. Magnetic Resonance Imaging Findings Linking Concussion and Emotional Regulation from the Existing Literature
There has been a paucity of studies examining the structural and functional neural changes accompanying emotional dysregulation in adolescent concussion, especially as to whether detecting concussion-related abnormalities in the brain relates to psychiatric vulnerability following concussion (Table 1). Important evidence of the relationship between brain structure and emotional dysregulation in concussion comes from animal studies [177,178].

Table 1.
Summary of Neuroimaging Studies on Concussion and Emotional Regulation.
Animal studies have shown increased anxiety-related behaviors after a concussion, as measured by the locomotor activity of concussed rats in a maze. These behaviors were associated with increased neuronal excitability of the amygdala, as demonstrated by decreased inhibitory synaptic transmission [177]. Another study focusing on the amygdala examined the freezing behavior of mice under a neutral condition in a fear conditioning paradigm. These mice showed decreased fear response after concussion and decreased amygdala activity in voltage-sensitive dye imaging [178]. Overall, these findings suggest that amygdala dysfunction after injury may explain part of the pathophysiological processes of concussion. Furthermore, it possibly represents the neural underpinnings of anxiety-related behaviors observed in animal models of concussion.
In the human literature, children and adolescents appear to have abnormal neural responses to emotional stimuli after injury [183,184]. Specifically, using functional magnetic resonance imaging, studies have found blunted amygdala responding to emotional stimuli in 14–18-year-old adolescents with a concussion, with a stronger effect in those with persistent symptomatology [183]. In addition to amygdala dysfunction, concussed adolescents—compared to non-concussed adolescents— have also shown a wider spread of frontal lobe activation when requested to process emotional stimuli during an inhibitory control task. This suggests needing to compensate more when emotional information interferes with an inhibitory control task [184]. Amygdala dysfunction and abnormal communication between the amygdala and frontal lobes have been consistently implicated in the pathophysiology of both depression and anxiety disorders [111,188,189,190,191,192,193]. Thus, concussion-related lesions affecting these regions may contribute to the emotional dysregulation often reported after a concussion.
Evidence from adults suggests that white matter abnormalities in those who developed depressive symptoms following concussion do not differ from those found in adults with depression alone [186]. Specifically, when comparing adults with depression to adults with concussion and depression, Maller et al. found that both groups had reduced collinearity in white matter tracts that are heavily involved in regulating emotions. These tracts include the forceps minor and superior longitudinal fasciculus [186]. Diffusion imaging abnormalities in emotional regulation circuits have been associated with new-onset depression following concussion [194], similar to those reported in depression [195,196].
To our knowledge, few studies have examined the relationship between the concussed brain and the emergence of depression or anxiety in children and adolescents. Max, Keatley [181] found evidence for a relationship between concussion-related abnormalities in brain regions involved in emotional regulation and the onset of subsequent psychiatric morbidity. Specifically, they found fronto-temporal lesions in gray (inferior frontal gyrus) and white (frontal and temporal) matter regions in a sample of children and adolescents (n = 15) who developed full or subclinical forms of depression or anxiety six months post-injury. Most of them (60%) had no prior psychiatric history [181]. Our recent work with concussed adolescents suggests that sex and pubertal status may also play a role [29]. Compared to non-concussed adolescents, concussed adolescents with more advanced pubertal maturation had lower neurite density index (a NODDI metric that represents water diffusivity in the intraneuritic space) [131] in the cingulum bundle and forceps minor [29]. These white matter tracts are involved in emotional regulation [64]. A lower neurite density index in these tracts was also associated with higher levels of anxiety shortly after the concussion, particularly in girls [29]. These findings suggest that girls at a more advanced stage of puberty might be at greater risk for developing psychological problems from concussion compared to boys at a similar pubertal stage or girls at a less mature stage of puberty. What remains unknown is if these structural abnormalities are associated with transient symptomatology (e.g., acute symptoms of emotional dysregulation that resolve within a few weeks) or if they relate to an increased vulnerability for psychiatric morbidity later in life.
6. Factors Contributing to a Knowledge Gap in the Current Literature
As noted above, adolescence is a sensitive developmental window characterized by structural and functional changes reflecting brain plasticity, and injury may induce a risk for psychiatric morbidity. A key aspect that needs further investigation is whether a concussion in the adolescent brain can affect the establishment of connections that support emotion regulation. This may lead to heightened vulnerability to anxiety and depression later in life. As noted above, adolescence is a sensitive developmental window characterized by structural and functional changes reflecting brain plasticity, and injury may induce risk. However, the lack of longitudinal studies, the paucity of studies in youth, and heterogeneity due to a lack of standardized protocols across studies contribute to this knowledge gap. These factors limit the interpretability of the existing findings and their clinical and translational relevance [197].
To properly address this question, longitudinal neuroimaging studies are needed to disentangle the effects of concussion on the adolescent brain from those associated with typical development or those related to preexisting psychiatric vulnerability (e.g., history of trauma, familial history) and morbidity (e.g., neural correlates of a psychiatric illness and/or the effects of psychotropic medications). Altogether, the literature reviewed in this section provides support for the employment of advanced neuroimaging protocols as a tool to determine the effect of concussions on the developing brain. Moreover, it further determines if a progression of concussion-related lesions in brain regions involved in emotional regulation relates to a heightened vulnerability for psychiatric morbidity in adolescents who sustain a concussion. However, the lack of longitudinal studies, the paucity of studies in youth, and heterogeneity due to a lack of standardized protocols across studies limit the interpretability of these findings and their clinical translational relevance [197].
7. Clinical Relevance
Understanding the neural mechanisms of emotional dysregulation following concussion in adolescents may promote early identification of at-risk adolescents. Specifically, based on the networks structurally and/or functionally affected by concussion, advanced neuroimaging techniques may help (1) assess risk for persistent emotional dysregulation symptoms and psychiatric morbidity, (2) tailor individualized treatment approaches (e.g., cognitive/behavioral therapies), (3) track recovery progress, and (4) inform decisions regarding return to activities. The identification of specific regions implicated in emotional dysregulation following concussion in adolescents might also pave the way for the development and inclusion of novel personalized treatments in clinical practice. One such example is neurofeedback, directed at modulating the functioning of identified targeted regions that may be key for recovery in adolescent concussion. Neurofeedback is a form of biofeedback technique that aims to train individuals to consciously control their brain activity [198]. Currently, there is limited evidence of the utility of neurofeedback in treating concussions [199,200]. However, this approach has been successfully implemented to strengthen emotional regulation networks [201] and treat emotional dysregulation disorders such as depression [202] in adolescents.
8. Recommendations for Future Research
There is a paucity of studies directly examining the relationship between concussion-related brain abnormalities and heightened vulnerability to emotional dysregulation in adolescents. The identification of structural and/or functional abnormalities in emotional regulation circuits may serve as biomarkers of emotional dysregulation in adolescent concussion and promote the identification of those adolescents who are most at risk for psychiatric morbidity following concussion. To achieve this, future studies should employ advanced neuroimaging techniques in longitudinal designs that allow for the identification of functional and structural changes in regions and networks involved with emotional regulation and the effects of environmental factors (e.g., sleep and physical activities) on brain recovery post-concussion. However, there are important steps to consider before contemplating their implementation into clinical practice: (1) Standardize neuroimaging protocols and analytic methods to ensure consistency and facilitate comparison across different sites; (2) establish data sharing protocols to promote replication of findings incorporating different socio–demographic factors (e.g., race, ethnicity, sex, income, and parent education); (3) foster communication between researchers and clinical providers to facilitate the translational aspect of concussion neuroimaging research; and (4) perform validation studies that address the clinical relevance and cost-effectiveness of these techniques in clinical settings.
9. Conclusions
Although variability in study design, age ranges, sample sizes, and post-injury time points limits conclusions, the literature reviewed above supports the employment of advanced magnetic resonance imaging techniques in adolescent concussions. These techniques have the potential to achieve several key objectives: (1) promote a better understanding of the different pathophysiologic mechanisms of acute, subacute, and chronic stages of concussion; (2) discern between structural and functional abnormalities associated with a concussion from those associated with normative development or preexisting psychopathology, which may overlap (3) foster early identification of risk for psychiatric outcomes; and (4) serve as a prognosis tool for returning to activities. These can be instrumental in improving clinical decision-making and fostering the identification of early intervention strategies aimed at strengthening emotional and behavioral regulation strategies in at-risk groups.
