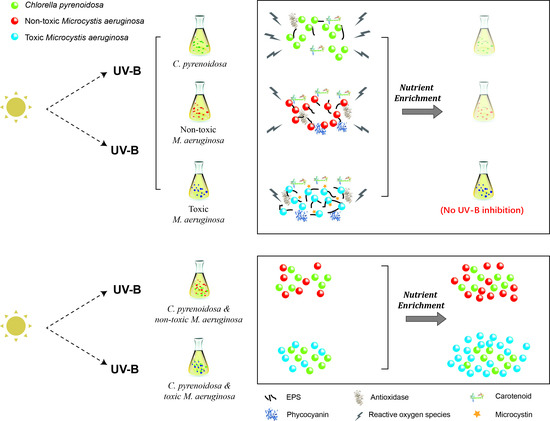
Comparative Study of Algal Responses and Adaptation Capability to Ultraviolet Radiation with Different Nutrient Regimes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Culture
2.2. Experimental Setup
2.3. Analytical Methods of Parameters
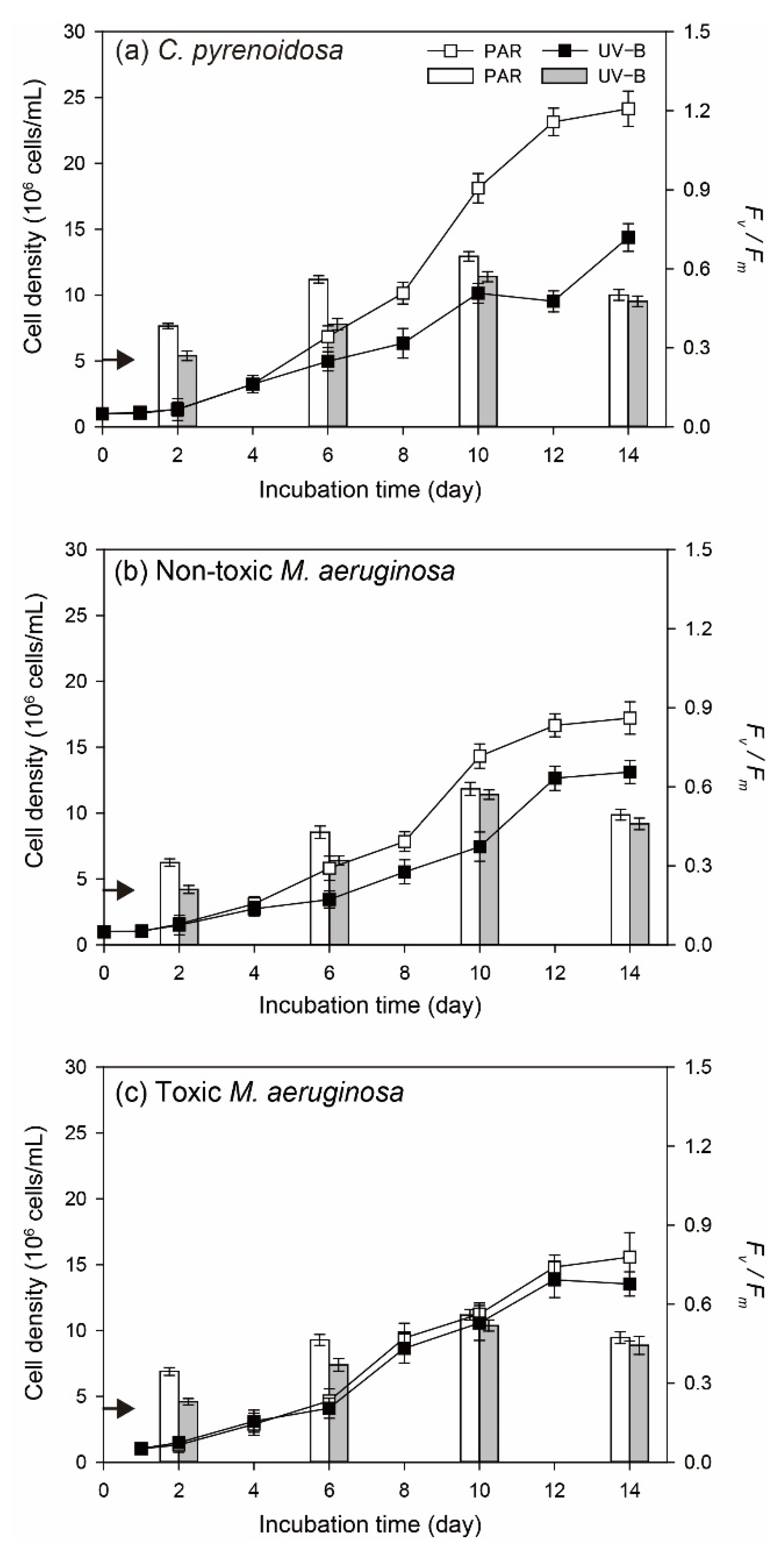
2.3.1. Cell Density and Photosynthetic Efficiency
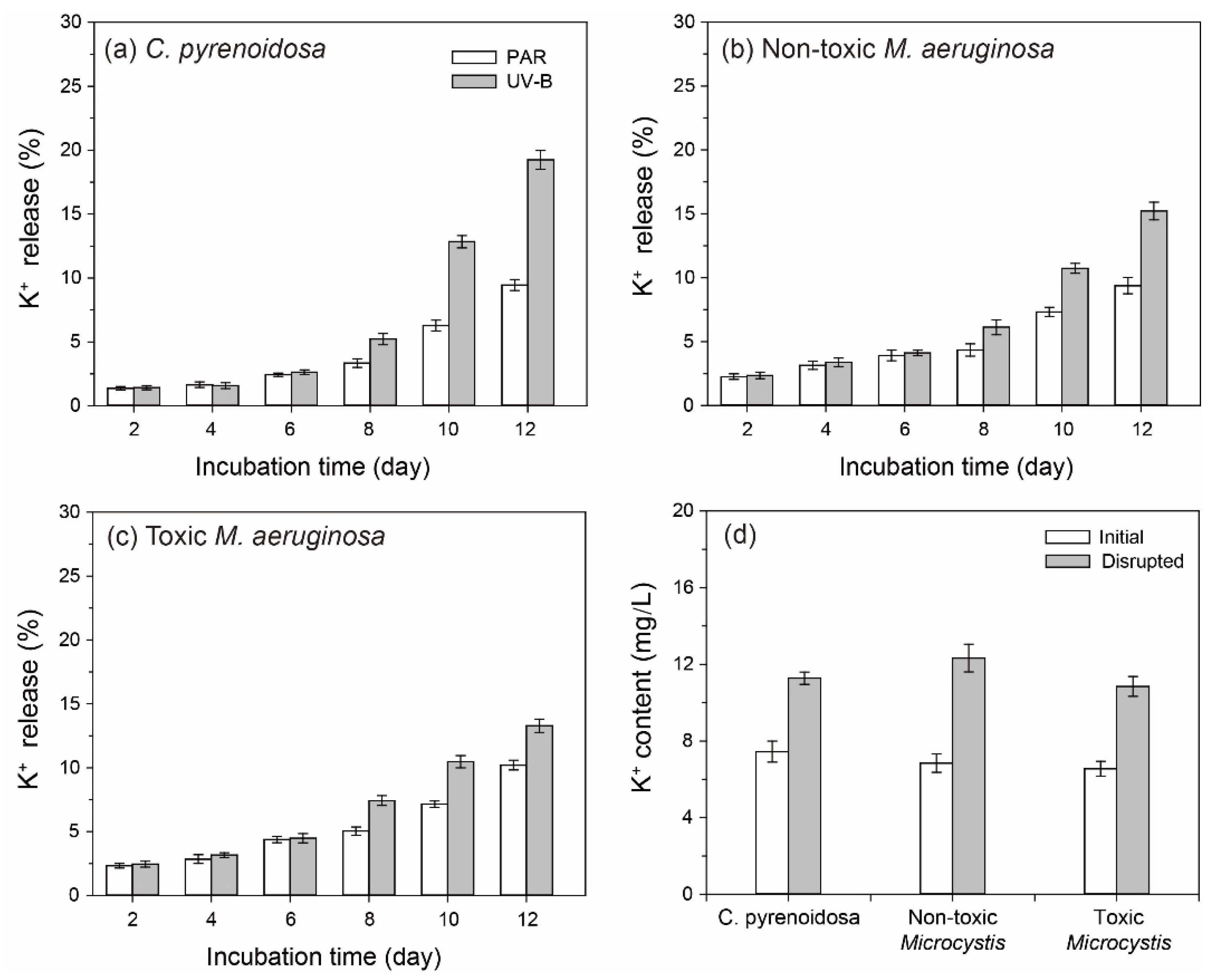
2.3.2. Release of K+ by Algal Cells
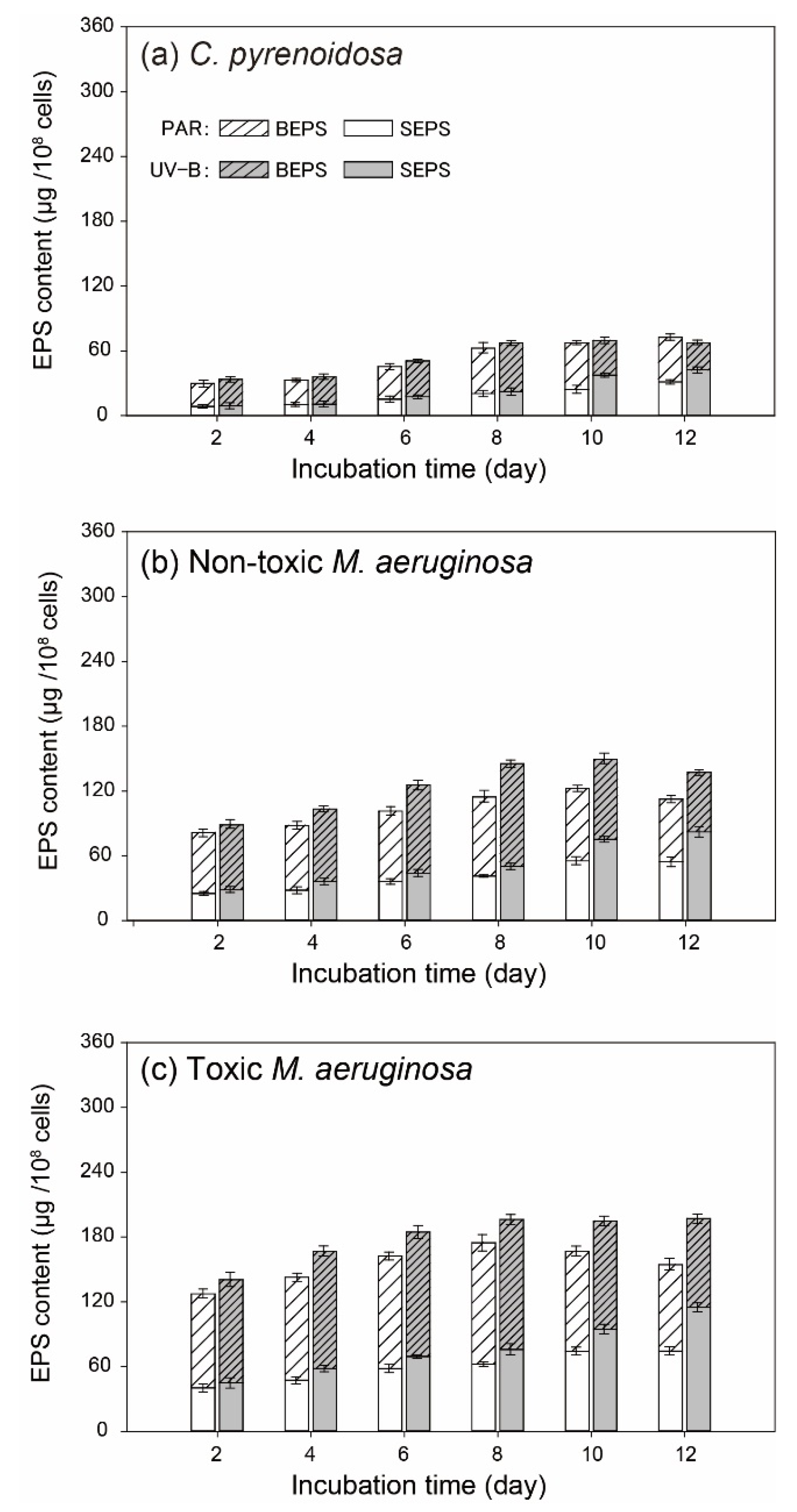
