Comparative Analysis of Age, Sex, and Viral Load in Outpatients during the Four Waves of SARS-CoV-2 in A Mexican Medium-Sized City
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Swabbing
2.3. qRT-PCR Procedure
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, P.; Ho, P.L.; Hota, S.S. Outbreak of a new coronavirus: What anaesthetists should know. Br. J. Anaesth. 2020, 124, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Siordia, J.A. Epidemiology and clinical features of COVID-19: A review of current literature. J. Clin. Virol. 2020, 10, 3–57. [Google Scholar] [CrossRef] [PubMed]
- Tsatsakis, A.; Calina, D.; Falzone, L.; Petrakis, D.; Mitrut, R.; Siokas, V.; Pennisi, M.; Lanza, G.; Libra, M.; Docea, A.O.; et al. SARS-CoV-2 pathophysiology and its clinical implications: An integrative overview of the pharmacotherapeutic management of COVID-19. Food Chem. Toxicol. 2020, 146, 111769. [Google Scholar] [CrossRef] [PubMed]
- Dixon, B.E.; Wools-Kaloustian, K.; Fadel, W.F.; Duszynski, T.J.; Yiannoutsos, C.; Halverson, P.K.; Menachemi, N. Symptoms and symptom clusters associated with SARS-CoV-2 infection in community-based populations: Results from a statewide epidemiological study. PLoS ONE 2021, 16, e0241875. [Google Scholar] [CrossRef] [PubMed]
- Girón-Pérez, D.A.; Benitez-Trinidad, A.B.; Ruiz-Manzano, R.A.; Toledo-Ibarra, G.A.; Ventura-Ramón, G.H.; Covantes-Rosales, C.E.; Ojeda-Durán, A.J.; Díaz-Reséndiz, K.; Mercado-Salgado, U.; Girón-Pérez, M.I. Correlation of hematological parameters and cycle threshold in ambulatory patients with SARS-CoV-2 infection. Int. J. Lab. Hematol. 2021, 43, 873–880. [Google Scholar] [CrossRef]
- Ranjan, R.; Sharma, A.; Verma, M.K. Characterization of the Second Wave of COVID-19 in India. MedRxiv 2021, 1–16. [Google Scholar] [CrossRef]
- Canals, M.; Cuadrado, C.; Canals, A.; Yohannessen, K.; Lefio, L.A.; Bertoglia, M.P.; Eguiguren, P.; Siches, I.; Iglesias, V.; Arteaga, O. Epidemic trends, public health response and health system capacity: The Chilean experience in four months of the COVID-19 pandemic. Rev. Panam. Salud Publica. 2020, 44, e99. [Google Scholar] [CrossRef]
- Seligmann, H.; Vuillerme, N.; Demongeot, J. Summer. COVID-19 third wave: Faster high altitude spread suggests high UV adaptation. MedRxiv. 2020, preprint. [Google Scholar] [CrossRef]
- Chongchitpaisan, W.; Bandhukul, A.; Prasan, S.; Omas, T.; Kornworanun, P. High Risk Bubble, Bubble and Seal program can help continue business of hospital in Fourth wave or COVID-19 pandemic in Thailand. Saf. Health Work. 2022, 13, S209–S210. [Google Scholar] [CrossRef]
- Popescu, M.; Ştefan, O.M.; Ştefan, M.; Văleanu, L.; Tomescu, D. ICU-Associated Costs during the Fourth Wave of the COVID-19 Pandemic in a Tertiary Hospital in a Low-Vaccinated Eastern European Country. Int. J. Environ. Res. Public Health 2022, 19, 1781. [Google Scholar] [CrossRef]
- Johns Hopkins University & Medicine. COVID-19 Dashboard. Coronavirus Resource Center [Johns Hopkins University website]. Available online: https://coronavirus.jhu.edu/map.html (accessed on 4 April 2022).