Author Contributions
Conceptualization, J.P.L.S. and A.V.; writing—original draft preparation, J.P.L.S. and A.V.; writing—review and editing, J.P.L.S., M.J.-R., A.P.K., M.W.C. and A.V.; supervision, A.P.K. and A.V.; funding acquisition, A.P.K. and A.V. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was supported by the National Institute of Mental Health (R01MH114881, PIs: Versace, Kontos) and the Chuck Noll Foundation for Brain Injury Research (FP00004146).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Kontos and Collins receive royalties from APA Books and research support through the University of Pittsburgh from the National Football League (NFL). Collins was a co-developer and a former shareholder (relationship ended 12/16/19) of ImPACT Applications, Inc. Other authors report no biomedical financial interests or potential conflicts of interest. The funders had no role in the design or in the decision to publish this review.
References
- McCrory, P.; Meeuwisse, W.; Dvorak, J.; Aubry, M.; Bailes, J.; Broglio, S.; Cantu, R.C.; Cassidy, D.; Echemendia, R.J.; Castellani, R.J. Consensus statement on concussion in sport—The 5th international conference on concussion in sport held in Berlin, October 2016. Br. J. Sport. Med. 2017, 51, 838–847. [Google Scholar]
- Kazl, C.; Torres, A. Definition, classification, and epidemiology of concussion. Semin. Pediatr. Neurol. 2019, 9, 13. [Google Scholar] [CrossRef]
- Bryan, M.A.; Rowhani-Rahbar, A.; Comstock, R.D.; Rivara, F.; on behalf of the Seattle Sports Concussion Research Collaborative. Sports-and recreation-related concussions in US youth. Pediatrics 2016, 138, e20154635. [Google Scholar] [CrossRef]
- Leddy, J.J.; Haider, M.N.; Noble, J.M.; Rieger, B.; Flanagan, S.; McPherson, J.I.; Shubin-Stein, K.; Saleem, G.T.; Corsaro, L.; Willer, B. Clinical Assessment of Concussion and Persistent Post-Concussive Symptoms for Neurologists. Curr. Neurol. Neurosci. Rep. 2021, 21, 1–14. [Google Scholar] [CrossRef]
- Anzalone, C.; Luedke, J.C.; Decker, S.L. Translating research practices in the cognitive assessment of mild traumatic brain injury into applied practice: A systematic review and assessment guide. Transl. Issues Psychol. Sci. 2023, 9, 6–24. [Google Scholar] [CrossRef]
- Ali, D.; Posas, J.H. A system-wide retrospective cohort analysis of psychiatric diagnoses and persistent symptoms following concussion. Neurology 2022, 98, S16–S17. [Google Scholar] [CrossRef]
- McLendon, L.A.; Kralik, S.F.; Grayson, P.A.; Golomb, M.R. The controversial second impact syndrome: A review of the literature. Pediatr. Neurol. 2016, 62, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Rotter, J.; Kamat, D. Concussion in children. Pediatr. Ann. 2019, 48, e182–e185. [Google Scholar] [CrossRef]
- Howell, D.R.; Kriz, P.; Mannix, R.C.; Kirchberg, T.; Master, C.L.; Meehan, W.P., III. Concussion symptom profiles among child, adolescent, and young adult athletes. Clin. J. Sport Med. 2019, 29, 391–397. [Google Scholar] [CrossRef]
- Field, M.; Collins, M.W.; Lovell, M.R.; Maroon, J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J. Pediatr. 2003, 142, 546–553. [Google Scholar] [CrossRef]
- Eisenberg, M.A.; Andrea, J.; Meehan, W.; Mannix, R. Time interval between concussions and symptom duration. Pediatrics 2013, 132, 8–17. [Google Scholar] [CrossRef]
- Chrisman, S.P.; Richardson, L.P. Prevalence of diagnosed depression in adolescents with history of concussion. J. Adolesc. Health 2014, 54, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, A.-A.; Tang, K.; Yeates, K.O.; Pusic, M.V.; Boutis, K.; Craig, W.R.; Gravel, J.; Freedman, S.B.; Gagnon, I.; Gioia, G.A.; et al. Natural progression of symptom change and recovery from concussion in a pediatric population. JAMA Pediatr. 2019, 173, e183820. [Google Scholar] [CrossRef]
- Ewing-Cobbs, L.; Cox, C.S.; Clark, A.E.; Holubkov, R.; Keenan, H.T. Persistent postconcussion symptoms after injury. Pediatrics 2018, 142, e20180939. [Google Scholar] [CrossRef]
- Fried, E.; Balla, U.; Catalogna, M.; Kozer, E.; Oren-Amit, A.; Hadanny, A.; Efrati, S. Persistent post-concussive syndrome in children after mild traumatic brain injury is prevalent and vastly underdiagnosed. Sci. Rep. 2022, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, L.; Sharma, M.J.; Madigan, S.; Callahan, B.L.; Yeates, K.O. Classification criteria and rates of persistent post-concussive symptoms in children: A systematic review and meta-analysis. J. Pediatr. 2022, 246, 131–137.e2. [Google Scholar] [CrossRef]
- Sheldrake, E.; Al-Hakeem, H.; Lam, B.; Goldstein, B.I.; Wheeler, A.L.; Burke, M.; Dunkley, B.T.; Reed, N.; Scratch, S.E. Mental health outcomes across the lifespan in individuals with persistent post-concussion symptoms: A scoping review. Front. Neurol. 2022, 13, 850590. [Google Scholar] [CrossRef]
- Lambert, M.; Sheldrake, E.; Deneault, A.-A.; Wheeler, A.; Burke, M.; Scratch, S. Depressive symptoms in individuals with persistent postconcussion symptoms: A systematic review and meta-analysis. JAMA Netw. Open 2022, 5, e2248453. [Google Scholar] [CrossRef]
- Casey, B.J.; Jones, R.M.; Hare , T.A. The adolescent brain. Ann. N. Y. Acad. Sci. 2008, 1124, 111–126. [Google Scholar] [CrossRef]
- Collins, M.W.; Kontos, A.P.; Reynolds, E.; Murawski, C.D.; Fu, F.H. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg. Sport. Traumatol. Arthrosc. 2014, 22, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Kontos, A.P.; Collins, M.W.; Holland, C.L.; Reeves, V.L.; Edelman, K.; Benso, S.; Schneider, W.; Okonkwo, D. Preliminary evidence for improvement in symptoms, cognitive, vestibular, and oculomotor outcomes following targeted intervention with chronic mTBI patients. Mil. Med. 2018, 183, 333–338. [Google Scholar] [CrossRef]
- Sandel, N.; Reynolds, E.; Cohen, P.E.; Gillie, B.L.; Kontos, A.P. Anxiety and mood clinical profile following sport-related concussion: From risk factors to treatment. Sport Exerc. Perform. Psychol. 2017, 6, 304. [Google Scholar] [CrossRef]
- Iverson, G.L.; Karr, J.E.; Maxwell, B.; Zafonte, R.; Berkner, P.D.; Cook, N.E. Examining criteria for defining persistent post-concussion symptoms in children and adolescents. Front. Neurol. 2021, 12, 614648. [Google Scholar] [CrossRef] [PubMed]
- Keltner, D.; Gross, J.J. Functional accounts of emotions. Cogn. Emot. 1999, 13, 467–480. [Google Scholar] [CrossRef]
- DiPiero, M.; Rodrigues, P.G.; Gromala, A.; Dean, D.C., III. Applications of advanced diffusion MRI in early brain development: A comprehensive review. Brain Struct. Funct. 2023, 228, 367–392. [Google Scholar] [CrossRef]
- Foulkes, L.; Blakemore, S.-J. Studying individual differences in human adolescent brain development. Nat. Neurosci. 2018, 21, 315–323. [Google Scholar] [CrossRef]
- Gilmore, J.H.; Knickmeyer, R.C.; Gao, W. Imaging structural and functional brain development in early childhood. Nat. Rev. Neurosci. 2018, 19, 123–137. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.E.; Pike, G.B. MRI of healthy brain aging: A review. NMR Biomed. 2021, 34, e4564. [Google Scholar] [CrossRef]
