Abstract
In youths, two cut-offs (133 and 155 mg/dL) have been proposed to identify high glucose levels at the 1 h (G60) mark during an oral glucose tolerance test (OGTT). We evaluated which cut-off was more closely associated with isolated impaired glucose tolerance (IGT) and cardiometabolic risk (CMR) in 1199 youth with overweight/obesity (OW/OB) and normal fasting glucose and/or HbA1c. The disposition index (DI) was available in 724 youths. The sample was divided by two cut-offs of G60: <133 mg/dL (n = 853) and ≥133 mg/dL (n = 346), or G60 < 155 mg/dL (n = 1050) and ≥155 mg/dL (n = 149). Independent of the cut-off, youths with high levels of G60 showed higher levels of G120, insulin resistance (IR), triglycerides to HDL ratio (TG/HDL), alanine aminotransferase (ALT), and lower insulin sensitivity (IS) and DI than youths with lower levels of G60. The percentage of youths showing IGT, IR, low IS, high TG/HDL ratio, high ALT, and low DI was 50% higher in the G60 ≥ 133 mg/dL group than in the G60 ≥ 155 mg/dL one. In youths with OW/OB and IGT, a cut-off of G60 ≥ 133 mg/dL is more useful than G60 ≥ 155 mg/dL to identify those at high risk of IGT and altered CMR profile.
1. Introduction
In recent decades, with the rising prevalence of obesity (OB) in pediatric age, the incidence of associated cardiometabolic comorbidities has also increased [1]. Glucose dysregulation ranging from prediabetes to overt type 2 diabetes (T2DM) has often been observed in youth with overweight (OW) or OB [2].
Prediabetes is a condition characterized by a concentration of glucose above the normal level but lower than values that identify overt diabetes. It encompasses both impaired glucose tolerance and impaired fasting glucose [3]. A recent meta-analysis reported that obese subjects have a three times higher prevalence of prediabetes than non-diabetic individuals. Additionally, T2DM, prediabetes, and indices of glucose dysmetabolism are positively correlated with body mass index (BMI) in obese children and adolescents [4].
Evidence suggests that prediabetes influences the development of atherosclerotic cardiovascular diseases (CVDs) in adults [5]. On the other hand, young individuals affected by T2DM display an accelerated decline of insulin secretion and are at risk of developing related complications; hence, preemptive risk-based screening to detect asymptomatic subjects is crucial to quickly manage the condition [3].
The oral glucose tolerance test (OGTT) represents the gold standard for the diagnosis of glucose dysregulation so that individuals at high risk can be referred for lifestyle intervention to prevent the progression to T2DM and associated complications.
The diagnosis of prediabetes, according to the American Diabetes Association (ADA), is based on the assessment of fasting glucose (G0), two-hour plasma glucose after OGTT (G120), or glycosylated hemoglobin (HbA1c) [3]. These methods differ in sensitivity and specificity, so they recognize distinct phenotypes with different risks of progression to overt T2DM [3].
Previous data suggest that in 30 to 40% of the T2DM-affected subjects, there is no evidence of any indicator suggesting the presence of the intermediate condition of impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) [6].
Recently, the one-hour post-load plasma glucose (G60) ≥ 155 mg/dL (8.6 mmol/L) has been demonstrated to be highly prognostic for alterations of β-cell function and progression to overt T2DM in adults with normal glucose tolerance (NGT) [7,8]. Furthermore, this threshold seems more predictive of cardiovascular events than fasting or two-hour plasma glucose [9]. From previous studies, it appears that the G60 level may help identify individuals at risk of diabetes better than traditional glucose tests [10,11].
Experts from the European Association for the Study of Diabetes (EASD) [7] have raised a petition to associate G60 levels above 155 mg/dl with altered glucose tolerance in adults on the grounds that G60 is a more time-effective diagnostic marker for prediabetes risk.
The relevance of G60 for identifying individuals at high risk for IGT or abnormal cardiometabolic risk (CMR) profile has also been previously suggested in children with OB using the same cut-off proposed in adults [12,13,14,15]. However, the assessment of the optimal cut-off value of G60 in young people with OB might be a goal to identify, to an acceptable level of accuracy, individuals at risk of developing prediabetes/T2DM and CMR factors.