- Ioannidis, J.P.A.; Axfors, C.; Contopoulos-Ioannidis, D.G. Population-Level COVID-19 Mortality Risk for Non-Elderly Individuals Overall and for Non-Elderly Individuals without Underlying Diseases in Pandemic Epicenters. Environ. Res. 2020, 188, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Smith, B.T.; Warren, C.; Shahidi, F.V.; Buchan, S.; Mustard, C. The prevalence and correlates of workplace infection control practices in Canada between July and September 2020. Health Rep. 2021, 32, 16–27. [Google Scholar] [PubMed]
- Danielsen, A.C.; Lee, K.M.; Boulicault, M.; Rushovich, T.; Gompers, A.; Tarrant, A.; Reiches, M.; Shattuck-Heidorn, H.; Miratrix, L.W.; Richardson, S.S. Sex disparities in COVID-19 outcomes in the United States: Quantifying and contextualizing variation. Soc Sci Med. 2022, 294, 114716. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Olsson, P.E. Sex differences in severity and mortality from COVID-19: Are males more vulnerable? Biol. Sex Differ. 2020, 11, 53. [Google Scholar] [CrossRef]
- Biswas, M.; Rahaman, S.; Biswas, T.K.; Haque, Z.; Ibrahim, B. Association of sex, age, and comorbidities with mortality in COVID-19 patients: A systematic review and meta-analysis. Intervirology 2021, 64, 36–47. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, L.M.; Wan, L.; Xiang, T.X.; Le, A.; Liu, J.M.; Peiris, M.; Poon, L.; Zhang, W. Viral dynamics in mild and severe cases of COVID-19. Lancet. Infect. Dis. 2020, 20, 656–657. [Google Scholar] [CrossRef] [Green Version]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro. Surveill. 2020, 25, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.R.; Kalikiri, M.; Mirza, F.; Sundararaju, S.; Sharma, A.; Xaba, T.; Lorenz, S.; Chemaitelly, H.; El-Kahlout, R.A.; Tsui, K.M.; et al. Real-time SARS-CoV-2 genotyping by high-throughput multiplex PCR reveals the epidemiology of the variants of concern in Qatar. Int. J. Infect. Dis. 2021, 112, 52–54. [Google Scholar] [CrossRef]
- Taboada, M.; González, M.; Alvarez, A.; Eiras, M.; Costa, J.; Álvarez, J.; Seoane-Pillado, T. First, second and third wave of COVID-19. What have we changed in the ICU management of these patients? J. Infect. 2021, 82, 14–15. [Google Scholar] [CrossRef]
- Gutierrez, E.; Rubli, A.; Tavares, T. Information and behavioral responses during a pandemic: Evidence from delays in COVID-19 death reports. J. Dev. Econ. 2020, 154, 102774. [Google Scholar] [CrossRef]
- Peci, A.; González, C.I.; Dussauge-Laguna, M.I. Presidential policy narratives and the (mis) use of scientific expertise: COVID-19 policy responses in Brazil, Colombia, and Mexico. Policy Stud. 2022, 1–22. [Google Scholar] [CrossRef]
- Galindo-Pérez, M.C.; Suárez, M.; Rosales-Tapia, A.R.; Sifuentes-Osornio, J.; Angulo-Guerrero, O.; Benítez-Pérez, H.; de Anda-Jauregui, G.; Díaz-de-León-Santiago, J.L.; Hernández-Lemus, E.; Alonso Herrera, L.; et al. Territorial Strategy of Medical Units for Addressing the First Wave of the COVID-19 Pandemic in the Metropolitan Area of Mexico City: Analysis of Mobility, Accessibility and Marginalization. Int. J. Environ. Res. Public Health 2022, 19, 665. [Google Scholar] [CrossRef] [PubMed]
- Páez-Osuna, F.; Valencia-Castañeda, G.; Rebolledo, U.A. The link between COVID-19 mortality and PM2. 5 emissions in rural and medium-size municipalities considering population density, dust events, and wind speed. Chemosphere 2022, 286, 131634. [Google Scholar] [CrossRef] [PubMed]
