Abstract
Smokefree laws are intended to protect against second-hand smoke (SHS) in outdoor areas. We examined if exposure to PM2.5 particles in outdoor smoking areas changed breathing rates in 60 patients with asthma (n = 30) or with COPD (n = 30), in an open, non-randomised, interventional study model in Czechia, Ireland and Spain. The patients wore a PM2.5 particle monitor (AirSpeck) and a breath monitor (RESpeck) for 24 h to determine changes in breathing rates (Br) at rest and during a visit to an outside smoking area. Spirometry and breath CO were measured before and the day after visiting an outdoor smoking area. The PM2.5 levels at the 60 venues were highly variable, ranging from ≥2000 µg/m3 (in 4 premises) to ≤10 µg/m3 (in 3 premises, which had only a single wall in the structure). At 39 venues, the mean PM 2.5 levels were ≥25 µg/m3. The breathing rate changed significantly in 57 of the 60 patients, resulting in an increase in some patients and a decrease in others. Comprehensive smokefree laws were ineffective in protecting asthma and COPD patients from exposure to high levels of SHS in outside areas of pubs and terraces, which should be avoided by these patients. These findings also support the extension of smokefree laws to outside areas.
1. Introduction
Smokefree legislation and policies have increased globally [1,2,3,4]. In most EU countries, smokefree laws have been implemented in public buildings and in private businesses [3,5,6]. Their aims, in general, have been to protect workers and customers from exposure to second-hand smoke (SHS) and improve health.
They have been successful in reducing exposure to SHS, with short- and long-term benefits, improving health, reducing illness, increasing smoking cessation, and denormalising smoking [4,7,8,9,10,11,12,13,14]. Specific health benefits have been shown to accrue to vulnerable populations, including children and those with underlying diseases [12,15].
Despite decreasing smoking prevalence in the last thirty years, the increased population growth in the same period has led to a significant increase in the total number of smokers. In 2019, more than 1 billion people smoked tobacco regularly, with almost 8 million deaths attributable to smoking [16].
While smokefree legislation and policies have led to a decline in smoking prevalence, as the global population grows and with an estimated 77% of the world’s population still vulnerable to SHS [17], more non-smokers are exposed to SHS hazards [16,18].
A systematic exploration of the global burden of disease attributable to SHS across 204 countries and territories from 1990 to 2019 found that SHS exposure increased the risk of tracheal, bronchus and lung cancers, breast cancer, ischemic heart disease, chronic obstructive pulmonary disease (COPD), stroke, lower respiratory infections and diabetes mellitus [18]. That analysis [18] also found that the number of years lived with disabilities (YLDs) as a result of SHS more than doubled between 1990 and 2019.
Regarding outdoor areas, the subject of this study, the details of the smokefree laws vary, and result in variable exposures. At entertainment venues, such as pubs, bars and clubs, an allowance is usually made within laws to permit an area (or terraces) outside the main premises where smoking is allowed, provided that these areas are separate and are not complete buildings to allow for increased ventilation, and that that there is no commercial activity [19].
However, it has become obvious in many instances that these smoking areas allow for the accumulation of SHS and cannot be considered safe [20,21]. Since we now also accept that there is no safe level of SHS exposure [22], it can be expected that the exposure in those areas causes adverse health effects in the long term. Nasal and oral sensory symptoms have been observed, and lung function measurements have shown deterioration from long-term exposure to SHS [23,24,25,26,27].
Chronic respiratory diseases cause an important worldwide health burden. It was estimated that, in 2017, they were the third leading cause of death, behind cardiovascular diseases and neoplasms. Globally, there were 3,914,196 deaths due to chronic respiratory diseases in 2017, an increase of 18.0% since 1990 [28].
While scientific evidence has accumulated linking SHS exposure to longer-term adverse health outcomes, including respiratory outcomes in children and adults, acute cardiovascular effects and lung cancer [13,27,29,30,31,32,33], knowledge about acute health effects of SHS on respiratory disease patients is scarce, although the present knowledge suggests that acute adverse SHS effects are the most likely to be seen in the upper or lower respiratory system or the cardiovascular system [27,31,34]. Furthermore, subjects with underlying diseases may be more likely to be more susceptible to acute effects, in addition to their increased risk of adverse long-term effects from SHS exposure [12].
The negative effects of SHS on respiratory function are thus well established. Moreover, it is well documented that SHS from combustible tobacco smoke outdoors results in poorer outdoor air quality [35,36]. With these known increased exposure and long-term health effects, we decided to monitor short-term exposure to SHS in outdoor areas and acute breathing responses of subjects with known doctor-diagnosed common respiratory diseases, asthma and COPD. Because of the possible, but undocumented, acute effects, only subjects who routinely visited outside smoking areas as part of their normal social life were considered for the inclusion.
