Young Adults with Negative Body Image at Fatness Subscale Are More Restrained Than Normal Adults during a Chocolate Discounting Task
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Materials
2.2.1. Self-Measurements
- (1)
- Hunger and Desire to Eat
- (2)
- Dutch Eating Behavior Questionnaire—Restraint Scale (DEBQ-RS)
- (3)
- Negative physical self scale at Fatness Subscale (NPSS-F)
- (4)
- Power of Food Scale (PFS)
2.2.2. Chocolate-Related Delayed Discounting Task
2.2.3. Procedure
2.2.4. Data Recording and Analysis
- (1)
- Behavior Analyses
- (2)
- EEG Recording and Analyses
3. Results
3.1. Behavioral Performance
- (1)
- Self-Reported Results
- (2)
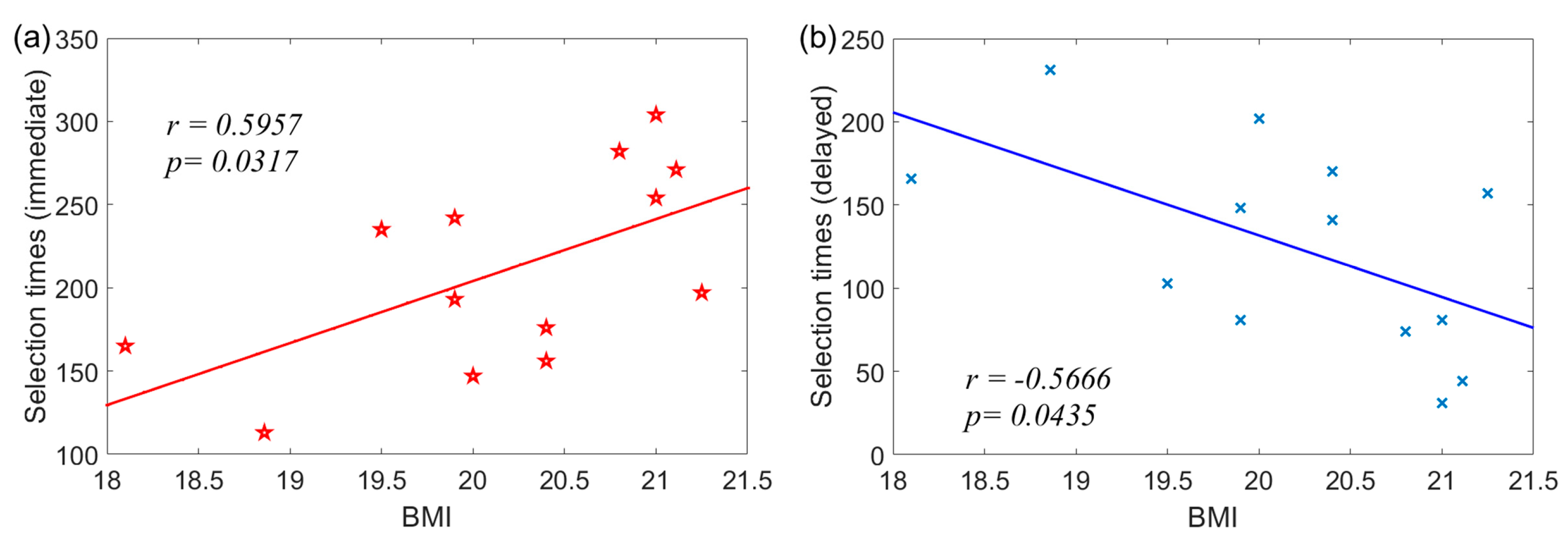
- Correlation between BMI and Selection Times
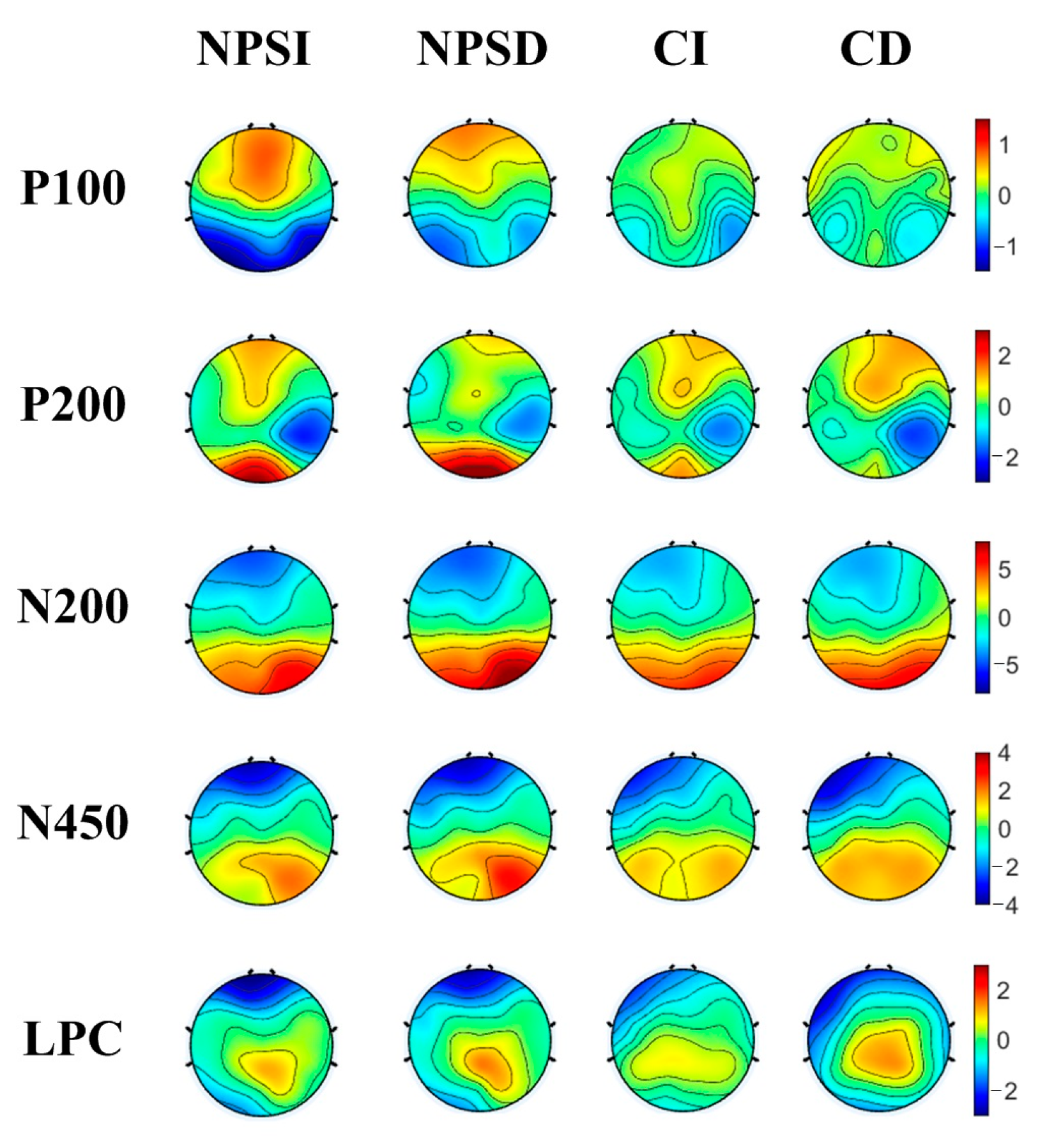
3.2. ERP Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, H.M.; Liu, Y.X.; Guo, X.; Liao, X.; Song, G.; Xian, Y.; Li, W.M. Investigation and analysis on the status quo of female college students’ weight loss behavior and cognition. Chin. Matern. Child Health Res. 2015, 4, 700–702. [Google Scholar]
- Zhang, X.D.; Wu, H.; Zhang, T.H. Research and analysis of female undergraduates’ fat cognition and weight-loss behavior. Matern. Child Health Care China 2008, 21, 3017–3019. [Google Scholar]
- Trédan, O.; Bajard, A.; Meunier, A.; Roux, P.; Fiorletta, I.; Gargi, T.; Bachelot, T.; Guastalla, J.P.; Lallemand, Y.; Faure, C.; et al. Body weight change in women receiving adjuvant chemotherapy for breast cancer: A French prospective study. Clin. Nutr. 2010, 29, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Gruszka, W.; Owczarek, A.J.; Glinianowicz, M.; Bąk-Sosnowska, M.; Chudek, J.; Olszanecka-Glinianowicz, M. Perception of body size and body dissatisfaction in adults. Sci. Rep. 2022, 12, 1159. [Google Scholar] [CrossRef]
- Kuchler, F.; Variyam, J.N. Mistakes were made: Misperception as a barrier to reducing overweight. Int. J. Obes. 2003, 27, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Seth, N.; Seal, A.; Ruchin, P.; McGirr, J. The accuracy of self-perception of obesity in a rural Australian population: A cross-sectional study. J. Prim. Care Community Health 2022, 13, 21501319221115256. [Google Scholar] [CrossRef]
- Baker, J.D.; Williamson, D.A.; Sylve, C. Body image disturbance, memory bias, and body dysphoria: Effects of negative mood induction. Behav. Ther. 1995, 26, 747–759. [Google Scholar] [CrossRef]
- Perpiñá, C.; Hemsley, D.; Treasure, J.; de Silva, P. Is the selective information processing of food and body words specific to patients with eating disorders? Int. J. Eat. Disord. 1993, 14, 359–366. [Google Scholar] [CrossRef]