- Santos, J.P.L.; Kontos, A.P.; Holland, C.L.; Suss, S.J., Jr.; Stiffler, R.S.; Bitzer, H.B.; Colorito, A.T.; Shaffer, M.; Skeba, A.; Iyengar, S. The role of puberty and sex on brain structure in adolescents with anxiety following concussion. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2022, in press. [Google Scholar] [CrossRef]
- Guenette, J.P.; Shenton, M.E.; Koerte, I.K. Imaging of concussion in young athletes. Neuroimaging Clin. 2018, 28, 43–53. [Google Scholar] [CrossRef]
- Fong, A.K.; Allen, M.D.; Waltzman, D.; Sarmiento, K.; Yeates, K.O.; Suskauer, S.; Wintermark, M.; Lindberg, D.M.; Tate, D.F.; Wilde, E.A.; et al. Neuroimaging in pediatric patients with mild traumatic brain injury: Relating the current 2018 Centers for Disease Control guideline and the potential of advanced neuroimaging modalities for research and clinical biomarker development. J. Neurotrauma 2021, 38, 44–52. [Google Scholar] [CrossRef]
- Kontos, A.P.; Covassin, T.; Elbin, R.; Parker, T. Depression and neurocognitive performance after concussion among male and female high school and collegiate athletes. Arch. Phys. Med. Rehabil. 2012, 93, 1751–1756. [Google Scholar] [CrossRef]
- Kontos, A.P.; Sufrinko, A.; Sandel, N.; Emami, K.; Collins, M.W. Sport-related Concussion Clinical Profiles: Clinical Characteristics, Targeted Treatments, and Preliminary Evidence. Curr. Sport. Med. Rep. 2019, 18, 82–92. [Google Scholar] [CrossRef]
- Lopez, D.A.; Christensen, Z.P.; Foxe, J.J.; Ziemer, L.R.; Nicklas, P.R.; Freedman, E.G. Association between mild traumatic brain injury, brain structure, and mental health outcomes in the Adolescent Brain Cognitive Development Study. NeuroImage 2022, 263, 119626. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, K.; Miller, G.F.; Jones, S.E. Sports-or physical activity–related concussions and feelings of sadness or hopelessness among US high school students: Results from the 2017 youth behavior risk survey. J. Sch. Nurs. 2022, 38, 203–209. [Google Scholar] [CrossRef]
- Knell, G.; Burkhart, S.O.; Caze, T.J.; Polousky, J.D.; Kohl III, H.W.; Messiah, S.E. Association between concussion history and factors relating to cognitive, behavioral, and emotional health among American high school athletes: A cross-sectional analysis. Am. J. Sport. Med. 2020, 48, 2534–2543. [Google Scholar] [CrossRef]
- Morissette, M.P.; Prior, H.J.; Tate, R.B.; Wade, J.; Leiter, J.R.S. Associations between concussion and risk of diagnosis of psychological and neurological disorders: A retrospective population-based cohort study. Fam. Med. Community Health 2020, 8, e000390. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.J.; Ritchie, L.J.; Koltek, M.; Hosain, S.; Cordingley, D.; Chu, S.; Selci, E.; Leiter, J.; Russell, K. Psychiatric outcomes after pediatric sports-related concussion. J. Neurosurg. Pediatr. 2015, 16, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Stazyk, K.; DeMatteo, C.; Moll, S.; Missiuna, C. Depression in youth recovering from concussion: Correlates and predictors. Brain Inj. 2017, 31, 631–638. [Google Scholar] [CrossRef]
- Chrisman, S.P.; Whelan, B.M.; Zatzick, D.F.; Hilt, R.J.; Wang, J.; Marcynyszyn, L.A.; Rivara, F.P.; McCarty, C.A. Prevalence and risk factors for depression, anxiety and suicidal ideation in youth with persistent post-concussive symptoms (PPCS). Brain Inj. 2021, 35, 1637–1644. [Google Scholar] [CrossRef]
- Broshek, D.K.; De Marco, A.P.; Freeman, J.R. A review of post-concussion syndrome and psychological factors associated with concussion. Brain Inj. 2015, 29, 228–237. [Google Scholar] [CrossRef]
- Brassil, H.E.; Salvatore, A.P. The frequency of post-traumatic stress disorder symptoms in athletes with and without sports related concussion. Clin. Transl. Med. 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Veliz, P.; McCabe, S.E.; Eckner, J.T.; Schulenberg, J.E. Concussion, sensation-seeking and substance use among US adolescents. Subst. Abus. 2021, 42, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Ilie, G.; Mann, R.E.; Boak, A.; Adlaf, E.M.; Hamilton, H.; Asbridge, M.; Rehm, J.; Cusimano, M.D. Suicidality, bullying and other conduct and mental health correlates of traumatic brain injury in adolescents. PLoS ONE 2014, 9, e94936. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.N.; Clements-Nolle, K.; Parrish, B.; Yang, W. Adolescent concussion and mental health outcomes: A population-based study. Am. J. Health Behav. 2019, 43, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Mantey, D.S.; Omega-Njemnobi, O.; Barroso, C.S.; Kelder, S.H. Self-reported history of concussions is associated with risk factors for suicide completion among high school students. J. Affect. Disord. 2020, 263, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Brent, D.A.; Max, J. Psychiatric sequelae of concussions. Curr. Psychiatry Rep. 2017, 19, 108. [Google Scholar] [CrossRef]
- Emery, C.A.; Barlow, K.M.; Brooks, B.L.; Max, J.E.; Villavicencio-Requis, A.; Gnanakumar, V.; Robertson, H.L.; Schneider, K.; Yeates, K.O. A systematic review of psychiatric, psychological, and behavioural outcomes following mild traumatic brain injury in children and adolescents. Can. J. Psychiatry 2016, 61, 259–269. [Google Scholar] [CrossRef]
- McLeod, T.C.V.; Leach, C. Psychometric properties of self-report concussion scales and checklists. J. Athl. Train. 2012, 47, 221–223. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Phillips, M.L.; Drevets, W.C.; Rauch, S.L.; Lane, R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol. Psychiatry 2003, 54, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Tanaka, S.C. Functions of the ventromedial prefrontal cortex in emotion regulation under stress. Sci. Rep. 2021, 11, 18225. [Google Scholar] [CrossRef] [PubMed]
- Šimić, G.; Tkalčić, M.; Vukić, V.; Mulc, D.; Španić, E.; Šagud, M.; Olucha-Bordonau, F.E.; Vukšić, M.; Hof, P.R. Understanding emotions: Origins and roles of the amygdala. Biomolecules 2021, 11, 823. [Google Scholar] [CrossRef]
- Pessoa, L.; Adolphs, R. Emotion processing and the amygdala: From a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat. Rev. Neurosci. 2010, 11, 773–782. [Google Scholar] [CrossRef]
- Andrewes, D.G.; Jenkins, L.M. The role of the amygdala and the ventromedial prefrontal cortex in emotional regulation: Implications for post-traumatic stress disorder. Neuropsychol. Rev. 2019, 29, 220–243. [Google Scholar] [CrossRef]
- Ferretti, V.; Papaleo, F. Understanding others: Emotion recognition in humans and other animals. Genes Brain Behav. 2019, 18, e12544. [Google Scholar] [CrossRef]
- Phillips, M.L.; Drevets, W.C.; Rauch, S.L.; Lane, R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol. Psychiatry 2003, 54, 504–514. [Google Scholar] [CrossRef]