2.3.3. Characterization of Extracellular Polymeric Substance (EPS)
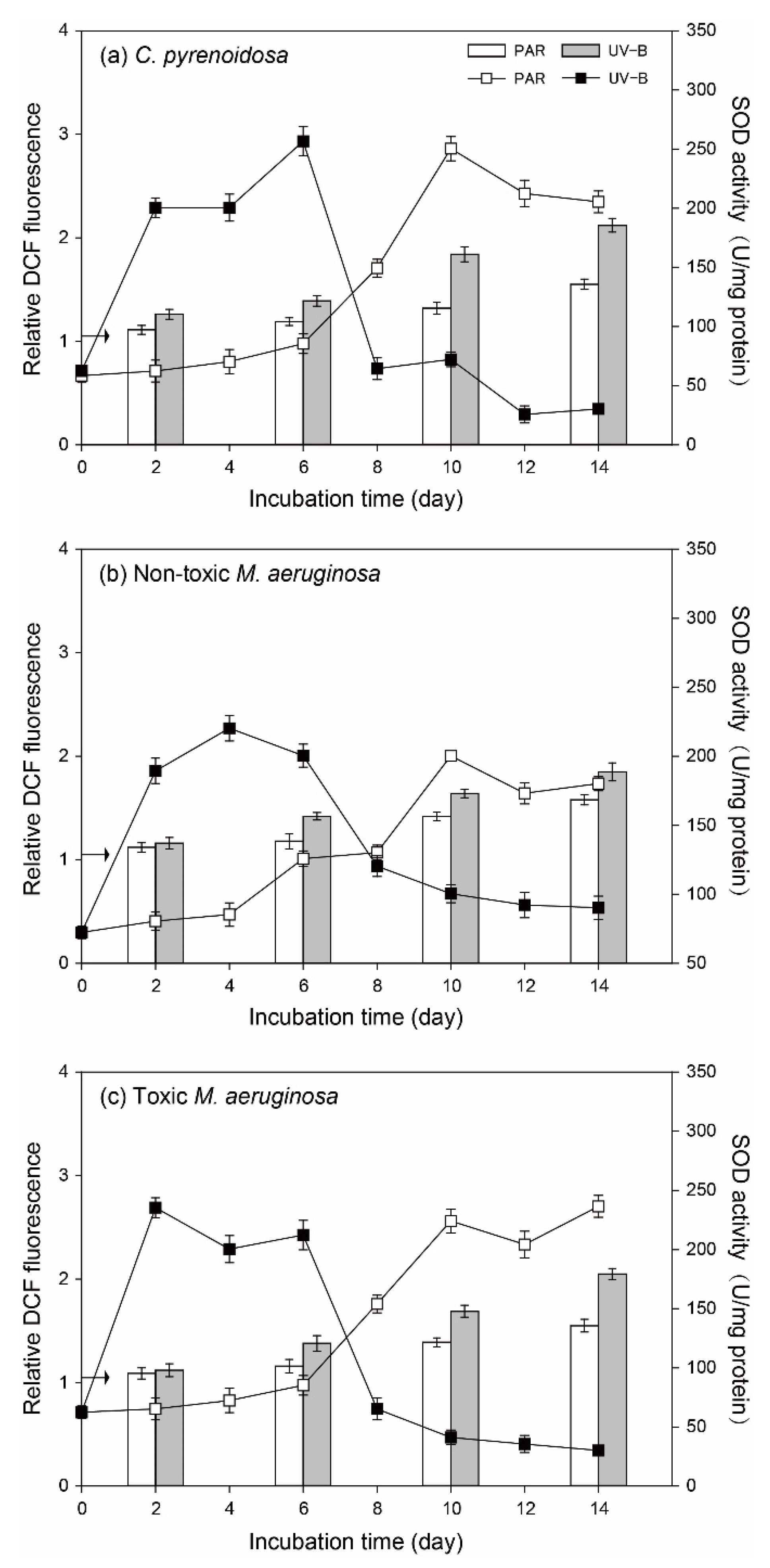
2.3.4. Reactive Oxygen Species (ROS) in Cells and Superoxide Dismutase (SOD) Activity
2.3.5. Cell Adsorption Spectra and Contents of Photosynthetic Pigments
2.4. Statistical Analysis
3. Results
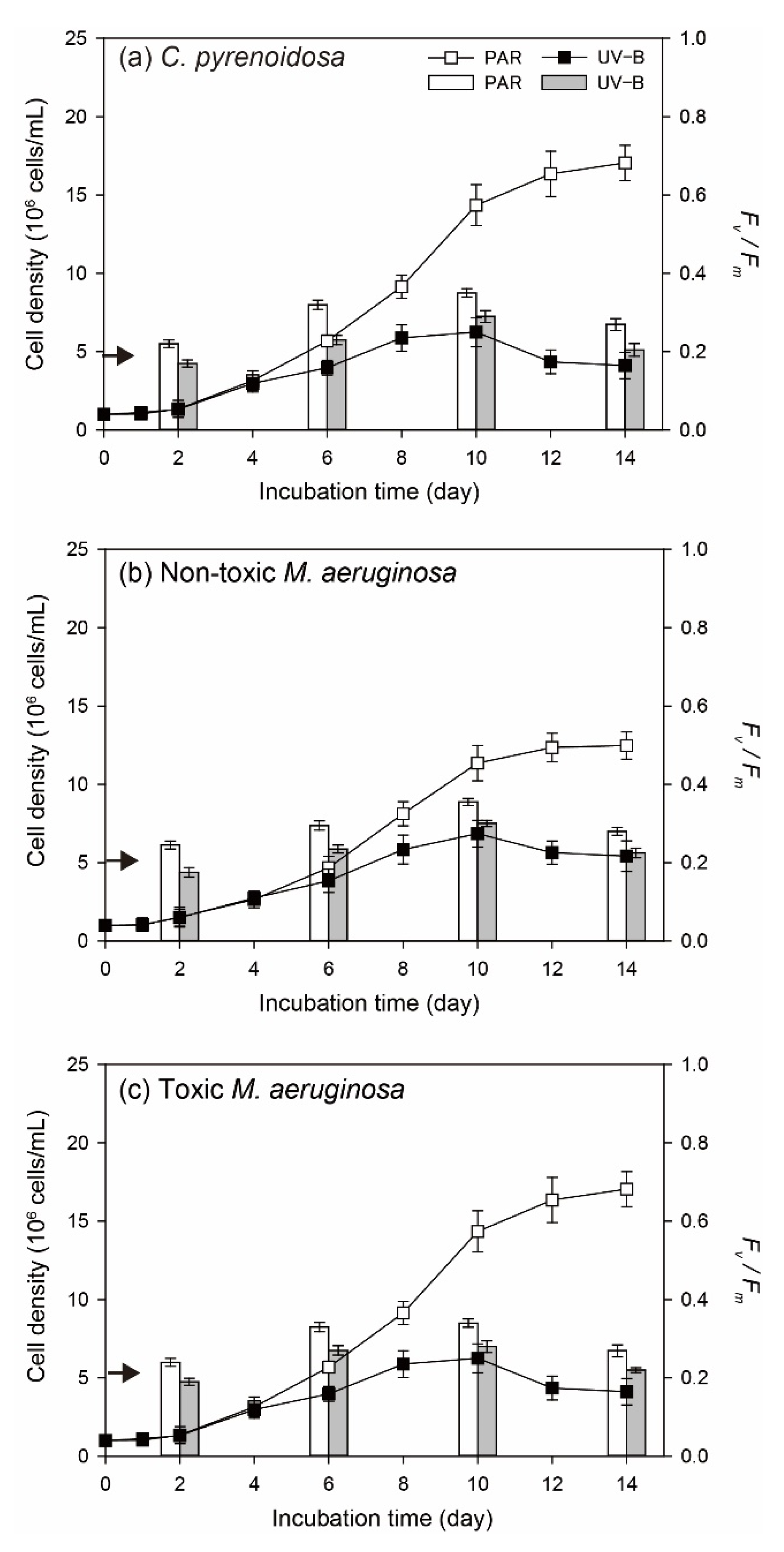
3.1. Algal Growth in the Mono-Cultures under Normal Growth Conditions
3.1.1. Cell Density and Algal Photosynthetic Efficiency
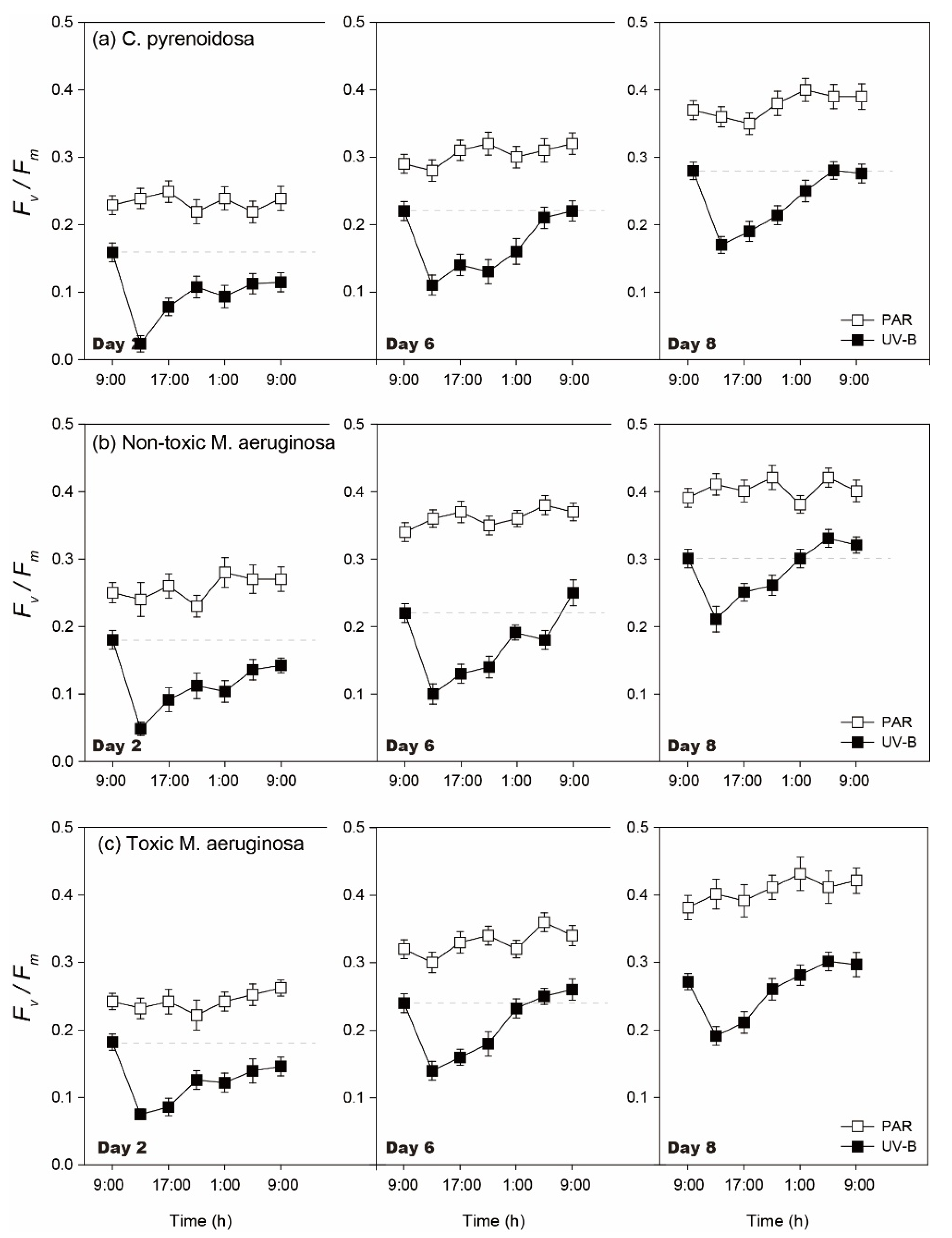