Three countries with statutory comprehensive smokefree laws, which have been in place for varying lengths of time—Czechia, Ireland and Spain—were selected. Ireland introduced its comprehensive smokefree laws in 2004 and was the first country in the world to do so; Spain introduced its smokefree laws initially in 2008 and strengthened them in 2012; and Czechia introduced its comprehensive laws in 2016 [37,38,39]. These countries reflect a geographic and temporal spread in the EU. Their laws also allow for smoking in special areas in a variety of structures which are outside the main premises.
2. Methods
The study is an open, multi-centre, non-randomised, interventional study model of the acute effects of exposure to SHS in outside smoking areas in 3 EU countries with comprehensive smokefree laws. All 60 patients (Figure 1, consort flow diagram) were assessed in a similar manner, with personal monitoring of particle exposure to PM 2.5 and breathing pattern on a visit, of at least one-hour duration, to an outside area/terrace of a pub. All the measurements reported were acquired with the subjects resting for at least 15 min before visiting the venue and during exposure to SHS in a legal outside smoking area.
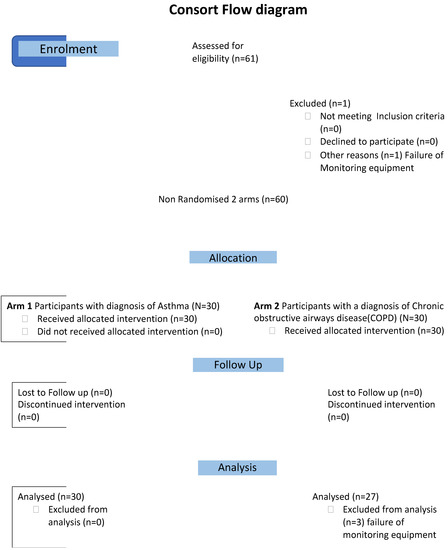
Figure 1.
Consort flow diagram.
2.1. Ethics
Ethical approval was awarded in Ireland by the Dublin Institute of Technology, Research Ethics Committee (approval ref. 13.103); in Spain by Comité de Ética de La Investigación con Medicamentos del Hospital Universitario de la Princesa, Madrid (Nº de registro: 3221); and in Czechia by the Ethics Committee of the Regional Hospital in Liberic (ref no. EK/22/2018) (Supplementary file S1 uploaded).
The study protocol (also included in the Supplementary file S1 uploaded) was registered on the ClinicalTrials.gov (Accessed on 26 May 2023), with identifier NCT03074734.
2.2. Recruitment
Preliminary discussions were held with patient representative groups in Ireland and, following these discussions, it was decided that recruitment through contact with established chest clinics would be more appropriate than a direct approach to patients for safety, ethical and consent considerations.
The study was discussed in each of the three countries, Czechia, Spain and Ireland, with hospital staff, and copies of the full protocol were made available, as well as patient information leaflets and copies of the consent forms.
Consent: informed written consent was obtained from each subject during an interview at a specially arranged visit to the centre, where the study was explained and each patient given written information. The voluntary nature of their consent was stressed and their right to withdraw at any stage was explained.
Criteria for eligibility: fully ambulant; minimum age 18 years; sex, all; doctor-diagnosed COPD patients who were current or ex-smokers, or doctor-diagnosed asthma patients, irrespective of smoking history; and established (at the interview) that it was usual practice for each participating patient to visit outside smoking areas of pubs and bars in their usual social life.
Exclusion criteria: under 18 years of age, on oxygen therapy, pregnant, and currently undergoing treatment for an acute exacerbation of their primary condition.
2.3. Group Assignment
It was explained that this study followed an interventional model with single-group assignment and that there was no randomisation.
2.4. Details of the Intervention
Monitoring devices: AirSpeck monitors employ a light-scattering nephelometer for recording real-time PM2.5 concentration data at 10 s intervals [40]. RESpeck monitors are light-weight—17 g (incl. battery)—unobtrusive devices, which use an encapsulated tri-axial accelerometer to identify the personal mode of the subject when wearing the device, i.e., stationary, lying or mobile, which is then used to derive a reliable measure of activity, of respiratory rate and geolocation [41]. Each pair of sensor readings was communicated wirelessly using Bluetooth connectivity to a smartphone, where it was GPS-stamped for later onward transmission to a secure server dashboard for display and later offline analysis. All the exposure measurements for each of the 5 AirSpeck and 5 RESpeck monitors used were adjusted according to the calibration factor derived in experimental studies in the Edinburgh laboratory and the National Physical Laboratory, Postcode: TW110LW. The data were analysed in consultation with Edinburgh University colleagues.
National research partners in Spain and Czechia were trained by the Irish research team in the use of AirSpeck and RESpeck monitors. The study was carried out sequentially at the three centres, one in each country, over a one-year period, allowing for the same calibrated sensors to be used at each centre.