Indeed, Manco et al. found that the best G60 cut-off associated with IGT corresponded to a value ≥ 133 mg/dL (7.4 mmol/L) in youths with OB. They also demonstrated that this cut-off was closely associated with low insulin sensitivity and altered β-cell function [16]. Subsequently, in a prospective study conducted in a multi-ethnic cohort of youths with OB and NGT, Tricò et al. confirmed that this threshold is associated with a worse clinical and metabolic phenotype, characterized by alterations in insulin sensitivity (IS), β-cell function, and insulin clearance [17]. In addition, they demonstrated that G60 ≥ 133 mg/dL might be an independent predictor of progression to prediabetes. Regarding the association with CMR, Manco et al. [16] demonstrated higher levels of triglycerides in youths with G60 ≥ 133 mg/dL compared to the group < 133 mg/dL, while this finding was not confirmed by Marcovecchio et al. [18].
The aim of this cross-sectional multicenter study was to compare the performance of G60 cut-off ≥133 mg/dL and ≥155 mg/dL to identify IGT, impaired insulin and β-cell function, and altered CMR profile in young people with OW/OB and normal levels of fasting glucose and HbA1c.
2. Materials and Methods
2.1. Study Design, Study Site, and Study Population
This is a cross-sectional study conducted on behalf of the “Childhood Obesity” study group of the Italian Society for Pediatric Endocrinology and Diabetology (ISPED). Nine Italian centers provided data from the clinical records of 1199 non-diabetic children and adolescents (mean age 11.7 years) with OW/OB consecutively observed in the period of June 2016–June 2020, as previously described [19].
For the purpose of this present study, youths with impaired fasting glucose or HbA1c ≥ 5.7%, defined according to the ADA criteria [3], were excluded. Other exclusion criteria were systemic and endocrine diseases and use of medications affecting glucose metabolism.
This study was conducted according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from the parents or tutors of all participants. This study was approved by the Ethics Committee of the AORN Santobono-Pausilipon (reference number 22877/2020).
2.2. Anthropometric, Clinical, and Biochemical Variables
Height and weight were measured for each patient using standard techniques. BMI was calculated as the ratio weight (kg)/height2 (m2) and transformed to age- and sex-specific standard deviation score (SDS) on the basis of reference data from Italian BMI percentiles [20].
Blood pressure (BP) was measured three times 2 min apart, in a sitting position after 5 min of rest, using aneroid sphygmomanometers with appropriately sized cuffs, according to standard procedures. The mean of the last two values was used [21].
Biochemical analyses were performed in the centralized laboratory of each center. All laboratories belong to the Italian National Health System and are certified according to International Standards ISO 9000 (www.iso9000.it/ accessed on 14 November 2022), undergoing semi-annual quality controls and inter-lab comparisons.
After overnight fasting, biochemical markers of lipid metabolism and alanine-aminotransferase (ALT) were analyzed. Triglycerides (TG) to HDL-cholesterol ratio was also calculated (TG/HDL ratio). HbA1c was assessed via high-performance liquid chromatography as elsewhere described [2]. OGTT was performed using 1.75 g/kg of glucose up to a maximum of 75 g [3,22]. Glucose and insulin levels at baseline (G0, I0), one-hour (G60), and two-hour post-load glucose (G120) were analyzed. Data on glucose and insulin at 30′ during OGTT were available in a subsample of 724 youths, as previously described [2].
Insulin resistance (IR) was measured using the homeostasis model assessment (HOMA-IR) index, calculated as insulin (μU/mL) × fasting glucose (mmol/L)/22.5 [23].
The insulin sensitivity (IS) was calculated as 1/ insulin at time 0 (1/I0) [24]. Insulinogenic index (IGI) was calculated as Δ(I0 − I30)/Δ(G0 − G30), where insulin was expressed as µU/mL and glucose as mg/dL. DI was calculated via the following formula: IGI × 1/I0, as described elsewhere [25].
2.3. Definition of Variables
OW and OB were defined on the basis of the finding of BMI ≥ 75th and ≥95th percentile, respectively, in accordance with the Italian BMI standards [18]. IR was defined using the 97th percentile of HOMA-IR distribution by age and gender in normal-weight Italian children [26]. Low IS or low DI was defined by the 25th percentile of, respectively, 1/I0 or DI as calculated in our sample [2]. High TG/HDL ratio was defined by the 75th percentile of TG/HDL ratio distribution in our sample. High ALT levels, as surrogate of nonalcoholic fatty liver disease (NAFLD), were defined using a cut-point > 25.8 IU/L in boys and 22.1 IU/L in girls, corresponding to the 95th percentile level for ALT in a healthy, normal weight pediatric population [27].