- Dixon, M.L.; Thiruchselvam, R.; Todd, R.; Christoff, K. Emotion and the prefrontal cortex: An integrative review. Psychol. Bull. 2017, 143, 1033. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Jbabdi, S.; Zhu, Z.; Cottaar, M.; Grisot, G.; Lehman, J.F.; Yendiki, A.; Haber, S.N. A connectional hub in the rostral anterior cingulate cortex links areas of emotion and cognitive control. eLife 2019, 8, e43761. [Google Scholar] [CrossRef]
- Kang, J.; Kim, H.; Hwang, S.H.; Han, M.; Lee, S.-H.; Kim, H.F. Primate ventral striatum maintains neural representations of the value of previously rewarded objects for habitual seeking. Nat. Commun. 2021, 12, 2100. [Google Scholar] [CrossRef]
- Filimon, F.; Nelson, J.D.; Sejnowski, T.J.; Sereno, M.I.; Cottrell, G.W. The ventral striatum dissociates information expectation, reward anticipation, and reward receipt. Proc. Natl. Acad. Sci. USA 2020, 117, 15200–15208. [Google Scholar] [CrossRef]
- Herbet, G.; Zemmoura, I.; Duffau, H. Functional anatomy of the inferior longitudinal fasciculus: From historical reports to current hypotheses. Front. Neuroanat. 2018, 12, 77. [Google Scholar] [CrossRef]
- Bubb, E.J.; Metzler-Baddeley, C.; Aggleton, J.P. The cingulum bundle: Anatomy, function, and dysfunction. Neurosci. Biobehav. Rev. 2018, 92, 104–127. [Google Scholar] [CrossRef]
- Schermuly, I.; Fellgiebel, A.; Wagner, S.; Yakushev, I.; Stoeter, P.; Schmitt, R.; Knickenberg, R.; Bleichner, F.; Beutel, M. Association between cingulum bundle structure and cognitive performance: An observational study in major depression. Eur. Psychiatry 2010, 25, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Bettcher, B.M.; Mungas, D.; Patel, N.; Elofson, J.; Dutt, S.; Wynn, M.; Watson, C.L.; Stephens, M.; Walsh, C.M.; Kramer, J.H. Neuroanatomical substrates of executive functions: Beyond prefrontal structures. Neuropsychologia 2016, 85, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.; Uchida, M.; Gaillard, S.L.; Woodworth, H.; Kelberman, C.; Capella, J.; Kadlec, K.; Goncalves, M.; Ghosh, S.; Yendiki, A.; et al. Cingulum-callosal white-matter microstructure associated with emotional dysregulation in children: A diffusion tensor imaging study. NeuroImage Clin. 2020, 27, 102266. [Google Scholar] [CrossRef]
- Vandekerckhove, M. Neural networks in bottom up ‘experiential emotion regulation’. Behav. Brain Res. 2020, 383, 111242. [Google Scholar] [CrossRef]
- Sasson, E.; Doniger, G.M.; Pasternak, O.; Tarrasch, R.; Assaf, Y. White matter correlates of cognitive domains in normal aging with diffusion tensor imaging. Front. Neurosci. 2013, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Kamali, A.; Sair, H.I.; Radmanesh, A.; Hasan, K.M. Decoding the superior parietal lobule connections of the superior longitudinal fasciculus/arcuate fasciculus in the human brain. Neuroscience 2014, 277, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Kamali, A.; Flanders, A.E.; Brody, J.; Hunter, J.V.; Hasan, K.M. Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct. Funct. 2014, 219, 269–281. [Google Scholar] [CrossRef]
- Larsen, B.; Luna, B. Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci. Biobehav. Rev. 2018, 94, 179–195. [Google Scholar] [CrossRef]
- Kolb, B.; Harker, A.; Gibb, R. Principles of plasticity in the developing brain. Dev. Med. Child. Neurol. 2017, 59, 1218–1223. [Google Scholar] [CrossRef]
- Sisk, C.L.; Zehr, J.L. Pubertal hormones organize the adolescent brain and behavior. Front. Neuroendocr. 2005, 26, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Ismail, F.Y.; Fatemi, A.; Johnston, M.V. Cerebral plasticity: Windows of opportunity in the developing brain. Eur. J. Paediatr. Neurol. 2017, 21, 23–48. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Steenerson, K.; Starling, A.J. Pathophysiology of sports-related concussion. Neurol. Clin. 2017, 35, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Laksari, K.; Kurt, M.; Wu, L.C. Concussion Mechanism: Biomechanical Perspectives. In Tackling the Concussion Epidemic: A Bench to Bedside Approach; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–24. [Google Scholar]
- Chancellor, S.E.; Franz, E.S.; Minaeva, O.V.; Goldstein, L.E. Pathophysiology of concussion. Semin. Pediatr. Neurol. 2019, 30, 14–25. [Google Scholar] [CrossRef]
- Romeu-Mejia, R.; Giza, C.C.; Goldman, J.T. Concussion pathophysiology and injury biomechanics. Curr. Rev. Musculoskelet. Med. 2019, 12, 105–116. [Google Scholar] [CrossRef]
- Churchill, N.W.; Hutchison, M.G.; Richards, D.; Leung, G.; Graham, S.J.; Schweizer, T.A. The first week after concussion: Blood flow, brain function and white matter microstructure. Neuroimage Clin. 2017, 14, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Seo, Y.S. Diffusion tensor tractography characteristics of axonal injury in concussion/mild traumatic brain injury. Neural Regen. Res. 2022, 17, 978. [Google Scholar] [PubMed]
- Churchill, N.W.; Hutchison, M.G.; Graham, S.J.; Schweizer, T.A. Acute and chronic effects of multiple concussions on midline brain structures. Neurology 2021, 97, e1170–e1181. [Google Scholar] [CrossRef]
- Rose, S.C.; Schaffer, C.E.; Young, J.A.; McNally, K.A.; Fischer, A.N.; Heyer, G.L. Utilization of conventional neuroimaging following youth concussion. Brain Inj. 2017, 31, 260–266. [Google Scholar] [CrossRef]
- Shetty, T.; Raince, A.; Manning, E.; Tsiouris, A.J. Imaging in chronic traumatic encephalopathy and traumatic brain injury. Sport. Health 2016, 8, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Heras, E.; Grussu, F.; Prados, F.; Solana, E.; Llufriu, S. Diffusion-weighted imaging: Recent advances and applications. Semin. Ultrasound CT MRI 2021, 42, 490–506. [Google Scholar] [CrossRef]
- Kamiya, K.; Hori, M.; Aoki, S. NODDI in clinical research. J. Neurosci. Methods 2020, 346, 108908. [Google Scholar] [CrossRef]
- Kraguljac, N.V.; Guerreri, M.; Strickland, M.J.; Zhang, H. Neurite Orientation Dispersion and Density Imaging (NODDI) in Psychiatric Disorders–A Systematic Literature Review and a Technical Note. Biol. Psychiatry Glob. Open Sci. 2022, 3, 10–21. [Google Scholar] [CrossRef]
- Nazeri, A.; Schifani, C.; Anderson, J.A.; Ameis, S.H.; Voineskos, A.N. In vivo imaging of gray matter microstructure in major psychiatric disorders: Opportunities for clinical translation. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020, 5, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Raichle, M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef]
- Heeger, D.J.; Ress, D. What does fMRI tell us about neuronal activity? Nat. Rev. Neurosci. 2002, 3, 142–151. [Google Scholar] [CrossRef]
- McKeown, M.J.; Hansen, L.K.; Sejnowsk, T.J. Independent component analysis of functional MRI: What is signal and what is noise? Curr. Opin. Neurobiol. 2003, 13, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zuo, X.; He, Y. Graph-based network analysis of resting-state functional MRI. Front. Syst. Neurosci. 2010, 4, 16. [Google Scholar] [CrossRef]