- Suárez, V.; Quezada, M.S.; Ruiz, S.O.; De Jesús, E.R. Epidemiología de COVID-19 en México: Del 27 de febrero al 30 de abril de 2020. Rev. Clin. Esp. 2020, 220, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Masters, N.B.; Shih, S.F.; Bukoff, A.; Akel, K.B.; Kobayashi, L.C.; Miller, A.L.; Harapan, H.; Lu, Y.; Wagner, A.L. Social distancing in response to the novel coronavirus (COVID-19) in the United States. PLoS ONE. 2020, 15, e0239025. [Google Scholar] [CrossRef] [PubMed]
- Aleta, A.; Moreno, Y. Age differential analysis of COVID-19 second wave in Europe reveals highest incidence among young adults. MedRxiv 2020, preprint. [Google Scholar] [CrossRef]
- Avadhanula, V.; Nicholson, E.G.; Ferlic-Stark, L.; Piedra, F.A.; Blunck, B.N.; Fragoso, S.; Bond, N.L.; Santarcangelo, P.L.; Ye, X.; McBride, T.J.; et al. Viral Load of Severe Acute Respiratory Syndrome Coronavirus 2 in Adults During the First and Second Wave of Coronavirus Disease 2019 Pandemic in Houston, Texas: The Potential of the Superspreader. J. Infect. Dis. 2021, 223, 1528–1537. [Google Scholar] [CrossRef]
- Pivonello, R.; Auriemma, R.S.; Pivonello, C.; Isidori, A.M.; Corona, G.; Colao, A.; Millar, R.P. Sex Disparities in COVID-19 Severity and Outcome: Are Men Weaker or Women Stronger? Neuroendocrinology 2021, 111, 1066–1085. [Google Scholar] [CrossRef]
- Mahallawi, W.H.; Alsamiri, A.D.; Dabbour, A.F.; Alsaeedi, H.; Al-Zalabani, A.H. Association of Viral Load in SARS-CoV-2 Patients With Age and Gender. Front. Med. 2021, 8, 39. [Google Scholar] [CrossRef]
- Ejima, K.; Kim, K.S.; Ludema, C.; Bento, A.I.; Iwanami, S.; Fujita, Y.; Ohashi, H.; Koizumi, Y.; Watashi, K.; Aihara, K.; et al. Estimation of the incubation period of COVID-19 using viral load data. Epidemics 2021, 35, 100454. [Google Scholar] [CrossRef]
- Zhao, W.; He, L.; Tang, H.; Xie, X.; Tang, L.; Liu, J. The relationship between chest imaging findings and the viral load of COVID-19. Front. Med. 2020, 7, 598. [Google Scholar] [CrossRef] [PubMed]
- Pujadas, E.; Chaudhry, F.; McBride, R.; Richter, F.; Zhao, S.; Wajnberg, A.; Nadkarni, G.; Glicksberg, B.S.; Houldsworth, J.; Cordon-Cardo, C. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet. Respir. Medicine 2020, 8, e70. [Google Scholar] [CrossRef]
- Tsukagoshi, H.; Shinoda, D.; Saito, M.; Okayama, K.; Sada, M.; Kimura, H.; Saruki, N. Relationships between Viral Load and the Clinical Course of COVID-19. Viruses 2021, 13, 304. [Google Scholar] [CrossRef]
- Kawasuji, H.; Morinaga, Y.; Tani, H.; Yoshida, Y.; Taekgoshi, Y.; Kaneda, M.; Murai, Y.; Kimoto, K.; Ueno, A.; Miyajima, Y.; et al. SARS-CoV-2 RNAemia with Higher Nasopharyngeal Viral Load Is Strongly Associated with Severity and Mortality in Patients with COVID-19. J. Med. Virol. 2020, 94, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Douaud, G.; Lee, S.; Alfaro-Almagro, F.; Arthofer, C.; Wang, C.; McCarthy, P.; Lange, F.; Andersson, J.L.R.; Griffanti, L.; Duff, E.; et al. SARS-CoV-2 Is Associated with Changes in Brain Structure in UK Biobank. Nature 2022, 604, 697–707. [Google Scholar] [CrossRef]
- Consorcio Mexicano de Vigilancia Genómica (CoViGen-Mex) 2022. Available online: http://mexcov2.ibt.unam.mx:8080/COVID-TRACKER/ (accessed on 16 March 2022).