2.4. Statistical Analysis
Continuous data were expressed as mean ± standard deviation (SD), numbers and proportions as percentages (%), and 95% confidence interval (CI). Variables with skewed distribution (i.e., HOMA-IR, 1/I0, ALT, and DI) were log-transformed for the analysis and expressed as median and interquartile range. Mean values were compared using Student’s t test. Distribution of categories was compared by χ2, and, when needed, exact tests were performed using the Monte Carlo method.
The relationships between two cut-offs of G60 (133 mg/dL or 155 mg/dL) with IGT, IR, low IS, high TG/HDL ratio, and ALT were analyzed using receiver operator curve (ROC) analysis. The area under curve (AUC) was obtained using IGT or the other metabolic variables as dependent variables and G60 ≥ 133 mg/dL or G60 ≥ 155 mg/dL as variables of interest. Sensitivity and specificity were calculated using 2 × 2 tables.
A p value < 0.05 was considered statistically significant. The statistical analysis was performed using IBM SPSS Statistics, Version 20.0. Armonk, NY, USA.
3. Results
A total of 1199 young people aged 5–18 years were included in this study. Of these, 595 were boys, and 604 were girls. The characteristic of the study population by sex is reported in Table 1. Boys showed slightly higher levels of fasting G0, ALT, and systolic BP and lower levels of IS compared to girls. The prevalence of isolated IGT did not significantly differ between boys and girls (5.4% vs. 7.6%, p = 0.116).

Table 1.
Characteristics of the overall sample and by sex.
In the overall sample, the prevalence of youths with G60 ≥ 133 mg/dL was 28.9% vs. 12.4% in youths with G60 ≥ 155 mg/dL (p < 0.0001). The features of the study sample divided by the two G60 cut-offs are reported in Table 2.

Table 2.
Features of children and adolescents without IFG and high HbA1c according to different cut-offs of G60.
Youths with G60 ≥ 133 mg/dL showed higher levels of G0, G60, G120, HbA1c, HOMA-IR, TG, HDL-C, TG/HDL-C, ALT, and lower IS and DI than the group with G60 < 133 mg/dL. Youths with G60 ≥ 155 mg/dL showed a similar profile to those with G60 < 155 mg/dL, except for the G0.
After removing youths with G60 ≥ 155 mg/dL, 1050 young people were reclassified according to the cut-off of G60 ≥ 133 mg/dL. Their features are reported in Table 3.

Table 3.
Characteristics of youths with G60 <155 mg/dL reclassified by cut-off of 133 mg/dL.
In this subsample, individuals with G60 ≥133 mg/dL showed similar features to that observed in the whole sample in terms of parameters of insulin, β-cell function, and CMR factors.
In the overall sample, the AUC (95%Cl) of G60 as a continuous variable was 0.86 (0.82–0.89) (p < 0.0001) compared to the isolated IGT. The G60 ≥ 133 mg/dL value showed an AUC of 0.76 (0.71–0.82) (p < 0.0001), while the cut-off at G60 ≥ 155 mg/dL showed an AUC of 0.75 (0.68–0.82) (p < 0.0001).
The percentage of youths with IGT, IR, low IS, high TG/HDL ratio, high ALT, and low DI according to the cut-offs of G60 ≥ 133 mg/dL or ≥155 mg/dL was significantly higher than their respective counterparts of G60 < 133 mg/dL or <155 mg/dL (Figure 1).
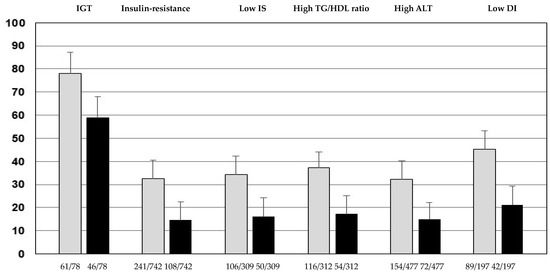
Figure 1.
Percentage of youths with impaired glucose tolerance (IGT), insulin resistance, low insulin sensitivity (IS), high triglycerides to HDL-cholesterol ratio (TG/HDL ratio), high alanine aminotransferase (ALT), and low disposition index (DI) in youths with G60 ≥ 133 mg/dL (grey bars) or G60 ≥ 155 mg/dL (black bars) (p < 0.0001 compared to their respective counterparts < 133 mg/dL or G60 < 155 mg/dL). Youths in the G60 ≥ 133 mg/dL group showed an increase of 33% for IGT, 123% for IR, 104% for low IS, 112% for high TG/HDL ratio, 114% for high ALT, and 103% for low DI, with respect to individuals in the G60 ≥ 155 mg/dL group.