- Yang, J.; Gohel, S.; Vachha, B. Current methods and new directions in resting state fMRI. Clin. Imaging 2020, 65, 47–53. [Google Scholar] [CrossRef]
- Lv, H.; Wang, Z.; Tong, E.; Williams, L.M.; Zaharchuk, G.; Zeineh, M.; Goldstein-Piekarski, A.N.; Ball, T.M.; Liao, C.; Wintermark, M. Resting-state functional MRI: Everything that nonexperts have always wanted to know. Am. J. Neuroradiol. 2018, 39, 1390–1399. [Google Scholar] [CrossRef]
- Farahani, F.V.; Karwowski, W.; Lighthall, N.R. Application of graph theory for identifying connectivity patterns in human brain networks: A systematic review. Front. Neurosci. 2019, 13, 585. [Google Scholar] [CrossRef]
- Plourde, V.; Rohr, C.S.; Virani, S.; Bray, S.; Yeates, K.O.; Brooks, B.L. Default Mode Network Functional Connectivity after Multiple Concussions in Children and Adolescents; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Sharma, B.; Nowikow, C.; DeMatteo, C.; Noseworthy, M.D.; Timmons, B.W. Sex-specific differences in resting-state functional brain activity in pediatric concussion. Sci. Rep. 2023, 13, 3284. [Google Scholar] [CrossRef] [PubMed]
- Murdaugh, D.L.; King, T.Z.; Sun, B.; Jones, R.A.; Ono, K.E.; Reisner, A.; Burns, T.G. Longitudinal changes in resting state connectivity and white matter integrity in adolescents with sports-related concussion. J. Int. Neuropsychol. Soc. 2018, 24, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Suss, S.J.; Manelis, A.; Lima Santos, J.P.; Holland, C.L.; Stiffler, R.S.; Bitzer, H.B.; Mailliard, S.; Shaffer, M.; Caviston, K.; Collins, M.W.; et al. Resting state functional connectivity between dorsal attentional network and right inferior frontal gyrus in concussed and control adolescents. J. Clin. Med. 2022, 11, 2293. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.L.; Knodt, A.R.; Cooke, M.; Kim, M.J.; Melzer, T.R.; Keenan, R.; Ireland, D.; Ramrakha, S.; Poulton, R.; Caspi, A.; et al. General functional connectivity: Shared features of resting-state and task fMRI drive reliable and heritable individual differences in functional brain networks. Neuroimage 2019, 189, 516–532. [Google Scholar] [CrossRef]
- Logan, G.D. On the ability to inhibit thought and action: A users’ guide to the stop signal paradigm. In Inhibitory Processes in Attention, Memory, and Language; Academic Press: Cambridge, MA, USA, 1994. [Google Scholar]
- Knutson, B.; Westdorp, A.; Kaiser, E.; Hommer, D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage 2000, 12, 20–27. [Google Scholar] [CrossRef]
- Ladouceur, C.D.; Silk, J.S.; Dahl, R.E.; Ostapenko, L.; Kronhaus, D.M.; Phillips, M.L. Fearful faces influence attentional control processes in anxious youth and adults. Emotion 2009, 9, 855. [Google Scholar] [CrossRef]
- Cook, M.J.; Gardner, A.J.; Wojtowicz, M.; Williams, W.H.; Iverson, G.L.; Stanwell, P. Task-related functional magnetic resonance imaging activations in patients with acute and subacute mild traumatic brain injury: A coordinate-based meta-analysis. Neuroimage Clin. 2020, 25, 102129. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.-T.; Hsu, H.-L.; Kuo, Y.-S.; Wu, C.W.; Chiu, W.-T.; Yan, F.-X.; Wang, W.-S.; Chen, C.-J.; Tseng, Y.-C. Effect of age on working memory performance and cerebral activation after mild traumatic brain injury: A functional MR imaging study. Radiology 2015, 278, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-L.; Chen, D.Y.-T.; Tseng, Y.-C.; Kuo, Y.-S.; Huang, Y.-L.; Chiu, W.-T.; Yan, F.-X.; Wang, W.-S.; Chen, C.-J. Sex differences in working memory after mild traumatic brain injury: A functional MR imaging study. Radiology 2015, 276, 828–835. [Google Scholar] [CrossRef]
- Chen, C.-J.; Wu, C.-H.; Liao, Y.-P.; Hsu, H.-L.; Tseng, Y.-C.; Liu, H.-L.; Chiu, W.-T. Working memory in patients with mild traumatic brain injury: Functional MR imaging analysis. Radiology 2012, 264, 844–851. [Google Scholar] [CrossRef]
- McAllister, T.W.; Saykin, A.; Flashman, L.; Sparling, M.; Johnson, S.; Guerin, S.; Mamourian, A.; Weaver, J.; Yanofsky, N. Brain activation during working memory 1 month after mild traumatic brain injury: A functional MRI study. Neurology 1999, 53, 1300. [Google Scholar] [CrossRef] [PubMed]
- Pardini, J.E.; Pardini, D.A.; Becker, J.T.; Dunfee, K.L.; Eddy, W.F.; Lovell, M.R.; Welling, J.S. Postconcussive symptoms are associated with compensatory cortical recruitment during a working memory task. Neurosurgery 2010, 67, 1020–1028. [Google Scholar] [CrossRef]
- Slobounov, S.M.; Zhang, K.; Pennell, D.; Ray, W.; Johnson, B.; Sebastianelli, W. Functional abnormalities in normally appearing athletes following mild traumatic brain injury: A functional MRI study. Exp. Brain Res. 2010, 202, 341–354. [Google Scholar] [CrossRef]
- McAllister, T.W.; Flashman, L.A.; McDonald, B.C.; Saykin, A.J. Mechanisms of working memory dysfunction after mild and moderate TBI: Evidence from functional MRI and neurogenetics. J. Neurotrauma 2006, 23, 1450–1467. [Google Scholar] [CrossRef]
- McAllister, T.W.; Sparling, M.B.; Flashman, L.A.; Guerin, S.J.; Mamourian, A.C.; Saykin, A.J. Differential working memory load effects after mild traumatic brain injury. Neuroimage 2001, 14, 1004–1012. [Google Scholar] [CrossRef]
- Newsome, M.R.; Steinberg, J.L.; Scheibel, R.S.; Troyanskaya, M.; Chu, Z.; Hanten, G.; Lu, H.; Lane, S.; Lin, X.; Hunter, J.V.; et al. Effects of traumatic brain injury on working memory-related brain activation in adolescents. Neuropsychology 2008, 22, 419. [Google Scholar] [CrossRef]
- Kramer, M.E.; Chiu, C.Y.; Walz, N.C.; Holland, S.K.; Yuan, W.; Karunanayaka, P.; Wade, S.L. Long-term neural processing of attention following early childhood traumatic brain injury: fMRI and neurobehavioral outcomes. J. Int. Neuropsychol. Soc. 2008, 14, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Tlustos, S.J.; Chiu, C.Y.; Walz, N.C.; Holland, S.K.; Bernard, L.; Wade, S.L. Neural correlates of interference control in adolescents with traumatic brain injury: Functional magnetic resonance imaging study of the counting stroop task. J. Int. Neuropsychol. Soc. 2011, 17, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Westfall, D.R.; West, J.D.; Bailey, J.N.; Arnold, T.W.; Kersey, P.A.; Saykin, A.J.; McDonald, B.C. Increased brain activation during working memory processing after pediatric mild traumatic brain injury (mTBI). J. Pediatr. Rehabil. Med. 2015, 8, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Iyer, K.K.; Khetani, A.M.; Barlow, K.M. Changes in working memory-related cortical responses following pediatric mild traumatic brain injury: A longitudinal fMRI study. J. Concussion 2021, 5, 20597002211006541. [Google Scholar] [CrossRef]
- Keightley, M.L.; Singh Saluja, R.; Chen, J.-K.; Gagnon, I.; Leonard, G.; Petrides, M.; Ptito, A. A functional magnetic resonance imaging study of working memory in youth after sports-related concussion: Is it still working? J. Neurotrauma 2014, 31, 437–451. [Google Scholar] [CrossRef]
