Neighborhood Deprivation and Racial Disparities in Early Pregnancy Impaired Glucose Tolerance
Abstract
1. Introduction
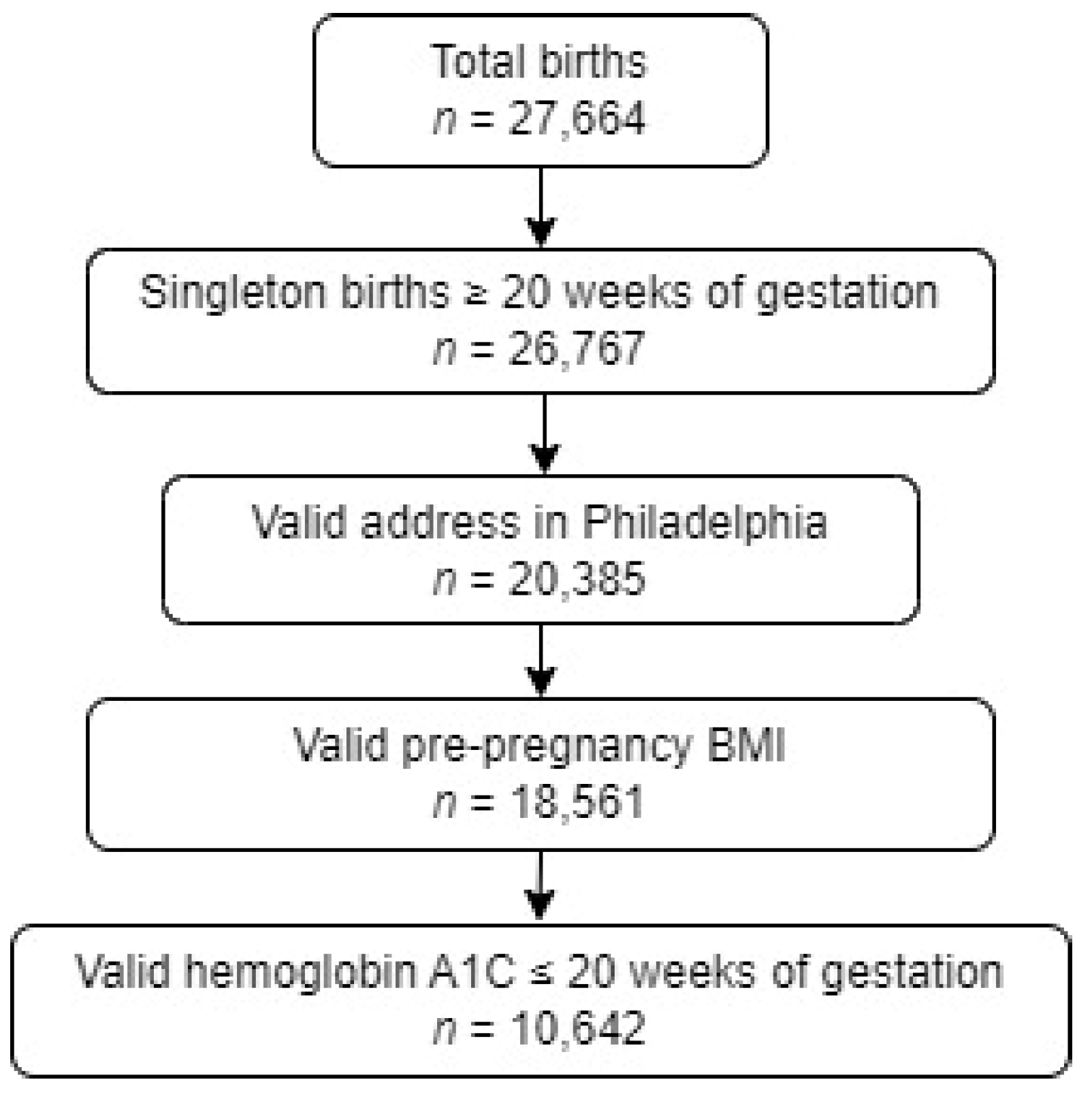
2. Materials and Methods
Statistical Analysis
3. Results
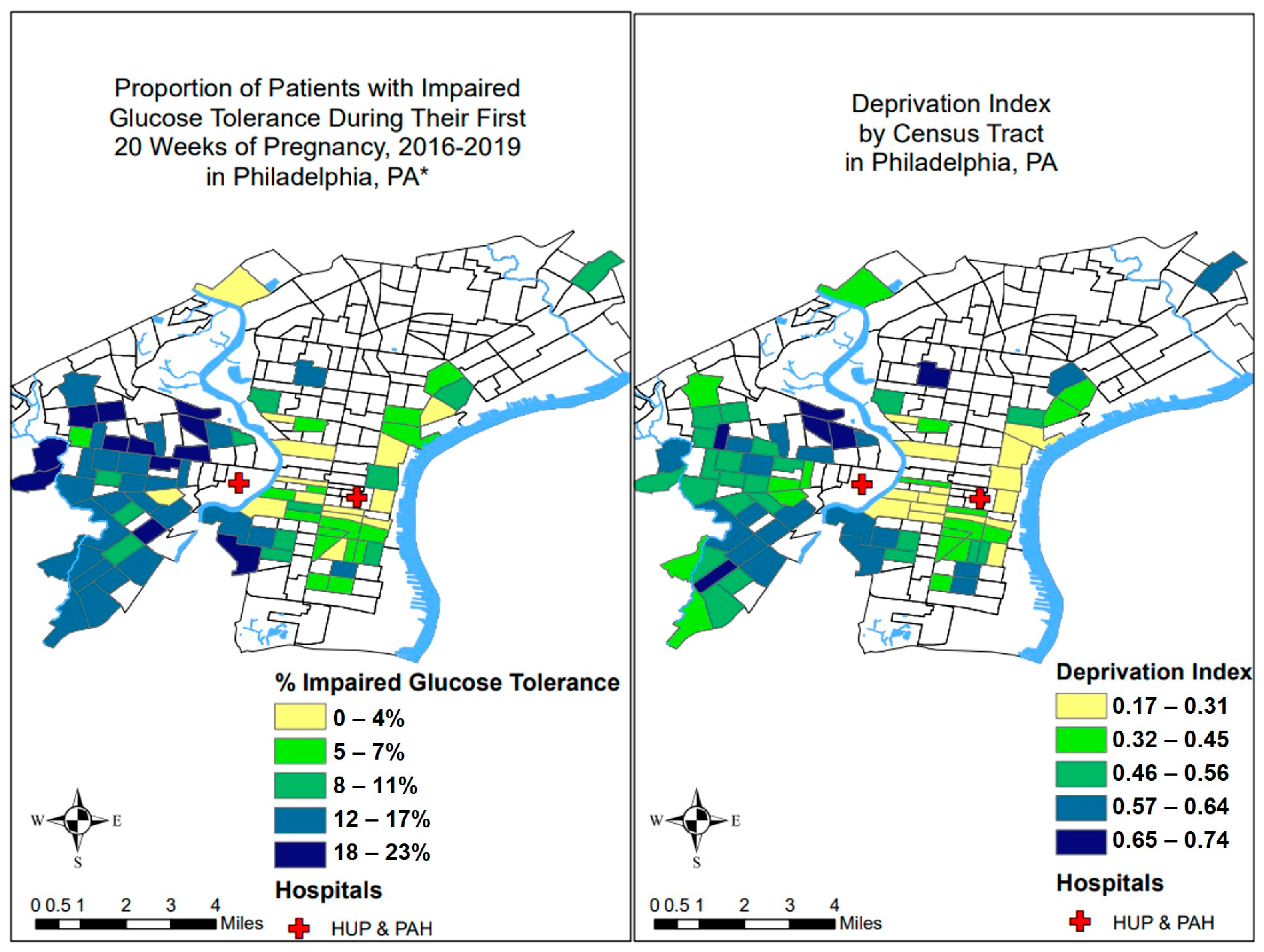
3.1. Associations of Neighborhood Deprivation with IGT and Obesity
3.2. Racial Disparities in IGT and Obesity
4. Discussion
4.1. Principal Findings
4.2. Results
4.3. Clinical and Research Implications
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021|Diabetes Care|American Diabetes Association. Available online: https://diabetesjournals.org/care/article/44/Supplement_1/S15/30859/2-Classification-and-Diagnosis-of-Diabetes (accessed on 27 July 2022).