The performance of the two cut-offs at G60 in relation to IGT, metabolic abnormalities, and CMRF is reported in Table 4. For each factor analyzed, the cut-off at G60 ≥ 133 mg/dL showed higher sensitivity and lower specificity with respect to G60 ≥ 155 mg/dL.

Table 4.
Performance of G60 ≥ 133 mg/dL and ≥155 mg/dL in relation to IGT, insulin resistance, low insulin sensitivity, high TG/HDL ratio, high ALT, and low disposition index.
4. Discussion
This study demonstrates that in young people with OW/OB and normal fasting glucose and HbA1c, a cut-off of G60 ≥ 133 mg/dL is more useful than G60 ≥ 155 mg/dL to identify individuals with isolated IGT, β-cell impairment, and altered CMR profile.
Prediabetes in youth has become a more frequent challenge for patients, families, and healthcare professionals. Knowledge of the natural history of prediabetes and T2DM have demonstrated differences between these conditions in juveniles and adults, making extrapolation from adult practice problematic. Furthermore, guidelines for the management and treatment of prediabetes in young people are lacking. The screening of prediabetes or T2DM in childhood is controversial. A recent statement from “The US Preventive Services Task Force” concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening for T2DM in children and adolescents [28]. In contrast, the ADA recommends screening for prediabetes/diabetes in youths with at least one risk factor among obesity, family history of diabetes, gestational diabetes, and other conditions associated with IR [3].
The one-hour plasma glucose during OGTT seems to be a useful early biomarker of glucose dysregulation [29]. A cut-off value of ≥155 mg/dl (8.6 mmol/L) was initially identified in the San Antonio Heart Study [30]. This finding was subsequently confirmed in the Botnia Study that demonstrated a better predictive power of the one-hour post-load plasma glucose for the risk of developing T2DM than fasting and post-load glucose at 120’ fasting glucose [31]. Several longitudinal studies have confirmed that individuals with NGT and one-hour post-load plasma glucose value ≥ 155 mg/dl (≥8.6 mmol/L) are at increased risk for T2DM [32,33,34,35,36].
Although a value of G60 ≥ 155 has been shown to be useful in predicting progression to prediabetes and β-cell deterioration in Latino youths with OB and a family history of T2DM [13], subsequent studies assessed the performance of diverse values of G60. For instance, using ROC analysis, Manco et al. demonstrated that the cut-off of G60 ≥ 133 mg/dL was able to identify IGT with higher sensitivity and specificity than G60 ≥ 155 mg/dL in Caucasian youths with OB [16]. The efficacy of this lower cut-point in predicting the progression to prediabetes was subsequently confirmed in a prospective study in normoglycemic youths with OB [17].
Recently, an international panel of experts supported a petition for redefining the current diagnostic criteria for prediabetes with the use of the elevated one-hour post-load plasma glucose level [7]. Given the paucity of pediatric studies on this issue, it is useful to provide more evidence about the most accurate cut-off of G60 for the screening of prediabetes among youths. Compared to the study by Manco et al., we obtained a similar performance in terms of accuracy to identify IGT using the G60 cut-off ≥ 133 mg/dL (sensitivity 0.81 vs. 0.78 and specificity 0.74 vs. 0.75, respectively) or the G60 ≥ 155 mg/dL (sensitivity 0.45 vs. 0.59 and specificity 0.91 vs. 0.91, respectively).
In our sample, 88.4% of youths showed at least one risk factor for diabetes, i.e., elevated BP, high TG, high ALT, low HDL-cholesterol, or a family history of T2DM. Indeed, according to the consensus position statement of the ISPED (that implemented the ADA guidelines), the presence of several associated cardiovascular risk factors suggests the assessment of OGTT in youths with OB [22].
We extended our comparative analysis regarding the accuracy of the two thresholds to identify altered insulin and β-cell function and abnormal CMR profile. The main finding of our study supported the higher predictive ability of G60 ≥ 133 mg/dL to detect individuals with several metabolic abnormalities and a worse CMR profile. This latter association was demonstrated only for higher levels of triglycerides by Manco et al. [16] but not by Marcovecchio et al. [18]. This discrepancy may be explained by the exclusion of individuals with IFG and IGT by the latter. This finding extends previous studies that confirmed the association between prediabetes, particularly IGT [2] and CMR [37,38]. In our study, the high predictive ability of G60 ≥ 133 mg/dL was confirmed also in the subsample of youths who were reclassified after the removal of individuals with G60 ≥ 155 mg/dL. The strong association between G60 ≥ 133 mg/dL and CMR factors contributes to strengthening the greater usefulness of this cut-off value in children and adolescents compared to that derived from adults.