2.5. Patients, Protocol and Training
The study population consisted of 60 patients (30 asthma and 30 COPD patients) in Czechia (30 patients), Ireland (10 patients) and Spain (20 patients). Each patient visited their local national study centre on two occasions. During the first visit, the study was explained to each participant, both in written (information sheet) and oral communication. They completed a recruitment questionnaire to ascertain personal smoking status, other sources of exposure, average weekly attendance and SHS exposure in hospitality premises and the experience of respiratory symptoms. All consented patients were trained in the use of monitoring equipment.
Diary cards were demonstrated and explained to the patients, and they were asked to fill in details at the first visit: medication consumed, any symptoms (e.g., cough, wheeze), doctor or hospital visits, exposure to SHS and the number of cigarettes smoked (if any).
Diary card entries were also made on the day of the exposure and included a description of the premises visited, number of smokers present during exposure time, as well as any change in their use of medication required during the 24 h period or unscheduled visits to the hospital or doctor.
The participants were also asked to note the time and date when the exposure to SHS occurred in outside areas.
2.6. Venues
At least one visit to an outdoor smoking area was scheduled during a one-hour visit to a premise. An outdoor smoking area was defined as a place or premise, or part of a place or premise that is fully uncovered by any roof, fixed or mobile, or an outdoor place or premise that is covered by a roof, so long as not more than 50% of the perimeter (outside) is covered by a wall, windows, gate or similar.
The study subjects were asked to spend at least 15 min in the outdoor smoking area, a preferable time of 30–60 min, and 15 min at rest was desirable.
2.7. Measurements
The patients wore the personal monitors for 24 h to continuously measure exposure to particulate matter PM2.5, with continuous geolocalisation monitoring (AirSpeck) and a RESpeck monitor to measure the breathing rate (Br), to detect activity and any acute changes in breathing before and during exposure to SHS. To have a standardised period for the measurement of breathing rates, we selected a period of 7 min when the patient was at rest before the exposure to SHS, as defined by the RESpeck measurements, and the PM2.5 was less than 10 µg/m3, and compared it to breathing rates for 7 min at rest, during the exposure and when the PM 2.5 was greater than 10 µg/m3.
At the second study centre visit on the day post-exposure, all data recorded by the devices were downloaded and checked, and any diary card anomalies were addressed and clarified with the patient.
Routine pulmonary function tests consisting of forced expiratory volume in the first second (FEV1), forced vital capacity (FVC) and peak expiratory flow rate (PEFR) were measured at the study centre pre- and post-exposure to SHS within 24 h (and are reported elsewhere) [34].
2.8. Statistical Analysis
Baseline characteristics of the participating patients by their diagnosis were compared using descriptive statistics (mean, standard deviation (SD), median, interquartile range (IQR) and percentages as appropriate). The Student t-test for continuous variables and Chi-square test for categorical variables were used to determine whether there was a difference in the breathing rates among the variables of interest, and a two-tailed p-value, with a less than 0.05 significance threshold, was chosen for all tests. Stata v16 (Stata Corp LP, College Station, TX, USA) was used for the statistical analysis.
3. Results
Table 1 shows the demographic characteristics of the 60 patients. The COPD participants were older (age 63.3 ± 10.2 yrs.) than the asthmatics (46.9 ± 18.7 yrs.), and there were more women (n = 35) than men (n = 25). Of the COPD group, 21 patients (70.0%) were current smokers, as were 8 of the asthmatics (26.7%), while 15 of the 60 (25.0%) were ex-smokers. Sixteen of the asthmatics (53.3%) had never smoked. No patient reported significant changes either of maintenance medication or unscheduled visits to hospital or doctor. The number of smokers in the outdoor areas was usually fewer than five. Mainly, there were three or four walls in the smoking areas, with fewer than 20% having one or two walls. The PM 2.5 levels (Table 2) varied wildly within the smoking areas, mainly depending on the number of walls in the facility and less on the number of smokers.

Table 1.
Demographic and clinical characteristics of 60 patients, number smoking in the facility and walls in the structure (mean ± SD/n (%)).

Table 2.
Exposure levels of PM2.5 µg/m3 during AirSpeck monitoring of 60 patients before outdoor smoking area visit (not in SHS area), and 57 patients during the outdoor smoking area visit (in SHS area).
3.1. Exposure Levels
Table 2 shows the mean and median PM 2.5 (µg/m3) exposure for all subjects during their visits to an outdoor smoking area (SHS exposure) and for the rest of the 24 h period (not in SHS area). While the level of exposure was greater in the SHS areas, many patients also had high exposure during the whole observed periods. It is of note that 29 of the patients were smokers.