- Ito, K.; Piantham, C.; Nishiura, H. Relative instantaneous reproduction number of Omicron SARS-CoV-2 variant with respect to the Delta variant in Denmark. J. Med. Virol. 2022, 94, 2265–2268. [Google Scholar] [CrossRef] [PubMed]
- Marc, A.; Kerioui, M.; Blanquart, F.; Bertrand, J.; Mitjà, O.; Corbacho-Monné, M.; Marks, M.; Guedj, J. Quantifying the relationship between SARS-CoV-2 viral load and infectiousness. Elife 2021, 10, e69302. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.A.; Kissler, S.M.; Fauver, J.R.; Mack, C.; Tai, C.G.; Samant, R.M.; Connelly, S.; Anderson, D.J.; Khullar, G.; MacKay, M.; et al. Viral Dynamics and Duration of PCR Positivity of the SARS-CoV-2 Omicron Variant. MedRxiv 2022, preprint. [Google Scholar] [CrossRef]
- Márquez, S.; Prado-Vivar, B.; José Guadalupe, J.; Becerra-Wong, M.; Gutierrez, B.; Clinical COVID-19 Ecuador Consortium; Fernández-Cadena, J.C.; Andrade-Molina, D.; Morey-Leon, G. SARS-CoV-2 Genome Sequencing from COVID-19 in Ecuadorian Patients: A Whole Country Analysis. MedRxiv 2021. [Google Scholar] [CrossRef]
- Mora, E.L.; Espinoza, J.; Dabanch, J.; Cruz, R. Emergencia de variante Delta-B. 1.617. 2. Su impacto potencial en la evolución de la pandemia por SARS-CoV-2. Bol. Micol. 2021, 361, 12–16. [Google Scholar] [CrossRef]