Although the determinations of fasting glucose and HbA1c are the most frequently used tests for screening of prediabetes/diabetes in the pediatric population [39], the assessment of G60 during the OGTT in children or adolescents might be considered an early biomarker of glucose metabolism impairment compared to the complete OGTT. Another consideration in favor of one-hour post-load glucose is relative to the difficulties in fulfilling the guidelines about sample centrifugation, storage, and transport in ice [40]. Delays in handling and analysis of samples might increase the risk of underestimation of prediabetes/diabetes [41]. Interestingly, the one-hour post-load plasma glucose level, among the high-risk OGTT-glucose phenotypes, represents a good biomarker in response to lifestyle intervention [42].
Concerning the cost-effectiveness of performing 1 h afterload glucose in obese subjects as a screening strategy to identify prediabetes, the data are heterogeneous as the success of a screening program depends on the context (epidemiology, social factors, political priorities, and budget constraints). A recent literature review shows that more cost-effective evaluations of national and regional prevention programs for non-communicable diseases are needed to guide policymakers [43]. We believe that good clinical practice would be to implement screening for young people at risk for prediabetes and lifestyle modification through improved nutrition and exercise. In some cases, pharmacological intervention may even be warranted, but always in the context of lifestyle and behavioral changes.
Limitation and Strength
Limitations of this study include the following: (1) the cross-sectional observational nature that precludes evaluation of the progression over time of children with the G60 ≥ 133 mg/dL; (2) the evaluation of a sample of youths with OW/OB that limits the extendibility of our results to the general population; (3) the derivation of the measures to estimate insulin sensitivity and β-cell function from the OGTT.
The strength of our study includes a large sample of young people without impaired fasting glucose and HbA1c ≥ 5.7% and the evaluation of metabolic and CMR profiles.
5. Conclusions
Our results confirm and extend the findings of previous studies about the use of one-hour post-load glucose in identifying children and adolescents with isolated IGT and metabolic abnormalities. An elevated one-hour post-load plasma glucose level, not measured with current diagnostic standards, may provide an opportunity for the early identification of a large population despite normal levels of fasting glucose and HbA1c. The finding of an elevated one-hour post-load plasma glucose level can allow lifestyle intervention that has the greatest benefit for preserving or reversing β-cell function and preventing further progression to prediabetes and diabetes.
We suggest that the higher accuracy in terms of sensitivity of a lower cut-off compared to that proposed in adults is more appropriate to identify youths at risk of altered glucose metabolism. Further studies are needed to assess the predictive value of the cut-off ≥133 mg/dL on the monitoring of the cardiometabolic health of youths with OB.
Author Contributions
Conceptualization, P.D.B. and G.V.; methodology, P.D.B., M.F.F. and G.V.; software, P.D.B.; formal analysis, P.D.B.; investigation, M.R.L., D.C., M.W., A.D.S., E.M.d.G., A.M., C.M., M.F.F., E.M., V.C., F.F. and G.M.; resources, M.R.L., D.C., M.W., A.D.S., E.M.d.G., A.M., C.M., M.F.F., E.M., V.C., F.F. and G.M.; data curation, P.D.B.; writing—original draft preparation, P.D.B., G.V. and M.F.F.; writing—review and editing, all authors. All authors have read, provided their intellectual contribution, and agreed to the published version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee) of AORN SANTOBONO-PAUSILIPON, Naples, Italy (protocol code 22877/2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Faienza, M.F.; Urbano, F.; Lassandro, G.; Valente, F.; D’Amato, G.; Portincasa, P.; Giordano, P. The Cardiovascular Disease (CVD) Risk Continuum from Prenatal Life to Adulthood: A Literature Review. Int. J. Environ. Res. Public Health 2022, 19, 8282. [Google Scholar] [CrossRef]