- Ralph-Nearman, C.; Achee, M.; Lapidus, R.; Stewart, J.L.; Filik, R. A systematic and methodological review of attentional biases in eating disorders: Food, body, and perfectionism. Brain Behav. 2019, 9, e01458. [Google Scholar] [CrossRef]
- Feng, W.F.; Luo, W.B.; Liao, Y.; Chen, H.; Luo, Y.J. Attention biases of undergraduate women with fat negative physical self: Orienting or maintenance. Acta Psychol. Sin. 2010, 42, 779–790. [Google Scholar] [CrossRef]
- Ahrberg, M.; Trojca, D.; Nasrawi, N.; Vocks, S. Body image disturbance in binge eating disorder: A review. Eur. Eat. Disord. Rev. 2011, 19, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Ahadzadeh, A.S.; Sharif, S.P.; Ong, F.S. Self-schema and self-discrepancy mediate the influence of Instagram usage on body image satisfaction among youth. Comput. Hum. Behav. 2017, 68, 8–16. [Google Scholar] [CrossRef]
- Mischel, W.; Shoda, Y.; Rodriguez, M.L. Delay of gratification in children. Science 1989, 244, 933–937. [Google Scholar] [CrossRef]
- Pang, X.; Lv, H.C.; Hua, S.X. Delayed gratification: Self-regulation based on future time insight. Psychol. Sci. 2014, 1, 78–82. [Google Scholar]
- Yang, Y.; Shields, G.S.; Guo, C.; Liu, Y. Executive function performance in obesity and overweight individuals: A meta-analysis and review. Neurosci. Biobehav. Rev. 2018, 84, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hu, Y.; Zhang, W.; Wang, J.; Ji, W.; Manza, P.; Volkow, N.D.; Zhang, Y.; Wang, G. Brain functional and structural magnetic resonance imaging of obesity and weight loss interventions. Mol. Psychiatry 2023, 28, 1466–1479. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, J.; Zhang, X.; Gao, X.; Xu, W.; Chen, H. Overweight adults are more impulsive than normal weight adults: Evidence from ERPs during a chocolate-related delayed discounting task. Neuropsychologia 2019, 133, 107181. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, X.; Zhao, J.; Zhang, L.; Chen, H. Neurocognitive correlates of food-related response inhibition in overweight/obese adults. Brain Topogr. 2020, 33, 101–111. [Google Scholar] [CrossRef]
- Guerrieri, R.; Nederkoorn, C.; Jansen, A. How impulsiveness and variety influence food intake in a sample of healthy women. Appetite 2007, 48, 119–122. [Google Scholar] [CrossRef]
- Jarmolowicz, D.P.; Cherry, J.B.C.; Reed, D.D.; Bruce, J.M.; Crespi, J.M.; Lusk, J.L.; Bruce, A.S. Robust relation between temporal discounting rates and body mass. Appetite 2014, 78, 63–67. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.; Fowler, J.; Tomasi, D.; Baler, R. Food and drug reward: Overlapping circuits in human obesity and addiction. Curr. Top. Behav. Neurosci. 2012, 11, 1–24. [Google Scholar] [PubMed]