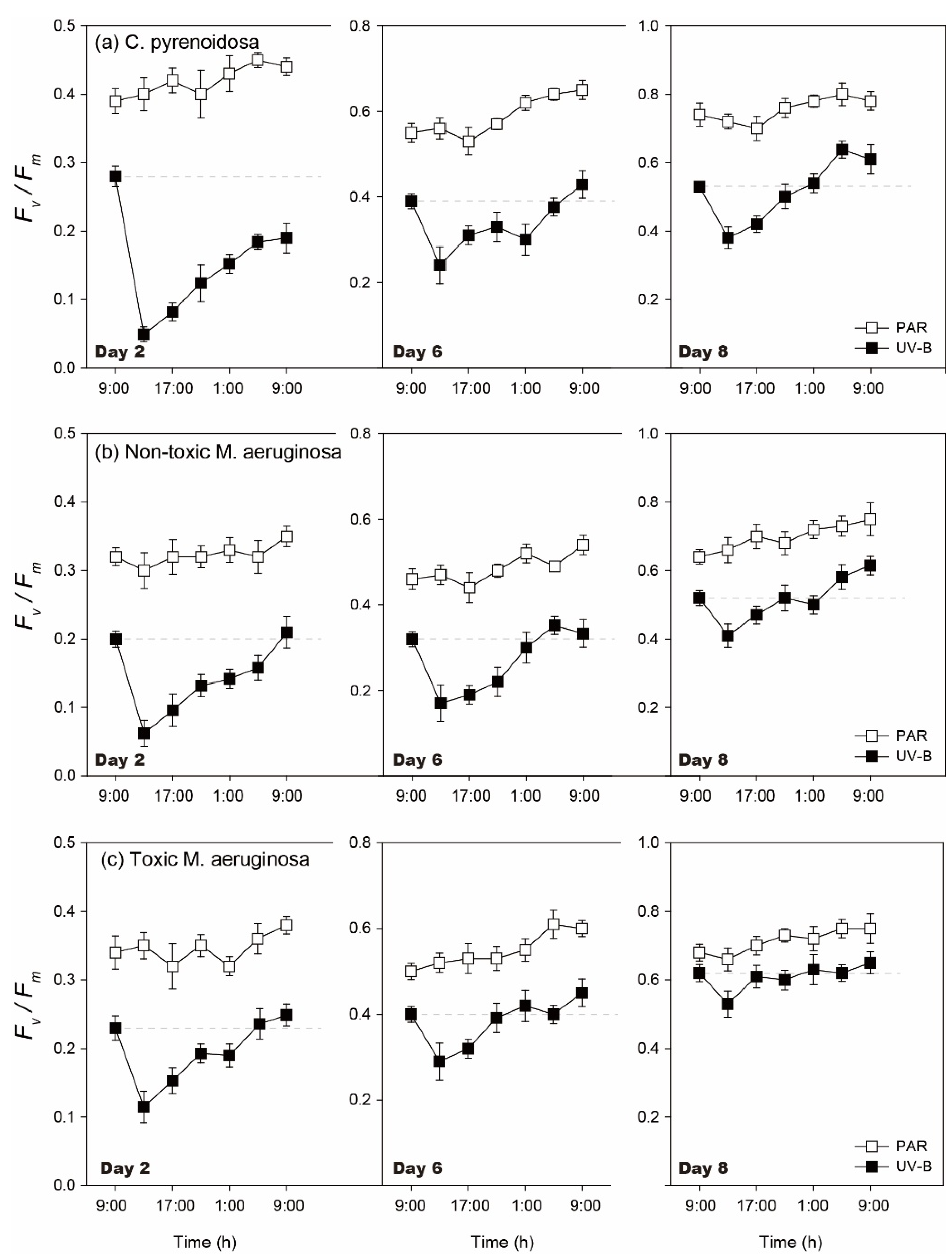
3.1.2. Diurnal Changes of Algal Fv/Fm
3.2. K+ Contents in the Algal Cultures
3.3. EPS Determination of Algal Cells
3.4. Antioxidant Responses of Algal Species under Normal Growth Conditions
3.4.1. ROS in Algal Cells and SOD Activity
3.4.2. Contents of Photosynthetic Pigments
3.5. Algal Growth in the Mono-Cultures under Nutrient Enrichment Conditions
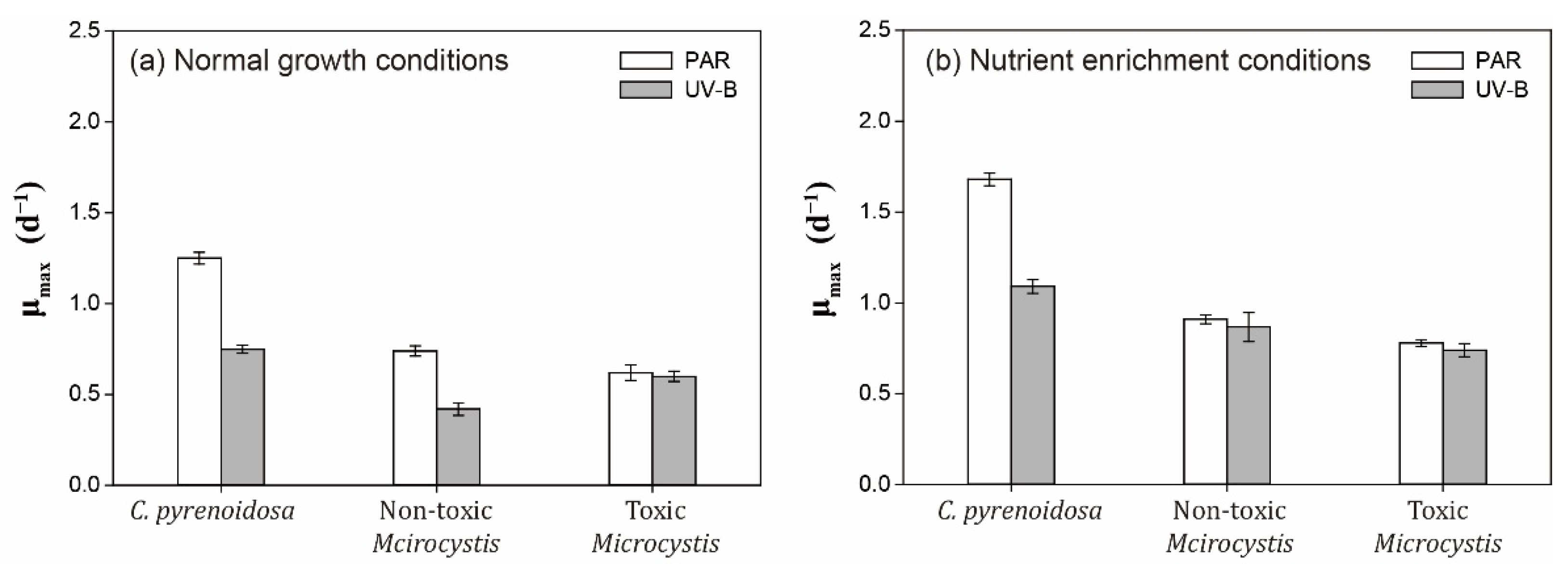
3.5.1. Cell Density and Algal Photosynthetic Efficiency