- Di Bonito, P.; Licenziati, M.R.; Corica, D.; Wasniewska, M.G.; Di Sessa, A.; Del Giudice, E.M.; Morandi, A.; Maffeis, C.; Faienza, M.F.; Mozzillo, E.; et al. Phenotypes of prediabetes and metabolic risk in Caucasian youths with overweight or obesity. J. Endocrinol. Investig. 2022, 45, 1719–1727. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and diagnosis of diabetes. Sec. 2. In standards of medical Care in Diabetes—2016. Diabetes. Care 2016, 39 (Suppl. S1), S13–S22. [Google Scholar]
- He, Q.X.; Zhao, L.; Tong, J.S.; Liang, X.Y.; Li, R.N.; Zhang, P.; Liang, X.H. The impact of obesity epidemic on type 2 diabetes in children and adolescents: A systematic review and meta-analysis. Prim. Care Diabetes 2022, 16, 736–744. [Google Scholar] [CrossRef]
- Liu, X.; Wu, S.; Song, Q.; Wang, X. Reversion from Pre-Diabetes Mellitus to Normoglycemia and Risk of Cardiovascular Disease and All-Cause Mortality in a Chinese Population: A Prospective Cohort Study. J. Am. Heart Assoc. 2021, 10, e019045. [Google Scholar] [CrossRef] [PubMed]
- Unwin, N.; Shaw, J.; Zimmet, P.; Alberti, K.G. Impaired glucose toleranceand impaired fasting glycaemia: The current status on definition and intervention. Diabet. Med. 2002, 19, 708–723. [Google Scholar] [PubMed]
- Bergman, M.; Manco, M.; Sesti, G.; Dankner, R.; Pareek, M. Petition to replace current OGTT criteria for diagnosing prediabetes with the 1-hour post-load plasma glucose ≥ 155 mg/dL (8.6 mmol/L). Diabetes Res. Clin. Pract. 2018, 146, 18–33. [Google Scholar] [CrossRef]
- Buysschaert, M.; Bergman, M.; Yanogo, D.; Jagannathan, R.; Buysschaert, B.; Preumont, V. An elevated 1-h post-load glucose level during the oral glucose tolerance test detects prediabetes. Diabetes Metab. Syndr. 2017, 11, 137–139. [Google Scholar] [CrossRef]
- Rong, L.; Cheng, X.; Yang, Z.; Gong, Y.; Li, C.; Yan, S.; Sun, B. One-hour plasma glucose as a long-term predictor of cardiovascular events and all-cause mortality in a Chinese older male population without diabetes: A 20-year retrospective and prospective study. Front. Cardiovasc. Med. 2022, 9, 947292. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, R.; Sevick, M.A.; Li, H.; Fink, D.; Dankner, R.; Chetrit, A.; Roth, J.; Bergman, M. Elevated 1-Hour Plasma Glucose Levels are Associated with Dysglycemia, Impaired Beta-Cell Function, and Insulin Sensitivity: A Pilot Study from a Real World Health Care Setting. Endocrine 2016, 52, 172–175. [Google Scholar] [CrossRef]
- Bergman, M. The 1-Hour Plasma Glucose: Common Link Across the Glycemic Spectrum. Front. Endocrinol. 2021, 12, 752329. [Google Scholar] [CrossRef] [PubMed]
- Yeşiltepe Mutlu, G.; Özsu, E.; Çizmecioğlu, F.M.; Hatun, Ş. Can HbA1c and one-hour glucose concentration in standard OGTT be used for evaluation of glucose homeostasis in childhood? J. Clin. Res. Pediatr. Endocrinol. 2013, 5, 80–84. [Google Scholar] [PubMed]
- Kim, J.Y.; Goran, M.I.; Toledo-Corral, C.M.; Weigensberg, M.J.; Choi, M.; Shaibi, G.Q. One-hour glucose during an oral glucose challenge prospectively predicts β-cell deterioration and prediabetes in obese Hispanic youth. Diabetes Care 2013, 36, 1681–1686. [Google Scholar] [CrossRef]
- Serbis, A.; Giapros, V.; Challa, A.; Chaliasos, N.; Siomou, E. Elevated 1-hour post-load plasma glucose identifies obese youth with abnormal glucose metabolism and an unfavourable inflammatory profile. Clin. Endocrinol. 2018, 89, 757–764. [Google Scholar] [CrossRef]