- Mlcochova, P.; Kemp, S.A.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I.; Datir, R.; Collier, D.A.; Albecka, A.; Singh, S.; et al. SARS-CoV-2 B. 1.617. 2 Delta variant replication and immune evasion. Nature 2021, 599, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.H.; Morris, C.P.; Sachithanandham, J.; Amadi, A.; Gaston, D.; Li, M.; Swanson, N.J.; Schwartz, M.; Klein, E.Y.; Pekosz, A.; et al. Infection with the SARS-CoV-2 Delta Variant is Associated with Higher Infectious Virus Loads Compared to the Alpha Variant in both Unvaccinated and Vaccinated Individuals. MedRxiv 2021, preprint. [Google Scholar] [CrossRef]
- Wang, C.; Han, J. Will the COVID-19 pandemic end with the Delta and Omicron variants? Environ. Chem. Lett. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Riediker, M.; Briceno-Ayala, L.; Ichihara, G.; Albani, D.; Poffet, D.; Tsai, D.H.; Iff, S.; Monn, C. Higher viral load and infectivity increase risk of aerosol transmission for Delta and Omicron variants of SARS-CoV-2. Swiss Med. Wkly. 2022, 152, w30133. [Google Scholar] [CrossRef] [PubMed]
- Levine-Tiefenbrun, M.; Yelin, I.; Katz, R.; Herzel, E.; Golan, Z.; Schreiber, L.; Wolf, T.; Nadler, V.; Ben-Tov, A.; Kuint, J.; et al. Initial Report of Decreased SARS-CoV-2 Viral Load after Inoculation with the BNT162b2 Vaccine. Nat. Med. 2021, 27, 790–792. [Google Scholar] [CrossRef] [PubMed]
- Matrajt, L.; Eaton, J.; Leung, T.; Brown, E.R. Vaccine Optimization for COVID-19: Who to Vaccinate First? Sci. Adv. 2021, 7, eabf1374. [Google Scholar] [CrossRef] [PubMed]
- Petter, E.; Mor, O.; Zuckerman, N.; Oz-Levi, D.; Younger, A.; Aran, D.; Erlich, Y. Initial real world evidence for lower viral load of individuals who have been vaccinated by BNT162b2. MedRxiv 2021, preprint. [Google Scholar] [CrossRef]