Individual venue measurements of PM2.5 were highly variable. One area reached 2500 µg/m3 for a short period during venue exposure and was sustained for 15 min at ≥2000 µg/m3. The PM2.5 levels in 4 premises were ≥500 (1933–539) µg/m3, in 11 premises ≥ 200 (480–203) µg/m3, in 9 premises ≥ 100 (170–108) µg/m3, in 10 premises ≥ 40 (80.5–40.1) µg/m3, in 9 premises ≥ 25(39.2–25.6) µg/m3, in 10 premises ≥ 11 (23.28–11.0) and ≤10 (8.6–5.8) µg/m3 in 3 premises, which had only a single wall.
3.2. Respiratory Responses
The mean breathing rate (Br) (Table 3(a)) tended to be lower during exposure to SHS, ranging from 17.88 to 28.58 in the non-SHS areas at rest, and 16.46 to 27.56 during the SHS areas at rest, but the difference was not statistically significant. The pattern was similar looking at the means and medians, asthma and COPD, men and women, smokers and non-smokers (not shown in table). Exposure to SHS changed the patients’ Br. For some subjects there was a significant increase in Br during exposure to SHS and for others a significant decrease (Table 3(b)).

Table 3.
Mean and median breathing rates (Br) for patients at rest before (PM 2.5 < 10 µg/m3) and during (PM 2.5 ≥ 10 µg/m3) SHS exposure.
Table 4(a) shows the results for the overall population of Br at rest before and during SHS exposure (i.e., not differentiated by whether patients had increased or decreased Br as a result of SHS exposure), according to gender, asthma/COPD, smoking status and duration of exposure. Table 4(b,c) [42] separately examines the increased and decreased Br subgroups, for both asthmatic and COPD patients. Overall, 19 female and 9 male patients had an increased Br, and 14 female and 15 male patients had a decreased Br. In Table 4(b), we see that the Br increased significantly during SHS exposure among 17 asthmatic patients, but the increase did not reach the threshold for statistical significance in males. Among 11 COPD patients, SHS exposure significantly increased the Br for both male and female patients. In Table 4(c), we see that for those whose Br decreased during exposure, there were statistically significant changes for both male and female patients in both asthma and COPD disease subgroups.

Table 4.
(a) Breathing rates/minute (Br) at rest, before and during exposure for the entire population (n = 60). (b) Increased Br during exposure (n = 28) and (c) decreased Br at rest during SHS exposure (n = 29), according to disease and gender.
Table 5 further examines the differences between the subgroups where the Br either increased or decreased. Table 5(a) shows differences in those where the Br either increased or decreased according to population characteristics. A younger age, female gender, lighter body weight, non-smokers, asthma, higher CAT/ACT grade and a shorter duration of exposure were more commonly associated with an increase in Br, whereas older age, male gender, heavier body weight, COPD, lower CAT/ACT grade and a longer time exposure to SHS tended to be associated with a decrease in Br, but these changes were not statistically significant.

Table 5.
(a) Breathing rates/minute (Br), decrease or increase, by gender, age, smoking status, weight and disease diagnosis, CAT/ACT score and the duration time of exposure in 57 patients and (b) changes in breathing rates/minute (Br), decrease or increase, in 19 female asthmatics via spirometry.
We also examined the differences in routine pulmonary function tests between those with a decreased and an increased Br. Routine pulmonary function tests consisting of forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and peak expiratory flow rate (PEFR) were measured at the study centre visit on the day of the pub visit, and repeated at a second study centre visit on the day following the pub visit. There were minor changes measured in lung function, which only showed a statistically significant deterioration in female asthma patients, which are reported elsewhere (34). In this study, only female asthmatics who had a decrease in the FEV1, FVC or PEFR had a statistically significant increase in the Br as a group, and this is shown in Table 5(b).
4. Discussion
This study confirms that exposure to SHS under the present legislation in legal outside areas in three EU countries with comprehensive smokefree laws still results in exposure to very high SHS levels [9,21]. There is no safe level of SHS [43] and chronic exposure to the SHS levels seen in this study has been shown to result in cancer, heart attacks and COPD in those who are chronically exposed [18,44]. Removal from SHS exposure in the short-to-medium term has resulted in improvements, not only in symptoms, but also in improved pulmonary function, even in asymptomatic bar workers whose pulmonary function was within normal limits [7,12,45,46]. Nevertheless, such reports of effects of short-term exposure to SHS on acute pulmonary function are scarce. We argue that any such effects are most likely to be of increased clinical importance to patients with already compromised airflow limitations. In that regard, we opted to measure the effects on breathing in patients with doctor-diagnosed asthma and COPD. We accepted only volunteer patients who had normally attended such venues where exposure to SHS was usual and had not noticed significant ill-effects on many such previous visits to pubs or bars with outdoor areas where customers are allowed to smoke. Many of the COPD patients were still smokers or ex-smokers. Of interest was also that when we approached asthma/COPD patient organisations to discuss participation, most of the members with severe diseases told us that they had abandoned visits to pubs because of SHS exposure and they did not take part in the study.