- Kılınç, S.; Demirbaş, T.; Atay, E.; Ceran, Ö.; Atay, Z. Elevated 1-h post-load plasma glucose levels in normal glucose tolerance children with obesity is associated with early carotid atherosclerosis. Obes. Res. Clin. Pract. 2020, 14, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Manco, M.; Del Giudice, E.M.; Spreghini, M.R.; Cappa, M.; Perrone, L.; Brufani, C.; Rustico, C.; Morino, G.; Caprio, S. 1-Hour plasma glucose in obese youth. Acta Diabetol. 2012, 49, 435–443. [Google Scholar] [CrossRef]
- Tricò, D.; Galderisi, A.; Mari, A.; Santoro, N.; Caprio, S. One-hour post-load plasma glucose predicts progression to prediabetes in a multi-ethnic cohort of obese youths. Diabetes Obes. Metab. 2019, 21, 1191–1198. [Google Scholar] [CrossRef]
- Marcovecchio, M.L.; Bagordo, M.; Marisi, E.; de Giorgis, T.; Chiavaroli, V.; Chiarelli, F.; Mohn, A. One-hour post-load plasma glucose levels associated with decreased insulin sensitivity and secretion and early makers of cardiometabolic risk. J. Endocrinol. Investig. 2017, 40, 771–778. [Google Scholar] [CrossRef]
- Di Bonito, P.; Licenziati, M.R.; Corica, D.; Wasniewska, M.; Di Sessa, A.; Del Giudice, E.M.; Morandi, A.; Maffeis, C.; Faienza, M.F.; Mozzillo, E.; et al. Which Is the Most Appropriate Cut-Off of HbA1c for Prediabetes Screening in Caucasian Youths with Overweight or Obesity? Int. J. Environ. Res. Public Health 2023, 20, 928. [Google Scholar] [CrossRef]
- Cacciari, E.; Milani, S.; Balsamo, A.; Spada, E.; Bona, G.; Cavallo, L.; Cerutti, F.; Gargantini, L.; Greggio, N.; Tonini, G.; et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Investig. 2006, 29, 581–593. [Google Scholar] [CrossRef]
- Di Bonito, P.; Valerio, G.; Pacifico, L.; Chiesa, C.; Invitti, C.; Morandi, A.; Licenziati, M.R.; Manco, M.; Giudice, E.M.D.; Baroni, M.G.; et al. Impact of the 2017 Blood Pressure Guidelines by the American Academy of Pediatrics in overweight/obese youth. J. Hypertens. 2019, 37, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Valerio, G.; Maffeis, C.; Saggese, G.; Ambruzzi, M.A.; Balsamo, A.; Bellone, S.; Bergamini, M.; Bernasconi, S.; Bona, G.; Calcaterra, V.; et al. Diagnosis, treatment and prevention of pediatric obesity: Consensus position statement of the Italian Society for Pediatric Endocrinology and Diabetology and the Italian Society of Pediatrics. Ital. J. Pediatr. 2018, 44, 88. [Google Scholar] [CrossRef] [PubMed]
- Cutfield, W.S.; Jefferies, C.A.; Jackson, W.E.; Robinson, E.M.; Hofman, P.L. Evaluation of HOMA and QUICKI as measures of insulin sensitivity in prepubertal children. Pediatr. Diabetes 2003, 4, 119–125. [Google Scholar] [CrossRef] [PubMed]
- George, L.; Bacha, F.; Lee, S.; Tfayli, H.; Andreatta, E.; Arslanian, S. Surrogate estimates of insulin sensitivity in obese youth along the spectrum of glucose tolerance from normal to prediabetes to diabetes. J. Clin. Endocrinol. Metab. 2011, 96, 2136–2145. [Google Scholar] [CrossRef]
- Sjaarda, L.G.; Bacha, F.; Lee, S.; Tfayli, H.; Andreatta, E.; Arslanian, S. Oral disposition index in obese youth from normal to prediabetes to diabetes: Relationship to clamp disposition index. J. Pediatr. 2012, 161, 51–57. [Google Scholar] [CrossRef]
- Shashaj, B.; Luciano, R.; Contoli, B.; Morino, G.S.; Spreghini, M.R.; Rustico, C.; Sforza, R.W.; Dallapiccola, B.; Manco, M. Reference ranges of HOMA-IR in normal-weight and obese young Caucasians. Acta Diabetol. 2016, 53, 251–260. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Dunn, W.; Norman, G.J.; Pardee, P.E.; Middleton, M.S.; Kerkar, N.; Sirlin, C.B. SAFETY study: Alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology 2010, 138, 1357–1364. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davidson, K.W.; Davis, E.M.; Donahue, K.E.; et al. Screening for Prediabetes and Type 2 Diabetes in Children and Adolescents: US Preventive Services Task Force. JAMA 2022, 328, 963–967. [Google Scholar] [CrossRef]