- Christensen, P.A.; Olsen, R.J.; Long, S.W.; Subedi, S.; Davis, J.J.; Hodjat, P.; Walley, D.R.; Kinskey, J.C.; Ojeda-Saavedra, M.; Pruitt, L.; et al. Delta variants of SARS-CoV-2 cause significantly increased vaccine breakthrough COVID-19 cases in Houston, Texas. Am. J. Pathol. 2022, 192, 320–331. [Google Scholar] [CrossRef]
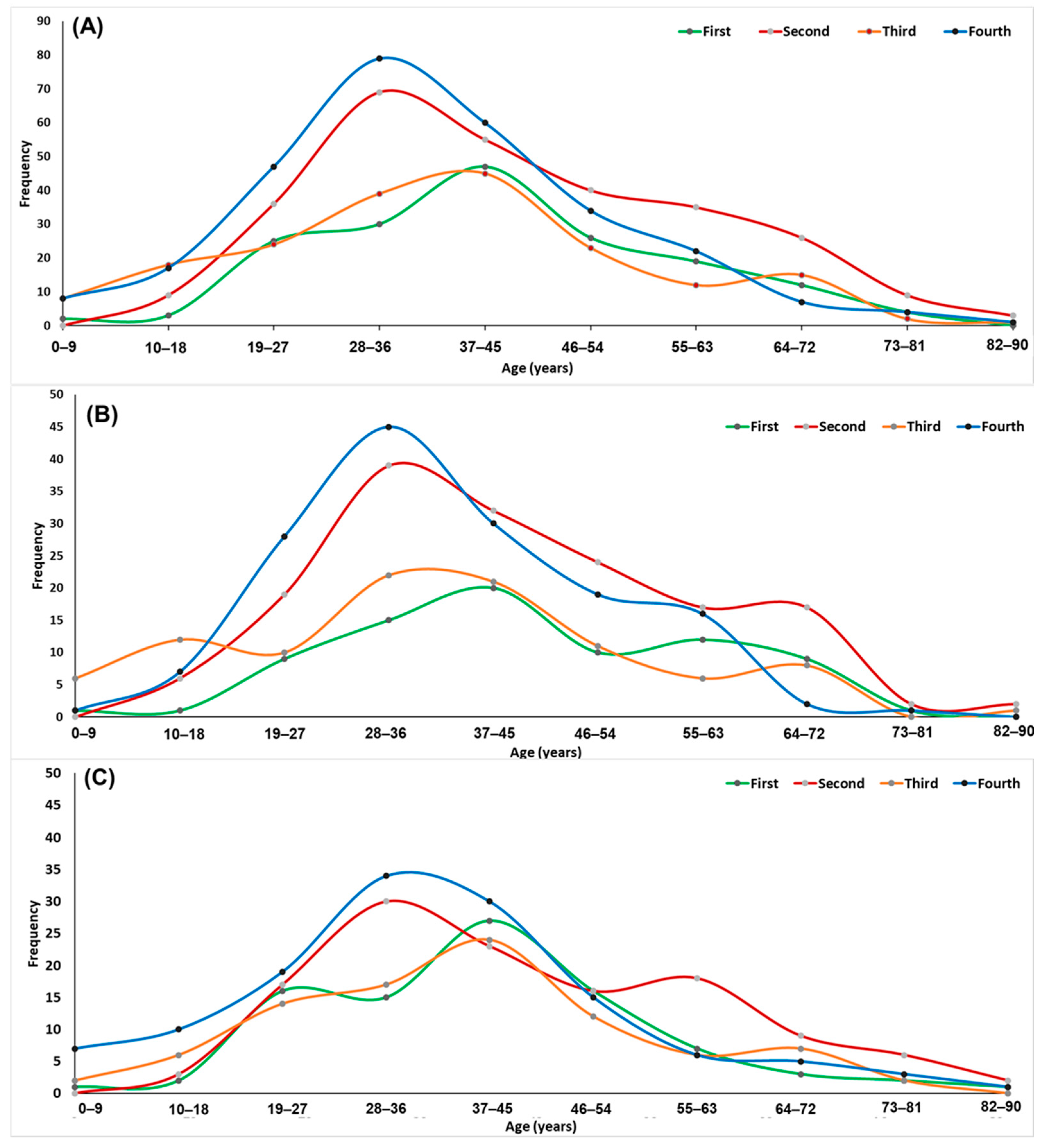
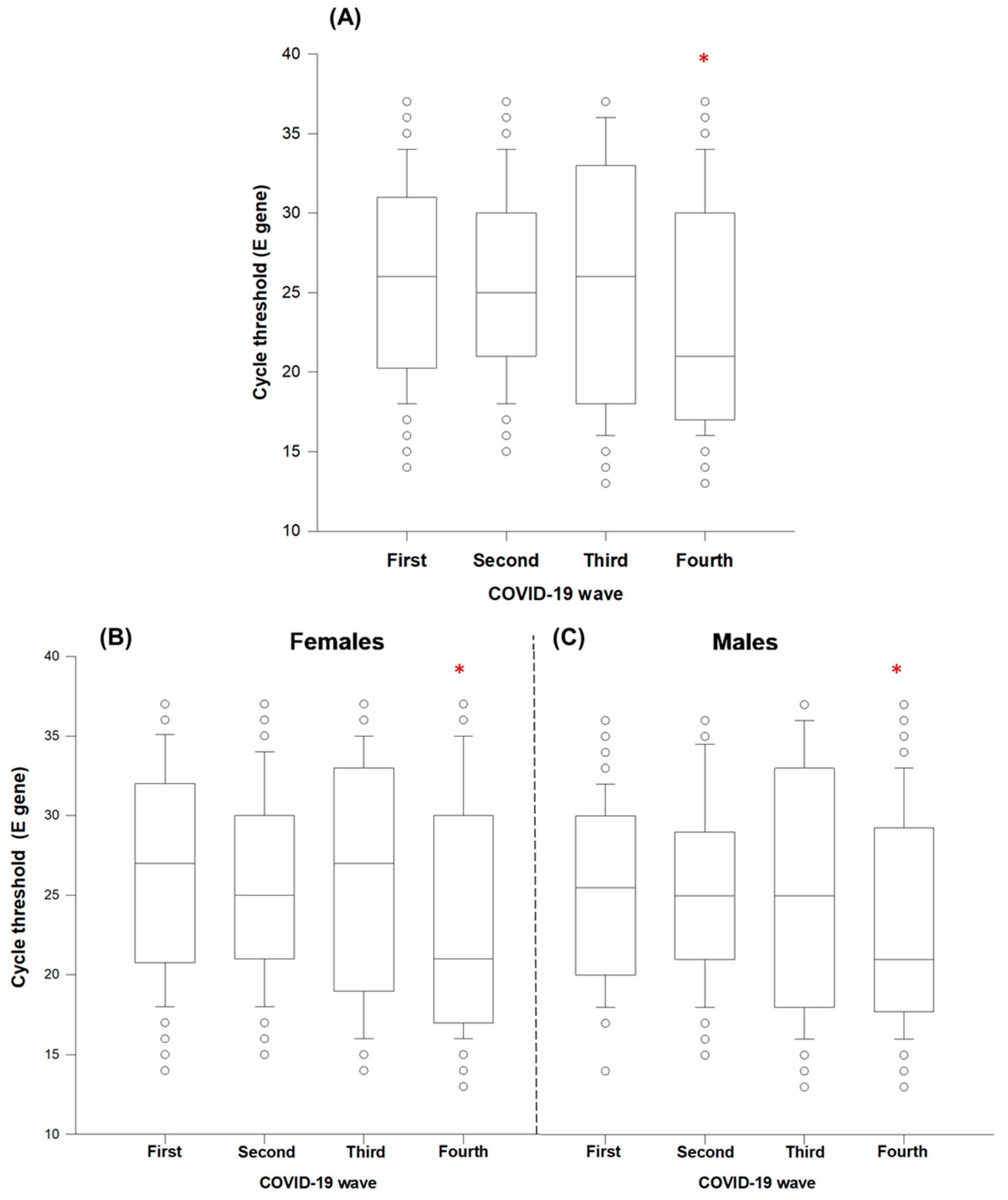
| COVID-19 Wave | |||||
|---|---|---|---|---|---|
| First | Second | Third | Fourth | ||
| Females | N | 78 | 158 | 97 | 149 |
| Age | |||||
| (Mean ± SD) | 44 ± 15 a | 43 ± 16 a | 36 ± 17 b | 40 ± 14 ab | |
| Range (year) | (7–75) | (10–87) | (2–87) | (6–82) | |
| Males | N | 90 | 124 | 90 | 130 |
| Age | |||||
| (Mean ± SD) | 41 ± 15 a | 44 ± 16 a | 39 ± 16 a | 39 ± 18 a | |
| Range (year) | (4–86) | (15–83) | (2–79) | (5–93) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Covantes-Rosales, C.E.; Barajas-Carrillo, V.W.; Girón-Pérez, D.A.; Toledo-Ibarra, G.A.; Díaz-Reséndiz, K.J.G.; Navidad-Murrieta, M.S.; Ventura-Ramón, G.H.; Pulido-Muñoz, M.E.; Mercado-Salgado, U.; Ojeda-Durán, A.J.; et al. Comparative Analysis of Age, Sex, and Viral Load in Outpatients during the Four Waves of SARS-CoV-2 in A Mexican Medium-Sized City. Int. J. Environ. Res. Public Health 2022, 19, 5719. https://doi.org/10.3390/ijerph19095719