- Kirk, J.K.; D’Agostino, R.B., Jr.; Bell, R.A.; Passmore, L.V.; Bonds, D.E.; Karter, A.J.; Narayan, K.M. Disparities in HbA1c Levels Between African-American and Non-Hispanic White Adults With Diabetes: A meta-analysis. Diabetes Care 2006, 29, 2130–2136. [Google Scholar] [CrossRef]
- Kattini, R.; Hummelen, R.; Kelly, L. Early Gestational Diabetes Mellitus Screening with Glycated Hemoglobin: A Systematic Review. J. Obstet. Gynaecol. Can. 2020, 42, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Fong, A.; Serra, A.E.; Gabby, L.; Wing, D.A.; Berkowitz, K.M. Use of hemoglobin A1c as an early predictor of gestational diabetes mellitus. Am. J. Obstet. Gynecol. 2014, 211, 641.e1–641.e7. [Google Scholar] [CrossRef]
- Amylidi, S.; Mosimann, B.; Stettler, C.; Fiedler, G.M.; Surbek, D.; Raio, L. First-trimester glycosylated hemoglobin in women at high risk for gestational diabetes. Acta Obstet. Gynecol. Scand. 2016, 95, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, S.N.; Tsai, M.Y.; Rawal, S.; Albert, P.S.; Zhang, C. HbA1c Measured in the First Trimester of Pregnancy and the Association with Gestational Diabetes. Sci. Rep. 2018, 8, 12249. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, A.N.; Ross, G.P.; Hyett, J.; Molyneaux, L.; Constantino, M.; Harding, A.J.; Wong, J. Gestational Diabetes Mellitus in Early Pregnancy: Evidence for Poor Pregnancy Outcomes Despite Treatment. Diabetes Care 2016, 39, 75–81. [Google Scholar] [CrossRef]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Committee on Health Care for Underserved Women. ACOG Committee Opinion No. 729: Importance of Social Determinants of Health and Cultural Awareness in the Delivery of Reproductive Health Care. Obstet. Gynecol. 2018, 131, e43–e48. [Google Scholar] [CrossRef]
- Blumenshine, P.; Egerter, S.; Barclay, C.J.; Cubbin, C.; Braveman, P.A. Socioeconomic disparities in adverse birth outcomes: A systematic review. Am. J. Prev. Med. 2010, 39, 263–272. [Google Scholar] [CrossRef]
- Tiako, M.J.N.; McCarthy, C.; Meisel, Z.F.; Elovitz, M.A.; Burris, H.H.; South, E. Association between Low Urban Neighborhood Greenness and Hypertensive Disorders of Pregnancy. Am. J. Perinatol. 2021. [Google Scholar] [CrossRef]
- Dolin, C.D.; Compher, C.C.; Oh, J.K.; Durnwald, C.P. Pregnant and hungry: Addressing food insecurity in pregnant women during the COVID-19 pandemic in the United States. Am. J. Obstet. Gynecol. MFM 2021, 3, 100378. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.H.; Hacker, M.R. Birth outcome racial disparities: A result of intersecting social and environmental factors. Semin. Perinatol. 2017, 41, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Messer, L.C.; Kaufman, J.S.; Dole, N.; Savitz, D.A.; Laraia, B.A. Neighborhood crime, deprivation, and preterm birth. Ann. Epidemiol. 2006, 16, 455–462. [Google Scholar] [CrossRef]
- Nardone, A.; Chiang, J.; Corburn, J. Historic Redlining and Urban Health Today in U.S. Cities. Environ. Justice 2020, 13, 109–119. [Google Scholar] [CrossRef]