The changes in breathing rates that we recorded were complex. Nearly half of the patients increased their breathing rates, and an almost equal number decreased their breathing rate at rest in comparison with resting rates during non-exposure, and these changes were statistically significantly different. Responses in younger, lower weight, non-smoking and female patients with asthma were associated with increases in the breathing rate, while older, heavier, smoker and ex-smokers, and male patients with COPD were more likely to decrease the breathing rate. This suggests that there are disease, gender, age, weight, and smoking effects in the responses, but these were directional changes only, which did not reach statistical significance except for female asthmatics who increased rates in line with a reduction in spirometry [34]. This increased response in asthmatics is in line with the increased bronchial responsiveness of asthmatics [47], but it did not happen in all asthmatics and was not significant in males. It is known that the Br is higher in women than in men [48]. It has also been reported [47] that the change in breathing rates leads to the possibility of hypoventilation and hyperventilation, since the low and high breathing rates seen in that study are known to be associated with hypercapnia and hypoxaemia, respectively. However, we have been unable to find any previous studies testing the effect of SHS on breathing rates in patients with asthma or COPD, or in subjects without disease.
Our findings also raise the question of possible alternative mechanisms at work [49,50]. The most obvious perhaps is the different regulation of breathing apart from bronchial responsiveness. We know of the blunting of the chemical drive to breathing in chronic hypoxia regarding the response to carbon dioxide (CO2) [51], but we know much less about the effect of the various chemicals in SHS on the regulation of breathing in different disease states. The chemical content, concentration and dispersion of SHS are likely to be very different in different settings, in different countries [52]. The dose inhaled is likely to vary widely and, if the susceptibility also varies, then this may account for or contribute to the variance in response that we saw in this study. The study was not designed to answer this question and the variation in the patient characteristics and sample size are also unsuitable to shed light on this aspect of the results. However, the main aim of the study was to determine if SHS exposure in legal outside smoking areas was associated with measurable changes in breathing. We believe this is an important question as the rationale for smokefree bars was to protect staff and patrons from harmful exposure to SHS. This has been largely achieved inside pubs in countries with comprehensive bans on smoking, but most legislation envisions an area outside the pub supplied by the owners of the pubs where smoking is allowed. It was anticipated when framing the smokefree legislation that these areas would be such that there was negligible or no exposure to SHS of staff or non-smokers, as they would not visit these areas for any length of time, as commercial activity would not be allowed and smokers would only use them short-term. The reality is different as commercial activity has crept back into these spaces and they are visited by smokers and non-smokers. Now that we know there is no safe level of long-term exposure to SHS, it is especially important to know there are significant respiratory changes due to short-term exposure. This is particularly important for patients such as those who took part in this study, who already have an impaired pulmonary function due to disease.
5. Conclusions
This study in patients with asthma and COPD shows high levels of SHS exposure in outside areas of hospitality premises in the three selected EU countries with comprehensive smokefree laws. This SHS exposure was shown to have acute effects on breathing in these patients. These real-world observations of high SHS exposure and acute breathing changes suggest that all such patients be advised to avoid these areas. These findings and the known long-term adverse effects of SHS should increase the demands for an extension of smokefree laws to outside areas, abandoning designated areas and redefining a smokefree pub as an establishment where smoking is not allowed in any part of its premises.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph20115978/s1, Supplementary file S1.
Author Contributions
Conceptualization, L.C., E.F.; methodology, S.K., L.C., E.F., S.S. (Sean Semple) and O.T.; software, D.K.A.; validation, L.C., E.F. and J.B.S.; formal analysis, S.S. (Salome Sunday), L.C., J.B.S., T.A., S.K. and J.H.; investigation, S.K., T.A., L.C., E.F. and J.B.S.; resources, S.K., L.C., C.R.-L., J.B.S., T.A. and D.K.A.; data curation, S.K., L.C., S.S. (Salome Sunday) and T.A.; writing—original draft preparation, L.C., J.B.S., T.A., S.K., J.H., S.S. (Sean Semple), M.J.L.; S.G., A.T., R.B., G.G. and A.L.-N.; writing—review and editing, L.C. and J.H.; visualization, S.S. (Salome Sunday), T.A. and S.K.; supervision, L.C., J.B.S. and E.F.; project administration, S.K., O.T., E.F., J.H., L.C. and J.B.S.; funding acquisition, E.F. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 681040. The Tobacco Control Research Group at ICO-IDIBELL (OT, EF) is partly supported by the Ministry of Universities and Research, Government of Catalonia (2021SGR00609). The work of SG was partially funded by the Italian League Against Cancer (LILT, Milan). SS was funded by Grant 167 from RCDHT. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved in Ireland by Dublin Institute of Technology, Research Ethics Committee (approval ref 13.103); in Spain by El Comité de Ética de La Investigación con Medicamentos del Hospital Universitario de la Princesa, Madrid, Nº de Registro: 3221; and, in Czechia by the Ethics Committee of the Regional Hospital in Liberic (ref no. EK/22/2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The authors are open to data-sharing of de-identified data.