- Rasmussen, E.B.; Lawyer, S.R.; Reilly, W. Percent body fat is related to delay and probability discounting for food in humans. Behav. Process. 2010, 83, 23–30. [Google Scholar] [CrossRef]
- Patton, J.H.; Stanford, M.S.; Barratt, E.S. Factor structure of the Barratt Impulsiveness Scale. J. Clin. Psychol. 1995, 51, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Bechara, A.; Damásio, A.R.; Damásio, H.; Anderson, S.W. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 1994, 50, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Fellows, L.K.; Farah, M.J. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cereb. Cortex 2005, 15, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Voges, M.M.; Giabbiconi, C.M.; Schöne, B.; Waldorf, M.; Hartmann, A.S.; Vocks, S. Gender differences in body evaluation: Do men show more self-serving double standards than women? Front. Psychol. 2019, 10, 544. [Google Scholar] [CrossRef]
- Chen, H.; Jackson, T.; Huang, X. The negative physical self scale: Initial development and validation in samples of Chinese adolescents and young adults. Body Image 2006, 3, 401–412. [Google Scholar] [CrossRef]
- Van Strien, T.; Frijters, J.E.; Van Staveren, W.A.; Defares, P.B.; Deurenberg, P. The predictive validity of the Dutch restrained eating scale. Int. J. Eat. Disord. 1986, 5, 747–755. [Google Scholar] [CrossRef]
- Bohrer, B.K.; Forbush, K.T.; Hunt, T.K. Are common measures of dietary restraint and disinhibited eating reliable and valid in obese persons? Appetite 2015, 87, 344–351. [Google Scholar] [CrossRef]
- Chen, L.L.; Zhou, A.B. The intervention of female negative physical self: The modification strategy of attention bias. Chin. J. Clin. Psychol. 2022, 30, 1240–1244. [Google Scholar]
- Cappelleri, J.C.; Bushmakin, A.G.; Gerber, R.A.; Leidy, N.K.; Sexton, C.C.; Karlsson, J.; Lowe, M.R. Evaluating the power of food scale in obese subjects and a general sample of individuals: Development and measurement properties. Int. J. Obes. 2009, 33, 913–922. [Google Scholar] [CrossRef]
- Dong, D.; Wang, Y.; Jackson, T.; Chen, S.; Wang, Y.; Zhou, F.; Chen, H. Impulse control and restrained eating among young women: Evidence for compensatory cortical activation during a chocolate-specific delayed discounting task. Appetite 2016, 105, 477–486. [Google Scholar] [CrossRef]
- Weygandt, M.; Mai, K.; Dommes, E.; Leupelt, V.; Hackmack, K.; Kahnt, T.; Rothemund, Y.; Spranger, J.; Haynes, J.D. The role of neural impulse control mechanisms for dietary success in obesity. Neuroimage 2013, 83, 669–678. [Google Scholar] [CrossRef]