- Howell, J.; Korver-Glenn, E. The Increasing Effect of Neighborhood Racial Composition on Housing Values, 1980–2015. Soc. Probl. 2021, 68, 1051–1071. [Google Scholar] [CrossRef]
- Do, D.P.; Finch, B.K.; Basurto-Davila, R.; Bird, C.; Escarce, J.; Lurie, N. Does place explain racial health disparities? Quantifying the contribution of residential context to the Black/white health gap in the United States. Soc. Sci. Med. 2008, 67, 1258–1268. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Mujahid, M.S.; Laraia, B.A.; Warton, E.M.; Blanchard, S.D.; Moffet, H.H.; Downing, J.; Karter, A.J. Association Between Neighborhood Supermarket Presence and Glycated Hemoglobin Levels Among Patients with Type 2 Diabetes Mellitus. Am. J. Epidemiol. 2017, 185, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Handley, S.C.; Mullin, A.M.; Elovitz, M.A.; Gerson, K.D.; Montoya-Williams, D.; Lorch, S.A.; Burris, H.H. Changes in Preterm Birth Phenotypes and Stillbirth at 2 Philadelphia Hospitals During the SARS-CoV-2 Pandemic, March–June 2020. JAMA 2021, 325, 87–89. [Google Scholar] [CrossRef]
- Burris, H.H.; Mullin, A.M.; Dhudasia, M.B.; Flannery, D.D.; Mukhopadhyay, S.; Pfeifer, M.R.; Woodford, E.C.; Briker, S.M.; Triebwasser, J.E.; Morris, J.S.; et al. Neighborhood Characteristics and Racial Disparities in Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Seropositivity in Pregnancy. Obstet. Gynecol. 2022, 139, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Williams, D.; Mullin, A.M.; Handley, S.C.; Flannery, D.D.; Lorch, S.A.; Elovitz, M.A.; Burris, H.H. Disparities in SARS-CoV-2 positivity among pregnant patients with limited English proficiency. J. Perinatol. 2021, 41, 2564–2565. [Google Scholar] [CrossRef] [PubMed]
- Weight Gain During Pregnancy. Available online: https://www.acog.org/en/clinical/clinical-guidance/committee-opinion/articles/2013/01/weight-gain-during-pregnancy (accessed on 27 July 2022).
- Brokamp, C.; Beck, A.F.; Goyal, N.K.; Ryan, P.; Greenberg, J.M.; Hall, E.S. Material community deprivation and hospital utilization during the first year of life: An urban population-based cohort study. Ann. Epidemiol. 2019, 30, 37–43. [Google Scholar] [CrossRef]
- Messer, L.C.; Laraia, B.A.; Kaufman, J.S.; Eyster, J.; Holzman, C.; Culhane, J.; Elo, I.; Burke, J.G.; O’Campo, P. The development of a standardized neighborhood deprivation index. J. Urban Health Bull. N. Y. Acad. Med. 2006, 83, 1041–1062. [Google Scholar] [CrossRef]
- Kind, A.J.H.; Buckingham, W.R. Making Neighborhood-Disadvantage Metrics Accessible—The Neighborhood Atlas. N. Engl. J. Med. 2018, 378, 2456–2458. [Google Scholar] [CrossRef] [PubMed]
- CDC/ATSDR Social Vulnerability Index (SVI). Published 13 June 2022. Available online: https://www.atsdr.cdc.gov/placeandhealth/svi/index.html (accessed on 1 August 2022).
- Bureau, U.C. American Community Survey (ACS). Census.gov. Available online: https://www.census.gov/programs-surveys/acs (accessed on 27 July 2022).