Acknowledgments
We wish to acknowledge the contribution of Milada Sipkova who was responsible for the administration of the project and data collection in Czechia. We wish to acknowledge the contributions of Maria Patricia Perez in training and data collection in Madrid Spain. We also thank Darius Fischer, University of Edinburgh for initial data management, visualisation and preliminary analysis. We are grateful to Aisling McGowan for her support in patient recruitment and facilitation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wakefield, T.D.; Glantz, S.A. Securing Smokefree Laws Covering Casinos and Bars in Louisiana via Messaging, Continuous Campaigning and Health Coalitions. Int. J. Environ. Res. Public Health 2022, 19, 3936. [Google Scholar] [CrossRef]
- Sendall, M.C.; Fox, L.; Wraith, D. University Staff and Students’ Attitudes towards a Completely Smoke-Free Campus: Shifting Social Norms and Organisational Culture for Health Promotion. Int. J. Environ. Res. Public Health 2021, 18, 7104. [Google Scholar] [CrossRef] [PubMed]
- Campaign for Tobacco-Free Kids. Tobacco Control Laws: Legislation: Campaign for Tobacco-Free Kids, 2021. Available online: https://www.tobaccocontrollaws.org/legislation (accessed on 14 March 2023).
- Hanafin, J.; Clancy, L. History of Tobacco Production and Use. In Tobacco Epidemic, 2nd ed.; Karger International: Basel, Switzerland, 2015; Volume 42, pp. 1–18. [Google Scholar]
- Martínez-Sánchez, J.M.; Fernández, E.; Fu, M.; Gallus, S.; Martínez, C.; Sureda, X.; La Vecchia, C.; Clancy, L. Smoking Behaviour, Involuntary Smoking, Attitudes towards Smoke-Free Legislations, and Tobacco Control Activities in the European Union. PLoS ONE 2010, 5, e13881. [Google Scholar] [CrossRef] [PubMed]
- Sunday, S.; Kabir, Z. Impact of Carers’ Smoking Status on Childhood Obesity in the Growing up in Ireland Cohort Study. Int. J. Environ. Res. Public Health 2019, 16, 2759. [Google Scholar] [CrossRef]
- Goodman, P.; Agnew, M.; McCaffrey, M.; Paul, G.; Clancy, L. Effects of the Irish smoking ban on respiratory health of bar workers and air quality in Dublin pubs. Am. J. Respir. Crit. Care Med. 2007, 175, 840–845. [Google Scholar] [CrossRef]
- Frazer, K.; Callinan, J.E.; Mchugh, J.; van Baarsel, S.; Clarke, A.; Doherty, K.; Kelleher, C. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database Syst. Rev. 2016, 2, CD005992. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, S.O.; Fernández, E.; Driezen, P.; Fu, M.; Tigova, O.; Castellano, Y.; Mons, U.; Herbeć, A.; Kyriakos, C.N.; Demjén, T. Secondhand smoke exposure in European countries with different smoke-free legislation: Findings from the EUREST-PLUS ITC Europe surveys. Nicotine Tob. Res. 2022, 24, 85–92. [Google Scholar] [CrossRef]
- Gao, M.; Li, Y.; Wang, F.; Zhang, S.; Qu, Z.; Wan, X.; Wang, X.; Yang, J.; Tian, D.; Zhang, W. The effect of smoke-free legislation on the mortality rate of acute myocardial infarction: A meta-analysis. BMC Public Health 2019, 19, 1269. [Google Scholar] [CrossRef] [PubMed]
- Semple, S.; Creely, K.S.; Naji, A.; Miller, B.G.; Ayres, J.G. Secondhand smoke levels in Scottish pubs: The effect of smoke-free legislation. Tob. Control 2007, 16, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Stallings-Smith, S.; Zeka, A.; Goodman, P.; Kabir, Z.; Clancy, L. Reductions in Cardiovascular, Cerebrovascular, and Respiratory Mortality Following the National Irish Smoking Ban: Interrupted Time-Series Analysis. PLoS ONE 2013, 8, e62063. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer. Tobacco Smoke and Involuntary Smoking; IARC: Lyon, French, 2004; Volume 83, ISBN 92-832-1283-5. [Google Scholar]
- CDC [Center for Disease Control]. Smokefree Policies Improve Health. Available online: https://www.cdc.gov/tobacco/secondhand-smoke/protection/improve-health.htm (accessed on 14 March 2023).