- Le Bihan, D.; Breton, E. Imagerie de diffusion in vivo par résonance magnétique nucléaire. Comptes Rendus De L’académie Des Sciences. Série 2 Mécanique Phys. Chim. Sci. De L’univers Sci. De La Terre 1985, 301, 1109–1112. [Google Scholar]
- Novikov, D.S.; Fieremans, E.; Jespersen, S.N.; Kiselev, V.G. Quantifying brain microstructure with diffusion MRI: Theory and parameter estimation. NMR Biomed. 2019, 32, e3998. [Google Scholar] [CrossRef]
- Meoded, A.; Orman, G.; Huisman, T.A. Diffusion weighted and diffusion tensor MRI in pediatric neuroimaging including connectomics: Principles and applications. Semin. Pediatr. Neurol. 2020, 33, 100797. [Google Scholar] [CrossRef]
- Ravikanth, R.; Majumdar, P. Prognostic Significance of Magnetic Resonance Imaging in Detecting Diffuse Axonal Injuries: Analysis of Outcomes and Review of Literature. Neurol. India 2022, 70, 2371. [Google Scholar] [CrossRef]
- Mendelson, S.J.; Prabhakaran, S. Diagnosis and management of transient ischemic attack and acute ischemic stroke: A review. Jama 2021, 325, 1088–1098. [Google Scholar] [CrossRef]
- Li, C.-X.; Patel, S.; Zhang, X. Evaluation of multi-shell diffusion MRI acquisition strategy on quantitative analysis using multi-compartment models. Quant. Imaging Med. Surg. 2020, 10, 824. [Google Scholar] [CrossRef]
- Tariq, M.; Schneider, T.; Alexander, D.C.; Wheeler-Kingshott, C.A.G.; Zhang, H. Bingham–NODDI: Mapping anisotropic orientation dispersion of neurites using diffusion MRI. Neuroimage 2016, 133, 207–223. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Mustafi, S.M.; Harezlak, J.; Kodiweera, C.; Flashman, L.A.; McAllister, T.W. Hybrid diffusion imaging in mild traumatic brain injury. J. Neurotrauma 2018, 35, 2377–2390. [Google Scholar] [CrossRef]
- Dell’Acqua, F.; Tournier, J.D. Modelling white matter with spherical deconvolution: How and why? NMR Biomed. 2019, 32, e3945. [Google Scholar] [CrossRef]
- De Luca, A.; Guo, F.; Froeling, M.; Leemans, A. Spherical deconvolution with tissue-specific response functions and multi-shell diffusion MRI to estimate multiple fiber orientation distributions (mFODs). NeuroImage 2020, 222, 117206. [Google Scholar] [CrossRef] [PubMed]
- Jeurissen, B.; Tournier, J.-D.; Dhollander, T.; Connelly, A.; Sijbers, J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. NeuroImage 2014, 103, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Schneider, T.; Wheeler-Kingshott, C.A.; Alexander, D.C. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 2012, 61, 1000–1016. [Google Scholar] [CrossRef]
- Lynch, K.M.; Cabeen, R.P.; Toga, A.W.; Clark, K.A. Magnitude and timing of major white matter tract maturation from infancy through adolescence with NODDI. NeuroImage 2020, 212, 116672. [Google Scholar] [CrossRef]
- Beck, D.; de Lange, A.-M.G.; Maximov, I.I.; Richard, G.; Andreassen, O.A.; Nordvik, J.E.; Westlye, L.T. White matter microstructure across the adult lifespan: A mixed longitudinal and cross-sectional study using advanced diffusion models and brain-age prediction. NeuroImage 2021, 224, 117441. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Guo, T.; Chen, J.; Guan, X.; Zhou, C.; Wu, J.; Liu, X.; Wu, H.; Wen, J.; Gu, L.; et al. Microstructural but not macrostructural cortical degeneration occurs in Parkinson’s disease with mild cognitive impairment. NPJ Park. Dis. 2022, 8, 151. [Google Scholar] [CrossRef]
- Lakhani, D.A.; Schilling, K.; Xu, J.; Bagnato, F. Advanced multicompartment diffusion MRI models and their application in multiple sclerosis. Am. J. Neuroradiol. 2020, 41, 751–757. [Google Scholar] [CrossRef]
- Kamagata, K.; Andica, C.; Hatano, T.; Ogawa, T.; Takeshige-Amano, H.; Ogaki, K.; Akashi, T.; Hagiwara, A.; Fujita, S.; Aoki, S. Advanced diffusion magnetic resonance imaging in patients with Alzheimer’s and Parkinson’s diseases. Neural Regen. Res. 2020, 15, 1590. [Google Scholar] [CrossRef] [PubMed]
- Barritt, A.W.; Gabel, M.C.; Cercignani, M.; Leigh, P.N. Emerging magnetic resonance imaging techniques and analysis methods in amyotrophic lateral sclerosis. Front. Neurol. 2018, 9, 1065. [Google Scholar] [CrossRef]
- Aoki, Y.; Inokuchi, R. A voxel-based meta-analysis of diffusion tensor imaging in mild traumatic brain injury. Neurosci. Biobehav. Rev. 2016, 66, 119–126. [Google Scholar] [CrossRef]
- Aoki, Y.; Inokuchi, R.; Gunshin, M.; Yahagi, N.; Suwa, H. Diffusion tensor imaging studies of mild traumatic brain injury: A meta-analysis. J. Neurol. Neurosurg. Psychiatry 2012, 83, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Shenton, M.E.; Hamoda, H.; Schneiderman, J.; Bouix, S.; Pasternak, O.; Rathi, Y.; Vu, M.-A.; Purohit, M.P.; Helmer, K.; Koerte, I.; et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012, 6, 137–192. [Google Scholar] [CrossRef]
- Yurgelun-Todd, D.A.; Bueler, C.E.; McGlade, E.C.; Churchwell, J.C.; Brenner, L.A.; Lopez-Larson, M.P. Neuroimaging correlates of traumatic brain injury and suicidal behavior. J. Head Trauma Rehabil. 2011, 26, 276–289. [Google Scholar] [CrossRef]
- Warner, M.A.; de la Plata, C.M.; Spence, J.; Wang, J.Y.; Harper, C.; Moore, C.; Devous, M.; Diaz-Arrastia, R. Assessing spatial relationships between axonal integrity, regional brain volumes, and neuropsychological outcomes after traumatic axonal injury. J. Neurotrauma 2010, 27, 2121–2130. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.; Houston, G.C.; Dippel, D.W.; Wielopolski, P.A.; Vernooij, M.W.; Koudstaal, P.J.; Hunink, M.M.; van der Lugt, A. Microstructural brain injury in post-concussion syndrome after minor head injury. Neuroradiology 2011, 53, 553–563. [Google Scholar] [CrossRef]
- Singh, M.; Jeong, J.; Hwang, D.; Sungkarat, W.; Gruen, P. Novel diffusion tensor imaging methodology to detect and quantify injured regions and affected brain pathways in traumatic brain injury. Magn. Reson. Imaging 2010, 28, 22–40. [Google Scholar] [CrossRef]
- Rutgers, D.; Toulgoat, F.; Cazejust, J.; Fillard, P.; Lasjaunias, P.; Ducreux, D. White matter abnormalities in mild traumatic brain injury: A diffusion tensor imaging study. Am. J. Neuroradiol. 2008, 29, 514–519. [Google Scholar] [CrossRef]
- Niogi, S.; Mukherjee, P.; Ghajar, J.; Johnson, C.; Kolster, R.; Sarkar, R.; Lee, H.; Meeker, M.; Zimmerman, R.; Manley, G.; et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: A 3T diffusion tensor imaging study of mild traumatic brain injury. Am. J. Neuroradiol. 2008, 29, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Miles, L.; Grossman, R.I.; Johnson, G.; Babb, J.S.; Diller, L.; Inglese, M. Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj. 2008, 22, 115–122. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.W.; Ford, J.C.; Ji, S.; Beckwith, J.G.; Flashman, L.A.; Paulsen, K.; Greenwald, R.M. Maximum principal strain and strain rate associated with concussion diagnosis correlates with changes in corpus callosum white matter indices. Ann. Biomed. Eng. 2012, 40, 127–140. [Google Scholar] [CrossRef]