- Kirk, J.K.; Bell, R.A.; Bertoni, A.G.; Arcury, T.A.; Quandt, S.A.; Goff, D.C., Jr.; Narayan, K.M. A Qualitative Review of Studies of Diabetes Preventive Care Among Minority Patients in the United States, 1993–2003. Am. J. Manag. Care 2005, 11, 349–360. [Google Scholar] [PubMed]
- Mezuk, B.; Lexima, E.; Kalesnikava, V.A.; Fleming, J.; Montgomery, J.; Tuktur, W.; Winston, J.; Perrin, P.B.; Green, T.; Wheeler, D.C. Stress Reactivity as a Contributor to Racial and Socioeconomic Disparities: Rationale and Baseline Results From the Richmond Stress and Sugar Study. Psychosom. Med. 2020, 82, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.S.; Shandas, V.; Pendleton, N. The Effects of Historical Housing Policies on Resident Exposure to Intra-Urban Heat: A Study of 108 US Urban Areas. Climate 2020, 8, 12. [Google Scholar] [CrossRef]
- Casey, J.A.; Morello-Frosch, R.; Mennitt, D.J.; Fristrup, K.; Ogburn, E.L.; James, P. Race/Ethnicity, Socioeconomic Status, Residential Segregation, and Spatial Variation in Noise Exposure in the Contiguous United States. Environ. Health Perspect. 2017, 125, 077017. [Google Scholar] [CrossRef]
- Runkle, J.D.; Matthews, J.L.; Sparks, L.; McNicholas, L.; Sugg, M.M. Racial and ethnic disparities in pregnancy complications and the protective role of greenspace: A retrospective birth cohort study. Sci. Total Environ. 2022, 808, 152145. [Google Scholar] [CrossRef]
- Shannon, M.M.; Clougherty, J.E.; McCarthy, C.; Elovitz, M.A.; Nguemeni Tiako, M.J.; Melly, S.J.; Burris, H.H. Neighborhood Violent Crime and Perceived Stress in Pregnancy. Int. J. Environ. Res. Public Health 2020, 17, 5585. [Google Scholar] [CrossRef]
- Eberly, L.A.; Julien, H.; South, E.C.; Venkataraman, A.; Nathan, A.S.; Anyawu, E.C.; Dayoub, E.; Groeneveld, P.W.; Khatana, S.A.M. Association Between Community-Level Violent Crime and Cardiovascular Mortality in Chicago: A Longitudinal Analysis. J. Am. Heart Assoc. 2022, 11, e025168. [Google Scholar] [CrossRef]
- Morello-Frosch, R.; Shenassa, E.D. The Environmental “Riskscape” and Social Inequality: Implications for Explaining Maternal and Child Health Disparities. Environ. Health Perspect. 2006, 114, 1150–1153. [Google Scholar] [CrossRef]
- Chandrabose, M.; Rachele, J.N.; Gunn, L.; Kavanagh, A.; Owen, N.; Turrell, G.; Giles-Corti, B.; Sugiyama, T. Built environment and cardio-metabolic health: Systematic review and meta-analysis of longitudinal studies. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2019, 20, 41–54. [Google Scholar] [CrossRef]