3.5.2. Diurnal Changes of Algal Fv/Fm
3.6. Antioxidant Responses of Algal Species under Nutrient Enrichment Conditions
3.6.1. ROS in Algal Cells and SOD Activity
3.6.2. Contents of Photosynthetic Pigments
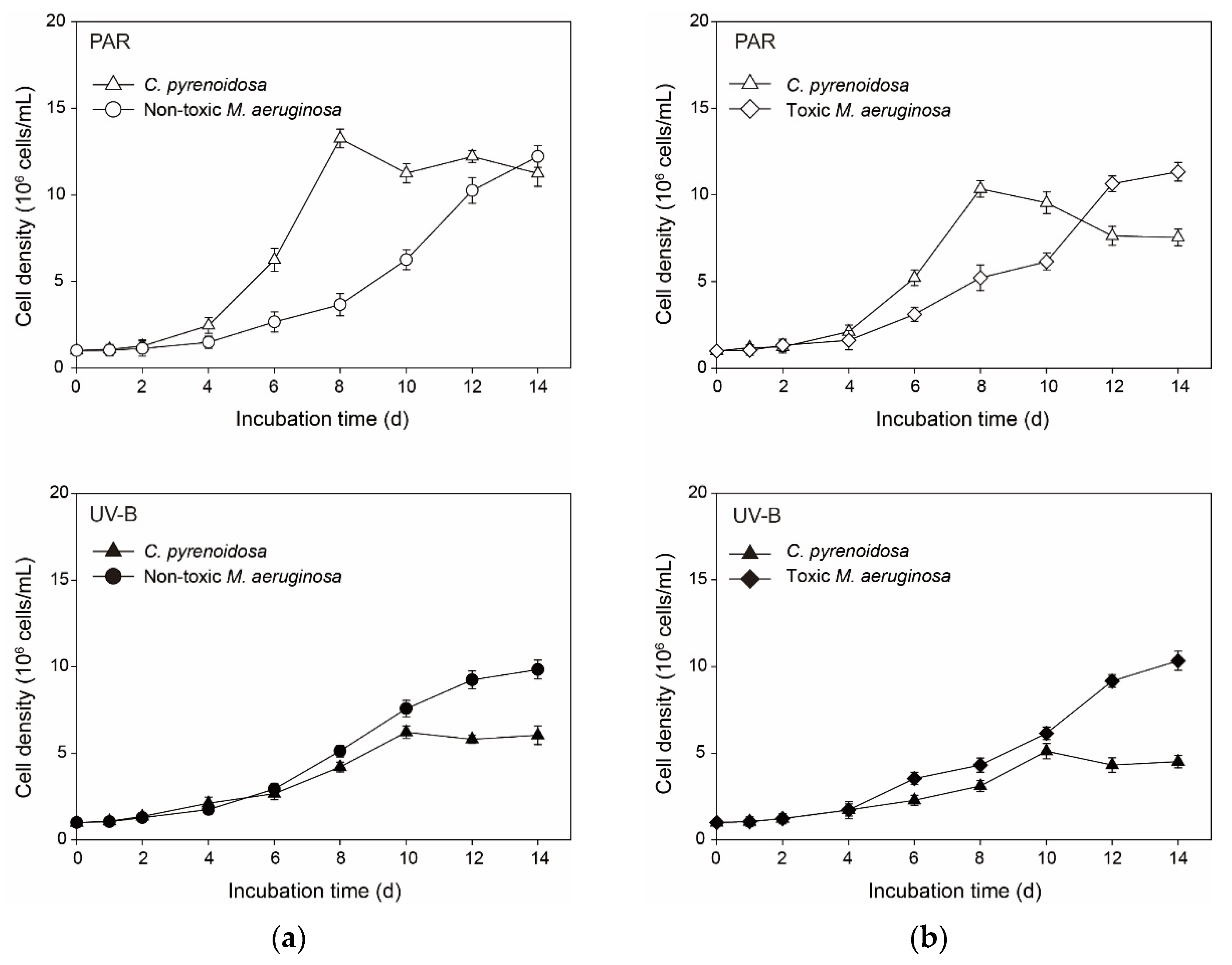
3.7. Interspecific Competition in the Co-Cultures
4. Discussion
4.1. Effects of UV-B Radiation and Algal Responses
4.2. Comparison of Algal Adaptation to UV-B Radiation
4.3. Effects of Nutrient Enrichment and Algal Competition Characteristics