- Mayer, A.; Ling, J.; Mannell, M.; Gasparovic, C.; Phillips, J.; Doezema, D.; Reichard, R.; Yeo, R. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology 2010, 74, 643–650. [Google Scholar] [CrossRef]
- Matthews, S.C.; Strigo, I.A.; Simmons, A.N.; O’connell, R.M.; Reinhardt, L.E.; Moseley, S.A. A multimodal imaging study in US veterans of Operations Iraqi and Enduring Freedom with and without major depression after blast-related concussion. Neuroimage 2011, 54, S69–S75. [Google Scholar] [CrossRef] [PubMed]
- Maruta, J.; Suh, M.; Niogi, S.N.; Mukherjee, P.; Ghajar, J. Visual tracking synchronization as a metric for concussion screening. J. Head Trauma Rehabil. 2010, 25, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Messé, A.; Caplain, S.; Paradot, G.; Garrigue, D.; Mineo, J.F.; Ares, G.S.; Ducreux, D.; Vignaud, F.; Rozec, G.; Desal, H.; et al. Diffusion tensor imaging and white matter lesions at the subacute stage in mild traumatic brain injury with persistent neurobehavioral impairment. Hum. Brain Mapp. 2011, 32, 999–1011. [Google Scholar] [CrossRef]
- Lo, C.; Shifteh, K.; Gold, T.; Bello, J.A.; Lipton, M.L. Diffusion tensor imaging abnormalities in patients with mild traumatic brain injury and neurocognitive impairment. J. Comput. Assist. Tomogr. 2009, 33, 293–297. [Google Scholar] [CrossRef]
- Ljungqvist, J.; Nilsson, D.; Ljungberg, M.; Sörbo, A.; Esbjörnsson, E.; Eriksson-Ritzén, C.; Skoglund, T. Longitudinal study of the diffusion tensor imaging properties of the corpus callosum in acute and chronic diffuse axonal injury. Brain Inj. 2011, 25, 370–378. [Google Scholar] [CrossRef]
- Little, D.; Kraus, M.; Joseph, J.; Geary, E.; Susmaras, T.; Zhou, X.; Pliskin, N.; Gorelick, P. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology 2010, 74, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Lipton, M.L.; Gellella, E.; Lo, C.; Gold, T.; Ardekani, B.A.; Shifteh, K.; Bello, J.A.; Branch, C.A. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: A voxel-wise analysis of diffusion tensor imaging. J. Neurotrauma 2008, 25, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gupta, R.K.; Husain, M.; Chaudhry, C.; Srivastava, A.; Saksena, S.; Rathore, R.K. Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: Its correlation with neuropsychometric tests. Brain Inj. 2009, 23, 675–685. [Google Scholar] [CrossRef]
- Inglese, M.; Makani, S.; Johnson, G.; Cohen, B.A.; Silver, J.A.; Gonen, O.; Grossman, R.I. Diffuse axonal injury in mild traumatic brain injury: A diffusion tensor imaging study. J. Neurosurg. 2005, 103, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Huisman, T.A.; Schwamm, L.H.; Schaefer, P.W.; Koroshetz, W.J.; Shetty-Alva, N.; Ozsunar, Y.; Wu, O.; Sorensen, A.G. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. Am. J. Neuroradiol. 2004, 25, 370–376. [Google Scholar]
- Holli, K.K.; Wäljas, M.; Harrison, L.; Liimatainen, S.; Luukkaala, T.; Ryymin, P.; Eskola, H.; Soimakallio, S.; Öhman, J.; Dastidar, P.; et al. Mild traumatic brain injury: Tissue texture analysis correlated to neuropsychological and DTI findings. Acad. Radiol. 2010, 17, 1096–1102. [Google Scholar] [CrossRef]
- Henry, L.C.; Tremblay, J.; Tremblay, S.; Lee, A.; Brun, C.; Lepore, N.; Theoret, H.; Ellemberg, D.; Lassonde, M. Acute and chronic changes in diffusivity measures after sports concussion. J. Neurotrauma 2011, 28, 2049–2059. [Google Scholar] [CrossRef]
- Grossman, E.J.; Ge, Y.; Jensen, J.H.; Babb, J.S.; Miles, L.; Reaume, J.; Silver, J.M.; Grossman, R.I.; Inglese, M. Thalamus and cognitive impairment in mild traumatic brain injury: A diffusional kurtosis imaging study. J. Neurotrauma 2012, 29, 2318–2327. [Google Scholar] [CrossRef]
- Bazarian, J.J.; Zhong, J.; Blyth, B.; Zhu, T.; Kavcic, V.; Peterson, D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: A pilot study. J. Neurotrauma 2007, 24, 1447–1459. [Google Scholar] [CrossRef]
- Cubon, V.A.; Putukian, M.; Boyer, C.; Dettwiler, A. A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J. Neurotrauma 2011, 28, 189–201. [Google Scholar] [CrossRef]
- Bazarian, J.J.; Zhu, T.; Blyth, B.; Borrino, A.; Zhong, J. Subject-specific changes in brain white matter on diffusion tensor imaging after sports-related concussion. Magn. Reson. Imaging 2012, 30, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Arfanakis, K.; Haughton, V.M.; Carew, J.D.; Rogers, B.P.; Dempsey, R.J.; Meyerand, M.E. Diffusion tensor MR imaging in diffuse axonal injury. Am. J. Neuroradiol. 2002, 23, 794–802. [Google Scholar]
- Lima Santos, J.P.; Kontos, A.P.; Mailliard, S.; Eagle, S.R.; Holland, C.L.; Suss, S.J., Jr.; Abdul-Waalee, H.; Stiffler, R.S.; Bitzer, H.B.; Blaney, N.A.; et al. White matter abnormalities associated with prolonged recovery in adolescents following concussion. Front. Neurol. 2021, 12, 681467. [Google Scholar] [CrossRef] [PubMed]
- Mac Donald, C.L.; Barber, J.; Wright, J.; Coppel, D.; De Lacy, N.; Ottinger, S.; Peck, S.; Panks, C.; Sun, S.; Zalewski, K.; et al. Longitudinal Clinical and Neuroimaging Evaluation of Symptomatic Concussion in 10-to 14-year-old Youth Athletes. J. Neurotrauma 2018, 36, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Kawata, K.; Steinfeldt, J.A.; Huibregtse, M.E.; Nowak, M.K.; Macy, J.T.; Kercher, K.; Rettke, D.J.; Shin, A.; Chen, Z.; Ejima, K.; et al. Association between proteomic blood biomarkers and DTI/NODDI metrics in adolescent football players: A pilot study. Front. Neurol. 2020, 11, 581781. [Google Scholar] [CrossRef]
- Santos, J.P.L.; Kontos, A.P.; Holland, C.L.; Stiffler, R.S.; Bitzer, H.B.; Caviston, K.; Shaffer, M.; Suss, S.J., Jr.; Martinez, L.; Manelis, A. The role of sleep quality on white matter integrity and concussion symptom severity in adolescents. Neuroimage Clin. 2022, 35, 103130. [Google Scholar] [CrossRef]
- Halefoglu, A.M.; Yousem, D.M. Susceptibility weighted imaging: Clinical applications and future directions. World J. Radiol. 2018, 10, 30. [Google Scholar] [CrossRef]
- Kirov, I.I.; Whitlow, C.T.; Zamora, C. Susceptibility-weighted imaging and magnetic resonance spectroscopy in concussion. Neuroimaging Clin. 2018, 28, 91–105. [Google Scholar] [CrossRef]
- Hageman, G.; Hof, J.; Nihom, J. Susceptibility-Weighted MRI and Microbleeds in Mild Traumatic Brain Injury: Prediction of Posttraumatic Complaints? Eur. Neurol. 2022, 85, 177–185. [Google Scholar] [CrossRef]
- Virani, S.; Barton, A.; Goodyear, B.G.; Yeates, K.O.; Brooks, B.L. Susceptibility-weighted magnetic resonance imaging (MRI) of microbleeds in pediatric concussion. J. Child Neurol. 2021, 36, 867–874. [Google Scholar] [CrossRef]