- Den Braver, N.R.; Lakerveld, J.; Rutters, F.; Schoonmade, L.J.; Brug, J.; Beulens, J.W.J. Built environmental characteristics and diabetes: A systematic review and meta-analysis. BMC Med. 2018, 16, 12. [Google Scholar] [CrossRef]
- Gebreab, S.Y.; Hickson, D.A.; Sims, M.; Wyatt, S.B.; Davis, S.K.; Correa, A.; Diez-Roux, A.V. Neighborhood social and physical environments and type 2 diabetes mellitus in African Americans: The Jackson Heart Study. Health Place 2017, 43, 128–137. [Google Scholar] [CrossRef]
- Holcomb, D.S.; Pengetnze, Y.; Steele, A.; Karam, A.; Spong, C.; Nelson, D.B. Geographic barriers to prenatal care access and their consequences. Am. J. Obstet. Gynecol. MFM 2021, 3, 100442. [Google Scholar] [CrossRef]
- Theall, K.P.; Drury, S.S.; Shirtcliff, E.A. Cumulative Neighborhood Risk of Psychosocial Stress and Allostatic Load in Adolescents. Am. J. Epidemiol. 2012, 176 (Suppl. 7), S164–S174. [Google Scholar] [CrossRef] [PubMed]
- Kurani, S.S.; Heien, H.C.; Sangaralingham, L.R.; Inselman, J.W.; Shah, N.D.; Golden, S.H.; McCoy, R.G. Association of Area-Level Socioeconomic Deprivation with Hypoglycemic and Hyperglycemic Crises in US Adults with Diabetes. JAMA Netw. Open 2022, 5, e2143597. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, J.; Sanbonmatsu, L.; Gennetian, L.; Adam, E.; Duncan, G.J.; Katz, L.F.; Kessler, R.C.; Kling, J.R.; Lindau, S.T.; Whitaker, R.C.; et al. Neighborhoods, Obesity, and Diabetes—A Randomized Social Experiment. N. Engl. J. Med. 2011, 365, 1509–1519. [Google Scholar] [CrossRef]
- Bender, W.; McCarthy, C.; Elovitz, M.; Parry, S.; Durnwald, C. Universal HbA1c screening and gestational diabetes: A comparison with clinical risk factors. J. Matern. Fetal Neonatal. Med. 2021, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lowe, L.P.; Metzger, B.E.; Dyer, A.R.; Lowe, J.; McCance, D.R.; Lappin, T.R.; Trimble, E.R.; Coustan, D.R.; Hadden, D.R.; Hod, M.; et al. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: Associations of maternal A1C and glucose with pregnancy outcomes. Diabetes Care 2012, 35, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.C.E.; Moore, M.P.; Gullam, J.E.; Mohamed, K.; Rowan, J. An early pregnancy HbA1c ≥ 5.9% (41 mmol/mol) is optimal for detecting diabetes and identifies women at increased risk of adverse pregnancy outcomes. Diabetes Care 2014, 37, 2953–2959. [Google Scholar] [CrossRef] [PubMed]
- Bender, W.R.; McCarthy, C.; Elovitz, M.; Parry, S.; Durnwald, C. Adverse Pregnancy Outcomes in Nondiabetic Patients with an Elevated Early Pregnancy HbA1c. Am. J. Perinatol. 2022, 29, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Robbins, C. Disparities in Preconception Health Indicators—Behavioral Risk Factor Surveillance System, 2013–2015, and Pregnancy Risk Assessment Monitoring System, 2013–2014. MMWR Surveill Summ. 2018, 67, 1–16. [Google Scholar] [CrossRef]