- Hawkins, S.S.; Hristakeva, S.; Gottlieb, M.; Baum, C.F. Reduction in emergency department visits for children’s asthma, ear infections, and respiratory infections after the introduction of state smoke-free legislation. Prev. Med. 2016, 89, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Reitsma, M.; Kendrick, P.; Ababneh, E.; Abbafati, C.; Abbasi-Kangevari, M.; Abdoli, A.; Abedi, A.; Abhilash, E.; Abila, D.; Aboyans, V.; et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet 2021, 397, 2337–2360. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Report on the Global Tobacco Epidemic 2021: Addressing New and Emerging Products; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Zhai, C.; Hu, D.; Yu, G.; Hu, W.; Zong, Q.; Yan, Z.; Wang, Y.; Wang, L.; Zhang, T.; Sun, H.; et al. Global, regional, and national deaths, disability-adjusted life years, years lived with disability, and years of life lost for the global disease burden attributable to second-hand smoke, 1990–2019: A systematic analysis for the Global Burden of Disease Study. Sci. Total Environ. 2023, 862, 160677. [Google Scholar] [CrossRef] [PubMed]
- Government of Ireland Public Health (Tobacco) Acts 2002 and 2004. Dublin: Stationery Office, 2004. Available online: https://www.irishstatutebook.ie/eli/2004/act/6/enacted/en/html (accessed on 14 March 2023).
- International Agency for Research on Cancer (IARC). Handbooks of Cancer Prevention, Tobacco Control. In Evaluating the Effectiveness of Smoke-Free Policies; IARC: Lyon, French, 2009; Volume 13. [Google Scholar]
- López, M.J.; Fernández, E.; Gorini, G.; Moshammer, H.; Polanska, K.; Clancy, L.; Dautzenberg, B.; Delrieu, A.; Invernizzi, G.; Muñoz, G. Exposure to secondhand smoke in terraces and other outdoor areas of hospitality venues in eight European countries. PLoS ONE 2012, 7, e42130. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Disparities in secondhand smoke exposure—United States, 1988–1994 and 1999–2004. MMWR Morb. Mortal. Wkly. Rep. 2008, 57, 744–747. [Google Scholar]
- Strulovici-Barel, Y.; Omberg, L.; O’Mahony, M.; Gordon, C.; Hollmann, C.; Tilley, A.E.; Salit, J.; Mezey, J.; Harvey, B.G.; Crystal, R.G. Threshold of biologic responses of the small airway epithelium to low levels of tobacco smoke. Am. J. Respir. Crit. Care Med. 2010, 182, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, B. Exposure-response relationship between passive smoking and adult pulmonary function. Am. J. Respir. Crit. Care Med. 1995, 151, 41–46. [Google Scholar] [CrossRef]
- Eisner, M.D.; Wang, Y.; Haight, T.J.; Balmes, J.; Hammond, S.K.; Tager, I.B. Secondhand Smoke Exposure, Pulmonary Function, and Cardiovascular Mortality. Ann. Epidemiol. 2007, 17, 364–373. [Google Scholar] [CrossRef]
- White, J.R.; Froeb, H.F. Small-airways dysfunction in nonsmokers chronically exposed to tobacco smoke. N. Engl. J. Med. 1980, 302, 720–723. [Google Scholar] [CrossRef]
- Masjedi, M.-R.; Kazemi, H.; Johnson, D.C. Effects of passive smoking on the pulmonary function of adults. Thorax 1990, 45, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Kabir, Z.; Manning, P.J.; Holohan, J.; Keogan, S.; Goodman, P.G.; Clancy, L. Second-hand smoke exposure in cars and respiratory health effects in children. Eur. Respir. J. 2009, 34, 629–633. [Google Scholar] [CrossRef]
- Öberg, M.; Jaakkola, M.S.; Woodward, A.; Peruga, A.; Prüss-Ustün, A. Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. Lancet 2011, 377, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Flouris, A.D.; Koutedakis, Y. Immediate and short-term consequences of secondhand smoke exposure on the respiratory system. Curr. Opin. Pulm. Med. 2011, 17, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Hu, K.R.; Jian, Z.; Ai, F.L.; Shi, Y.L.; Wang, X.W.; Yang, W.Y.; Wang, J.X.; Ai, L.M.; Xia, W. The Association between Exposure to Second-Hand Smoke and Disease in the Chinese Population: A Systematic Review and Meta-Analysis. Biomed. Environ. Sci. 2023, 36, 24–37. [Google Scholar]
- Zhao, B.; Bai, L.; Wan, R.; Wang, Y.; Qin, L.; Xiao, Q.; Pan, P.; Hu, C.; Jiang, J. Exposure to second-hand smoke is an independent risk factor of small airway dysfunction in non-smokers with chronic cough: A retrospective case-control study. Front. Public Health 2022, 2308, 912100. [Google Scholar] [CrossRef]