- Maudsley, A.A.; Andronesi, O.C.; Barker, P.B.; Bizzi, A.; Bogner, W.; Henning, A.; Nelson, S.J.; Posse, S.; Shungu, D.C.; Soher, B.J. Advanced magnetic resonance spectroscopic neuroimaging: Experts’ consensus recommendations. NMR Biomed. 2021, 34, e4309. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.J.; Stout, J.N.; Chung, A.W.; Grant, P.E.; Mannix, R.; Gagoski, B. Longitudinal changes in magnetic resonance spectroscopy in pediatric concussion: A pilot study. Front. Neurol. 2019, 10, 556. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Suhett, C.P.; Prager, E.M.; Pidoplichko, V.; Figueiredo, T.H.; Marini, A.M.; Li, Z.; Eiden, L.E.; Braga, M.F. Reduced GABAergic inhibition in the basolateral amygdala and the development of anxiety-like behaviors after mild traumatic brain injury. PLoS ONE 2014, 9, e102627. [Google Scholar] [CrossRef]
- Palmer, C.P.; Metheny, H.E.; Elkind, J.A.; Cohen, A.S. Diminished amygdala activation and behavioral threat response following traumatic brain injury. Exp. Neurol. 2016, 277, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Dégeilh, F.; Tilmann, S.; Lia, F.; Joanne, C.B.; Malo, G.; Inga, K.K.; Christian, K.T. Social problems and brain structure trajectories following pediatric mild traumatic brain injury. In Proceedings of the Organisation of Human Brain Mapping Annual Meeting, Glasgow, UK, 19–23 June 2022. [Google Scholar]
- Vasa, R.A.; Stacy, J.S.; Julia, M.T.; Luther, K.; Marco, A.G.; Beth, S.S.; Cynthia, F.S.; Joan, P.G. Prevalence and predictors of affective lability after paediatric traumatic brain injury. Brain Inj. 2015, 29, 921–928. [Google Scholar] [CrossRef]
- Max, J.E.; Keatley, E.; Wilde, E.A.; Bigler, E.D.; Schachar, R.J.; Saunders, A.E.; Ewing-Cobbs, L.; Chapman, S.B.; Dennis, M.; Yang, T.T.; et al. Depression in children and adolescents in the first 6 months after traumatic brain injury. Int. J. Dev. Neurosci. 2012, 30, 239–245. [Google Scholar] [CrossRef]
- Wilde, E.A.; Tricia, L.M.; Erin, D.B.; Jeffrey, E.M.; Adam, T.S.; Kareem, W.A.; Stephen, R.M.; Jill, V.H.; Gerri, H.; Li, X.; et al. Longitudinal changes in cortical thickness in children after traumatic brain injury and their relation to behavioral regulation and emotional control. Int. J. Dev. Neurosci. 2012, 30, 267–276. [Google Scholar] [CrossRef]
- Bohorquez-Montoya, L.; España, L.Y.; Nader, A.M.; Furger, R.E.; Mayer, A.R.; Meier, T.B. Amygdala response to emotional faces in adolescents with persistent post-concussion symptoms. Neuroimage Clin. 2020, 26, 102217. [Google Scholar] [CrossRef]
- Ho, R.A.; Hall, G.B.; Noseworthy, M.D.; DeMatteo, C. An Emotional Go/No-Go fMRI study in adolescents with depressive symptoms following concussion. Int. J. Psychophysiol. 2018, 132, 62–73. [Google Scholar] [CrossRef]
- Alhilali, L.M.; Joseph, A.D.; Serter, G.; Saeed, F. Evaluation of white matter injury patterns underlying neuropsychiatric symptoms after mild traumatic brain injury. Radiology 2015, 277, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Maller, J.J.; Thomson, R.H.; Pannek, K.; Rose, S.E.; Bailey, N.; Lewis, P.M.; Fitzgerald, P.B. The (Eigen) value of diffusion tensor imaging to investigate depression after traumatic brain injury. Hum. Brain Mapp. 2014, 35, 227–237. [Google Scholar] [CrossRef]
- Rao, V.; Michelle, M.; Xin, X.; Gwenn, S.S.; Una, D.M.; Alyssa, B.; Vishal, D.; Dzung, L.P.; David, Y.; Susumi, M. Diffusion tensor imaging atlas-based analyses in major depression after mild traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2012, 24, 309–315. [Google Scholar] [CrossRef]
- Lu, Q.; Li, H.; Luo, G.; Wang, Y.; Tang, H.; Han, L.; Yao, Z. Impaired prefrontal–amygdala effective connectivity is responsible for the dysfunction of emotion process in major depressive disorder: A dynamic causal modeling study on MEG. Neurosci. Lett. 2012, 523, 125–130. [Google Scholar] [CrossRef]
- Prater, K.E.; Hosanagar, A.; Klumpp, H.; Angstadt, M.; Luan Phan, K. Aberrant amygdala–frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress. Anxiety 2013, 30, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.A.; Wise, S.P.; Drevets, W.C. Localization of dysfunction in major depressive disorder: Prefrontal cortex and amygdala. Biol. Psychiatry 2011, 69, e43–e54. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.P. Neural systems underlying approach and avoidance in anxiety disorders. Dialogues Clin. Neurosci. 2022, 12, 517–531. [Google Scholar]
- Ferri, J.; Eisendrath, S.J.; Fryer, S.L.; Gillung, E.; Roach, B.J.; Mathalon, D.H. Blunted amygdala activity is associated with depression severity in treatment-resistant depression. Cogn. Affect. Behav. Neurosci. 2017, 17, 1221–1231. [Google Scholar] [CrossRef]
- Drevets, W.C. Neuroimaging abnormalities in the amygdala in mood disorders. Ann. N. Y. Acad. Sci. 2003, 985, 420–444. [Google Scholar] [CrossRef]
- Maller, J.J.; Thomson, R.H.; Lewis, P.M.; Rose, S.E.; Pannek, K.; Fitzgerald, P.B. Traumatic brain injury, major depression, and diffusion tensor imaging: Making connections. Brain Res. Rev. 2010, 64, 213–240. [Google Scholar] [CrossRef] [PubMed]
- Ota, M.; Noda, T.; Sato, N.; Hattori, K.; Hori, H.; Sasayama, D.; Teraishi, T.; Nagashima, A.; Obu, S.; Higuchi, T.; et al. White matter abnormalities in major depressive disorder with melancholic and atypical features: A diffusion tensor imaging study. Psychiatry Clin. Neurosci. 2015, 69, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Zuo, N.; Fang, J.; Lv, X.; Zhou, Y.; Hong, Y.; Li, T.; Tong, H.; Wang, X.; Wang, W.; Jiang, T. White matter abnormalities in major depression: A tract-based spatial statistics and rumination study. PLoS ONE 2012, 7, e37561. [Google Scholar] [CrossRef] [PubMed]
- Eierud, C.; Craddock, R.C.; Fletcher, S.; Aulakh, M.; King-Casas, B.; Kuehl, D.; LaConte, S.M. Neuroimaging after mild traumatic brain injury: Review and meta-analysis. NeuroImage Clin. 2014, 4, 283–294. [Google Scholar] [CrossRef]
- Marzbani, H.; Marateb, H.R.; Mansourian, M. Neurofeedback: A comprehensive review on system design, methodology and clinical applications. Basic Clin. Neurosci. 2016, 7, 143. [Google Scholar]
- Hershaw, J.; Hill-Pearson, C.A.; Arango, J.I.; Souvignier, A.R.; Pazdan, R.M. Changes in attentional processing following neurofeedback in patients with persistent post-concussive symptoms: A pilot study. Brain Inj. 2020, 34, 1723–1731. [Google Scholar] [CrossRef]
- Mollica, A.; Greben, R.; Oriuwa, C.; Siddiqi, S.H.; Burke, M.J. Neuromodulation treatments for mild traumatic brain injury and post-concussive symptoms. Curr. Neurol. Neurosci. Rep. 2022, 22, 171–181. [Google Scholar] [CrossRef]
- Kadosh, K.C.; Luo, Q.; de Burca, C.; Sokunbi, M.O.; Feng, J.; Linden, D.E.; Lau, J.Y. Using real-time fMRI to influence effective connectivity in the developing emotion regulation network. Neuroimage 2016, 125, 616–626. [Google Scholar] [CrossRef]
- Quevedo, K.; Liu, G.; Teoh, J.Y.; Ghosh, S.; Zeffiro, T.; Ahrweiler, N.; Zhang, N.; Wedan, R.; Oh, S.; Guercio, G.; et al. Neurofeedback and neuroplasticity of visual self-processing in depressed and healthy adolescents: A preliminary study. Dev. Cogn. Neurosci. 2019, 40, 100707. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).