Covantes-Rosales CE, Barajas-Carrillo VW, Girón-Pérez DA, Toledo-Ibarra GA, Díaz-Reséndiz KJG, Navidad-Murrieta MS, Ventura-Ramón GH, Pulido-Muñoz ME, Mercado-Salgado U, Ojeda-Durán AJ, et al. Comparative Analysis of Age, Sex, and Viral Load in Outpatients during the Four Waves of SARS-CoV-2 in A Mexican Medium-Sized City. International Journal of Environmental Research and Public Health. 2022; 19(9):5719. https://doi.org/10.3390/ijerph19095719
Chicago/Turabian StyleCovantes-Rosales, Carlos Eduardo, Victor Wagner Barajas-Carrillo, Daniel Alberto Girón-Pérez, Gladys Alejandra Toledo-Ibarra, Karina Janice Guadalupe Díaz-Reséndiz, Migdalia Sarahy Navidad-Murrieta, Guadalupe Herminia Ventura-Ramón, Mirtha Elena Pulido-Muñoz, Ulises Mercado-Salgado, Ansonny Jhovanny Ojeda-Durán, and et al. 2022. "Comparative Analysis of Age, Sex, and Viral Load in Outpatients during the Four Waves of SARS-CoV-2 in A Mexican Medium-Sized City" International Journal of Environmental Research and Public Health 19, no. 9: 5719. https://doi.org/10.3390/ijerph19095719
APA StyleCovantes-Rosales, C. E., Barajas-Carrillo, V. W., Girón-Pérez, D. A., Toledo-Ibarra, G. A., Díaz-Reséndiz, K. J. G., Navidad-Murrieta, M. S., Ventura-Ramón, G. H., Pulido-Muñoz, M. E., Mercado-Salgado, U., Ojeda-Durán, A. J., Argüero-Fonseca, A., & Girón-Pérez, M. I. (2022). Comparative Analysis of Age, Sex, and Viral Load in Outpatients during the Four Waves of SARS-CoV-2 in A Mexican Medium-Sized City. International Journal of Environmental Research and Public Health, 19(9), 5719. https://doi.org/10.3390/ijerph19095719