- Fiorentino, T.V.; Marini, M.A.; Succurro, E.; Andreozzi, F.; Perticone, M.; Hribal, M.L.; Sciacqua, A.; Perticone, F.; Sesti, G. One-Hour Postload Hyperglycemia: Implications for Prediction and Prevention of Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 3131–3143. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Abdul-Ghani, T.; Ali, N.; Defronzo, R.A. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care 2008, 31, 1650–1655. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Lyssenko, V.; Tuomi, T.; DeFronzo, R.A.; Groop, L. Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes: Results from the Botnia Study. Diabetes Care 2009, 32, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.; Chetrit, A.; Roth, J.; Jagannathan, R.; Sevick, M.; Dankner, R. One-hour post-load plasma glucose level during the OGTT predicts dysglycemia: Observations from the 24year follow-up of the Israel Study of Glucose Intolerance, Obesity and Hypertension. Diabetes Res. Clin. Pract. 2016, 120, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Priya, M.; Anjana, R.M.; Chiwanga, F.S.; Gokulakrishnan, K.; Deepa, M.; Mohan, V. 1-hour venous plasma glucose and incident prediabetes and diabetes in Asian indians. Diabetes Technol. Ther. 2013, 15, 497–502. [Google Scholar] [CrossRef]
- Fiorentino, T.V.; Marini, M.A.; Andreozzi, F.; Arturi, F.; Succurro, E.; Perticone, M.; Sciacqua, A.; Hribal, M.L.; Perticone, F.; Sesti, G. One-Hour Postload Hyperglycemia Is a Stronger Predictor of Type 2 Diabetes Than Impaired Fasting Glucose. J. Clin. Endocrinol. Metabol. 2015, 100, 3744–3751. [Google Scholar] [CrossRef]
- Pareek, M.; Bhatt, D.L.; Nielsen, M.L.; Jagannathan, R.; Eriksson, K.F.; Nilsson, P.M.; Bergman, M.; Olsen, M.H. Enhanced Predictive Capability of a 1-Hour Oral Glucose Tolerance Test: A Prospective Population-Based Cohort Study. Diabetes Care 2018, 41, 171–177. [Google Scholar] [CrossRef]
- Manco, M.; Mari, A.; Petrie, J.; Mingrone, G.; Balkau, B. One hour post-load plasma glucose and 3 year risk of worsening fasting and 2 hour glucose tolerance in the RISC cohort. Diabetologia 2019, 62, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Andes, L.J.; Cheng, Y.J.; Rolka, D.B.; Gregg, E.W.; Imperatore, G. Prevalence of prediabetes among adolescents and young adults in the United States, 2005–2016. JAMA Pediatr. 2020, 74, e194498. [Google Scholar] [CrossRef]
- Liu, C.; Wu, S.; Pan, X. Clustering of cardio-metabolic risk factors and pre-diabetes among U.S. adolescents. Sci. Rep. 2021, 11, 5015. [Google Scholar] [CrossRef]
- Wallace, A.S.; Wang, D.; Shin, J.I.; Selvin, E. Screening and Diagnosis of Prediabetes and Diabetes in US Children and Adolescents. Pediatrics 2020, 146, e20200265. [Google Scholar] [CrossRef]
- Sacks, D.B.; Arnold, M.; Bakris, G.L.; Bruns, D.E.; Horvath, A.R.; Kirkman, M.S.; Lernmark, A.; Metger, B.E.; Nathan, D.M. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Clin. Chem. 2011, 57, e1–e47. [Google Scholar] [CrossRef]
- Potter, J.M.; Hickman, P.E.; Oakman, C.; Woods, C.; Nolan, C.J. Strict Preanalytical Oral Glucose Tolerance Test Blood Sample Handling Is Essential for Diagnosing Gestational Diabetes Mellitus. Diabetes Care 2020, 43, 1438–1441. [Google Scholar] [CrossRef] [PubMed]
- Peña, A.; Kim, J.Y.; Reyes, J.A.; Vander Wyst, K.B.; Ayers, S.L.; Olson, M.L.; Williams, A.N.; Shaibi, G.Q. Changes in OGTT-derived biomarkers in response to lifestyle intervention among Latino adolescents with obesity. Pediatr. Obes. 2022, 17, e12867. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; John, R.; Afrin, S.; Zhang, X.; Wang, T.; Tian, M.; Sahu, K.S.; Mash, R.; Praveen, D.; Saif-Ur-Rahman, K.M. Cost-Effectiveness of Population Screening Programs for Cardiovascular Diseases and Diabetes in Low- and Middle-Income Countries: A Systematic Review. Front. Public Health. 2022, 10, 820750. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).