- Gemmill, A.; Berger, B.O.; Crane, M.A.; Margerison, C.E. Mortality Rates Among U.S. Women of Reproductive Age, 1999–2019. Am. J. Prev. Med. 2022, 62, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Maternal Mortality Rates in the United States. 2020; Published 22 February 2022. Available online: https://www.cdc.gov/nchs/data/hestat/maternal-mortality/2020/maternal-mortality-rates-2020.htm (accessed on 27 July 2022).
- Cai, X.; Zhang, Y.; Li, M.; Wu, J.H.; Mai, L.; Li, J.; Yang, Y.; Hu, Y.; Huang, Y. Association between prediabetes and risk of all cause mortality and cardiovascular disease: Updated meta-analysis. BMJ 2020, 370, m2297. [Google Scholar] [CrossRef]
- Benmarhnia, T.; Huang, J.; Basu, R.; Wu, J.; Bruckner, T.A. Decomposition Analysis of Black-White Disparities in Birth Outcomes: The Relative Contribution of Air Pollution and Social Factors in California. Environ. Health Perspect. 2017, 125, 107003. [Google Scholar] [CrossRef] [PubMed]
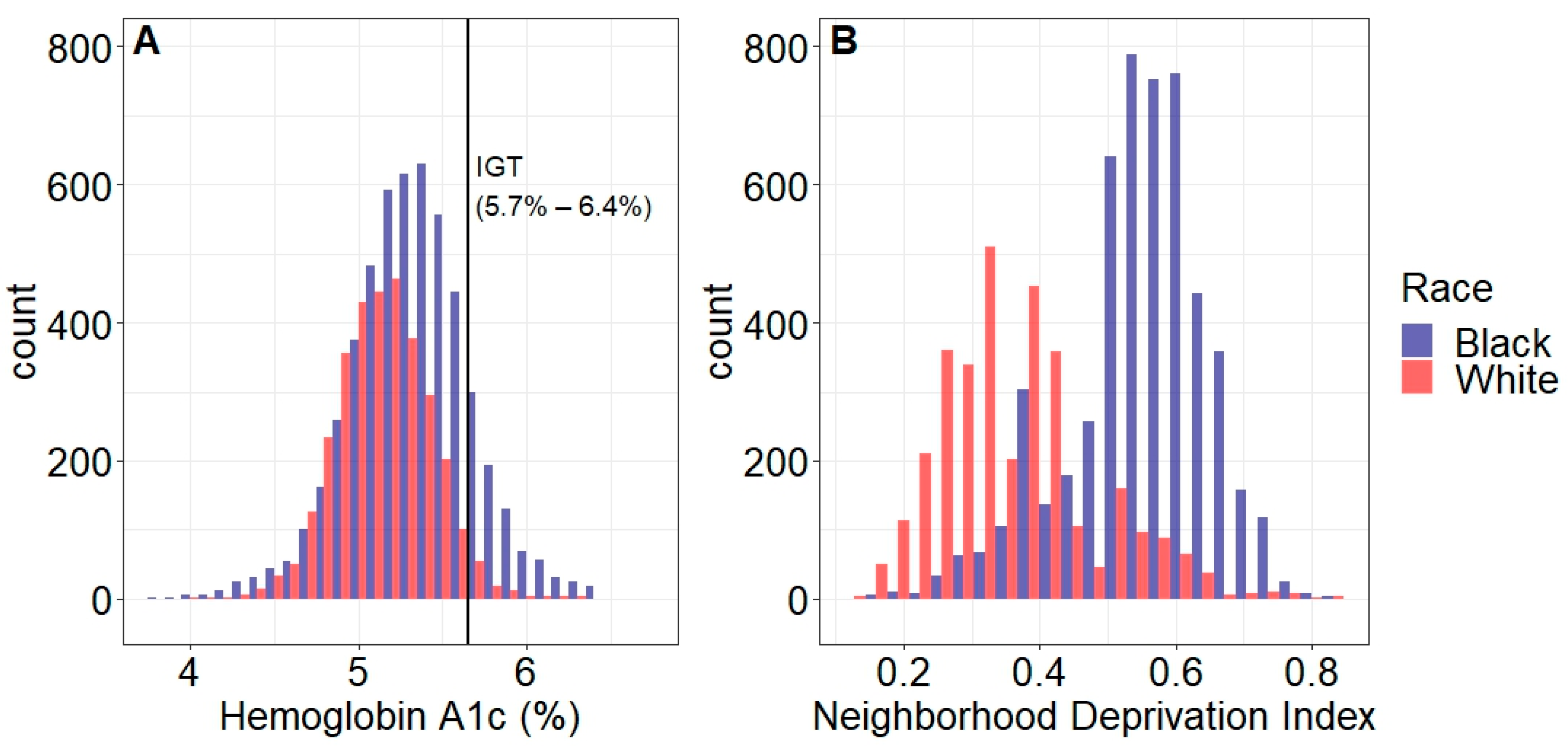
| Normal Glucose Tolerance (HbA1c < 5.7%) | Impaired Glucose Tolerance (HbA1c 5.7–6.4%) | ||
|---|---|---|---|
| Characteristics | n (row %) | n (row %) | p-value |
| All | 9510 (89.4) | 1132 (10.6) | |
| Race and ethnicity | <0.001 | ||
| Non-Hispanic Black | 4396 (84.2) | 823 (15.8) | |
| Non-Hispanic White | 3136 (96.9) | 101 (3.1) | |
| Another race or ethnicity | 1978 (90.5) | 208 (9.5) | |
| Insurance | <0.001 | ||
| Private | 5005 (91.6) | 456 (8.4) | |
| Public | 4505 (87.0) | 676 (13.0) | |
| Pre-pregnancy BMI (kg/m2) | <0.001 | ||
| <30 (not obese) | 6795 (93.6) | 463 (6.4) | |
| ≥30 (obese) | 2715 (80.2) | 669 (19.8) | |
| Smoked during pregnancy * | 0.18 | ||
| Yes | 554 (87.5) | 79 (12.5) | |
| No | 8827 (89.4) | 1042 (10.6) | |
| Parity | <0.001 | ||
| 0 | 4425 (93.1) | 330 (6.9) | |
| >0 | 5085 (86.4) | 802 (13.6) | |
| mean (SD) | mean (SD) | ||
| Age (years) | 30.0 (5.7) | 31.4 (5.8) | <0.001 |
| Pre-Pregnancy BMI (kg/m2) | 27.2 (7.2) | 32.9 (8.7) | <0.001 |
| Neighborhood deprivation index | 0.47 (0.14) | 0.52 (0.13) | <0.001 |
| Models of Neighborhood Deprivation with IGT | OR | (95% CI) |
|---|---|---|
| M0 = Unadjusted | 1.44 | (1.25, 1.67) |
| M1 = M0 + age, insurance, parity | 1.47 | (1.35, 1.61) |
| M2 = M1 + obesity | 1.33 | (1.23, 1.43) |
| M3 = M1 + race | 1.15 | (1.07, 1.24) |
| M4 = M1 + race + obesity | 1.08 | (1.00, 1.15) |