- Weygandt, M.; Mai, K.; Dommes, E.; Ritter, K.; Leupelt, V.; Spranger, J.; Haynes, J.D. Impulse control in the dorsolateral prefrontal cortex counteracts post-diet weight regain in obesity. Neuroimage 2015, 109, 318–327. [Google Scholar] [CrossRef]
- Kable, J.W.; Glimcher, P. The neural correlates of subjective value during inter-temporal choice. Nat. Neurosci. 2007, 10, 1625–1633. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, D.; Todd, J.; Du, J.; Yang, Z.; Lu, H.; Chen, H. Neural correlates of restrained eaters’ high susceptibility to food cues: An fMRI study. Neurosci. Lett. 2016, 631, 56–62. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, D.; Yu, C.; Zhang, J.; Tian, X.; von Deneen, K.M.; Zang, Y.; Walter, M.; Liu, Y. Altered baseline brain activities before food intake in obese men: A resting state fMRI study. Neurosci. Lett. 2015, 584, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, Y.; Zhang, J.; Liu, Y.; Long, Z.; Ran, H.; Li, J.; Qin, Y.; Zhao, J. Neural responses to rapidly selecting color names with different novelty. Cogn. Neurodyn. 2021, 15, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.S.; Knight, R.T. Mechanisms of human attention: Event-related potentials and oscillations. Neurosci. Biobehav. Rev. 2001, 25, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Luck, S.J.; Woodman, G.F.; Vogel, E.K. Event-related potential studies of attention. Trends Cogn. Sci. 2000, 4, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, P.D.; Stoerig, P.; Falkenstein, M. ERP correlations of response selection in a response conflict paradigm. Brain Res. 2008, 1189, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Nijs, I.M.; Muris, P.; Euser, A.S.; Franken, I.H. Differences in attention to food and food intake between overweight/obese and normal-weight females under conditions of hunger and satiety. Appetite 2010, 54, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.M.; Battlay, P.; Gledhill-Smith, R.S.; Good, R.T.; Lumb, C.; Fournier-Level, A.; Robin, C. Insights into DDT resistance from the drosophila melanogaster genetic reference panel. Genetics 2017, 207, 1181–1193. [Google Scholar] [CrossRef] [PubMed]
- Szűcs, D.; Soltész, F. Functional definition of the N450 event-related brain potential marker of conflict processing: A numerical stroop study. BMC Neurosci. 2012, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Imbir, K.K.; Spustek, T.; Duda, J.; Bernatowicz, G.; Żygierewicz, J. N450 and LPC event-related potential correlates of an emotional Stroop task with words differing in valence and emotional origin. Front. Psychol. 2017, 8, 880. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, J.; Asthana, M.K.; Zuo, K.; Comfort, W.E.; Xu, Z. Electrophysiological evidence that release from proactive inhibition reflects late semantic processing. Psychophysiology 2020, 57, e13639. [Google Scholar] [CrossRef]