- Keogan, S.; Alonso, T.; Sunday, S.; Tigova, O.; Fernández, E.; López, M.J.; Gallus, S.; Semple, S.; Tzortzi, A.; Boffi, R. Lung function changes in patients with chronic obstructive pulmonary disease (COPD) and asthma exposed to secondhand smoke in outdoor areas. J. Asthma 2021, 58, 1169–1175. [Google Scholar] [CrossRef]
- Cammalleri, V.; Marotta, D.; Protano, C.; Vitali, M.; Villari, P.; Cattaruzza, M.S. Smoke-free department working group how do combustion and non-combustion products used outdoors affect outdoor and indoor particulate matter levels? A field evaluation near the entrance of an Italian university library. Int. J. Environ. Res. Public Health 2020, 17, 5200. [Google Scholar] [CrossRef]
- Kaplan, B.; Carkoglu, A.; Ergor, G.; Hayran, M.; Sureda, X.; Cohen, J.E.; Navas-Acien, A. Evaluation of Secondhand smoke using PM2.5 and observations in a random stratified sample in hospitality venues from 12 cities. Int. J. Environ. Res. Public Health 2019, 16, 1381. [Google Scholar] [CrossRef]
- Currie, L.M.; Clancy, L. The road to smoke-free legislation in Ireland. Addiction 2011, 106, 15–24. [Google Scholar] [CrossRef]
- Kulhánek, A.; Lukavská, K.; Švancarová, I.; Fidesová, H.; Gabrhelík, R. Changes in tobacco use patterns and motivation to quit related to the new smoke-free legislation in the Czech Republic. J. Public Health 2021, 43, 348–354. [Google Scholar] [CrossRef]
- Fernández, E.; Fu, M.; Pérez-Ríos, M.; Schiaffino, A.; Sureda, X.; López, M.J. Changes in secondhand smoke exposure after smoke-free legislation (Spain, 2006–2011). Nicotine Tob. Res. 2017, 19, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Arvind, D.K.; Mann, J.; Bates, A.; Kotsev, K. The AirSpeck Family of Static and Mobile Wireless Air Quality Monitors. In Proceedings of the 19th Euromicro Conference on Digital System Design, Limassol, Cyprus, 31 August–2 September 2016. [Google Scholar]
- Drummond, G.; Bates, A.; Mann, J.; Arvind, D. Validation of a new non-invasive automatic monitor of respiratory rate for postoperative subjects. Br. J. Anaesth. 2011, 107, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Clancy, L.; Keogan, S.; Sunday, S.; Šípková, M.; Sanz, M.T.P.; Soriano, J.B. Changes in breathing during exposure to SHS in outside areas of pubs in patients with asthma and COPD in three EU countries. Tob. Prev. Cessat. 2020, 6, A78. [Google Scholar] [CrossRef]
- Potera, C. Smoking and secondhand smoke. Study finds no level of SHS exposure free of effects. Environ. Health Perspect. 2010, 118, 474. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (US); U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General; Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2006; ISBN 0-16-076152-2. [Google Scholar]
- Eagan, T.M.L.; Hetland, J.; Aarø, L.E. Decline in respiratory symptoms in service workers five months after a public smoking ban. Tob. Control 2006, 15, 242–246. [Google Scholar] [CrossRef]
- Eisner, M.D.; Smith, A.K.; Blanc, P.D. Bartenders’ respiratory health after establishment of smoke-free bars and taverns. J. Am. Med. Assoc. 1998, 280, 1909–1914. [Google Scholar] [CrossRef]
- Stewart, I.C.; Parker, A.; Catterall, J.R.; Douglas, N.J.; Flenley, D.C. Effect of bronchial challenge on breathing patterns and arterial oxygenation in stable asthma. Chest 1989, 95, 65–70. [Google Scholar] [CrossRef]
- Becklake, M.; Kauffmann, F. Gender differences in airway behaviour over the human life span. Thorax 1999, 54, 1119–1138. [Google Scholar] [CrossRef]
- Noble, M.I.M. Abraham Guz memorial: Still unresolved hypotheses: Lung reflexes and perceptions of breathing. Respir. Physiol. Neurobiol. 2015, 217, 46–53. [Google Scholar] [CrossRef]
- Hernandez, L.; Manning, J.; Zhang, S. Voluntary control of breathing affects center of pressure complexity during static standing in healthy older adults. Gait Posture 2019, 68, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Flenley, D.C.; Cooke, N.J.; King, A.J.; Leitch, A.G.; Brash, H.M. The hypoxic drive to breathing during exercise in normal man and in hypoxic patients with chronic bronchitis and emphysema. Bull. Physiopathol. Respir. 1973, 9, 689–693. [Google Scholar] [CrossRef]
- Soleimani, F.; Dobaradaran, S.; De-la-Torre, G.E.; Schmidt, T.C.; Saeedi, R. Content of toxic components of cigarette, cigarette smoke vs cigarette butts: A comprehensive systematic review. Sci. Total Environ. 2022, 813, 152667. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).