| Black Patients | ||
| M0 = Unadjusted | 1.06 | (0.96, 1.17) |
| M1 = M0 + age, insurance, parity | 1.17 | (1.06, 1.29) |
| M2 = M1 + obesity | 1.13 | (1.02, 1.25) |
| White Patients | ||
| M0 = Unadjusted | 1.16 | (0.97, 1.38) |
| M1 = M0 + age, insurance, parity | 1.09 | (0.91, 1.32) |
| M2 = M1 + obesity | 1.02 | (0.84, 1.24) |
| Models of Neighborhood Deprivation with Obesity | ||
| M0 = Unadjusted | 1.78 | (1.51, 2.11) |
| M1 = M0 + age, insurance, parity | 1.59 | (1.42, 1.78) |
| M2 = M1 + race | 1.39 | (1.28, 1.52) |
| Black Patients | ||
| M0 = Unadjusted | 1.10 | (1.03, 1.18) |
| M1 = M0 + age, insurance, parity | 1.15 | (1.07, 1.24) |
| White Patients | ||
| M0 = Unadjusted | 1.70 | (1.46, 1.99) |
| M1 = M0 + age, insurance, parity | 1.47 | (1.25, 1.73) |
| Models of IGT | OR | (95% CI) |
|---|---|---|
| M0 = Unadjusted | 5.81 | (4.80, 7.04) |
| M1 = M0 + age, insurance, parity | 8.23 | (6.60, 10.28) |
| M2 = M1 + deprivation index | 6.97 | (5.53, 8.79) |
| M3 = M1 + deprivation index + obesity | 5.26 | (4.17, 6.65) |
| Models of Obesity | ||
| M0 = Unadjusted | 4.31 | (3.11, 5.98) |
| M1 = M0 + age, insurance, parity | 4.14 | (3.22, 5.33) |
| M2 = M1 + deprivation index | 3.24 | (2.63, 3.98) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolin, C.D.; Mullin, A.M.; Ledyard, R.F.; Bender, W.R.; South, E.C.; Durnwald, C.P.; Burris, H.H. Neighborhood Deprivation and Racial Disparities in Early Pregnancy Impaired Glucose Tolerance. Int. J. Environ. Res. Public Health 2023, 20, 6175. https://doi.org/10.3390/ijerph20126175
Dolin CD, Mullin AM, Ledyard RF, Bender WR, South EC, Durnwald CP, Burris HH. Neighborhood Deprivation and Racial Disparities in Early Pregnancy Impaired Glucose Tolerance. International Journal of Environmental Research and Public Health. 2023; 20(12):6175. https://doi.org/10.3390/ijerph20126175
Chicago/Turabian StyleDolin, Cara D., Anne M. Mullin, Rachel F. Ledyard, Whitney R. Bender, Eugenia C. South, Celeste P. Durnwald, and Heather H. Burris. 2023. "Neighborhood Deprivation and Racial Disparities in Early Pregnancy Impaired Glucose Tolerance" International Journal of Environmental Research and Public Health 20, no. 12: 6175. https://doi.org/10.3390/ijerph20126175
APA StyleDolin, C. D., Mullin, A. M., Ledyard, R. F., Bender, W. R., South, E. C., Durnwald, C. P., & Burris, H. H. (2023). Neighborhood Deprivation and Racial Disparities in Early Pregnancy Impaired Glucose Tolerance. International Journal of Environmental Research and Public Health, 20(12), 6175. https://doi.org/10.3390/ijerph20126175