- Westenhoefer, J.; von Falck, B.; Stellfeldt, A.; Fintelmann, S. Behavioural correlates of successful weight reduction over 3 year. Results from the lean habits study. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 334–335. [Google Scholar] [CrossRef]
- Westenhoefer, J.; Engel, D.; Holst, C.; Lorenz, J.; Peacock, M.; Stubbs, J.; Whybrow, S.; Raats, M. Cognitive and weight-related correlates of flexible and rigid restrained eating behaviour. Eat. Behav. 2013, 14, 69–72. [Google Scholar] [CrossRef]



| Variable | Group with Negative Body Image at Fatness Subscale (M ± SD) N = 13 | Control Group (M ± SD) N = 13 |
|---|---|---|
| Age | 19.21 (1.31) | 18.85 (1.07) |
| DEBQ-RS *** | 38.07 (7.35) | 22.62 (5.33) |
| NPSS-F *** | 2.92 (0.30) | 1.62 (0.28) |
| PFS | 55.00 (11.57) | 48.69 (6.22) |
| Hunger | 3.43 (1.95) | 4.08 (1.27) |
| Desire to eat | 2.03 (0.37) | 2.13 (0.47) |
| BMI | 20.81 (1.62) | 20.64 (2.03) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, S.; Li, J.; Guo, J.; Yan, H.; Deng, X.; Liu, Y.; Zhao, J. Young Adults with Negative Body Image at Fatness Subscale Are More Restrained Than Normal Adults during a Chocolate Discounting Task. Int. J. Environ. Res. Public Health 2023, 20, 6122. https://doi.org/10.3390/ijerph20126122
Huo S, Li J, Guo J, Yan H, Deng X, Liu Y, Zhao J. Young Adults with Negative Body Image at Fatness Subscale Are More Restrained Than Normal Adults during a Chocolate Discounting Task. International Journal of Environmental Research and Public Health. 2023; 20(12):6122. https://doi.org/10.3390/ijerph20126122
Chicago/Turabian StyleHuo, Sirui, Jun Li, Jiaqi Guo, Han Yan, Xiaoyi Deng, Yong Liu, and Jia Zhao. 2023. "Young Adults with Negative Body Image at Fatness Subscale Are More Restrained Than Normal Adults during a Chocolate Discounting Task" International Journal of Environmental Research and Public Health 20, no. 12: 6122. https://doi.org/10.3390/ijerph20126122
APA StyleHuo, S., Li, J., Guo, J., Yan, H., Deng, X., Liu, Y., & Zhao, J. (2023). Young Adults with Negative Body Image at Fatness Subscale Are More Restrained Than Normal Adults during a Chocolate Discounting Task. International Journal of Environmental Research and Public Health, 20(12), 6122. https://doi.org/10.3390/ijerph20126122