5. Conclusions
- (1)
- Compared with PAR, 4 h of ambient UV-B radiation could exert oxidative stresses and negative effects on the photosynthesis and growth of three algal species under normal growth conditions. The adopted UV- B treatment did not cause lethal effects on algae, and three species could grow with adaptive responses, including EPS production, regulation of SOD activity, synthesis of photosynthetic pigments, and Fv/Fm recovery.
- (2)
- Three species exhibited strain-specific responses to ambient UV-B radiation in the mono-cultures, when toxic M. aeruginosa was more tolerant and showed a higher adaptation capability to UV-B, including lower sensitivity and better self-repair efficiency. In addition to stable μmax in two treatments, higher production of EPS, and enhanced production of CAR and PC under UV-B radiation, toxic M. aeruginosa showed a better recovery of its photosynthetic efficiency.
- (3)
- Nutrient enrichment could alleviate the negative effects of UV-B radiation on algae, and the growth of toxic M. aeruginosa was comparable between PAR and UV-B treatment. In the co-cultures with nutrient enrichment, M. aeruginosa gradually outcompeted C. pyrenoidosa in the PAR treatment, and UV-B treatment enhanced the growth advantages of M. aeruginosa, when toxic M. aeruginosa showed a greater competitiveness to maintain high μmax and inhibit the growth of C. pyrenoidosa.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paerl, H.W.; Huisman, J. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Shang, J.H.; Zhang, C.; Zhang, W.L.; Niu, L.H.; Wang, L.F.; Zhang, H.J. The role of freshwater eutrophication in greenhouse gas emissions: A review. Sci. Total Environ. 2021, 768, 144582. [Google Scholar] [CrossRef]
- Paerl, H.W. Mitigating harmful cyanobacterial blooms in a human- and climatically-impacted world. Life 2014, 4, 988–1012. [Google Scholar] [CrossRef] [Green Version]
- Rigosi, A.; Carey, C.C.; Ibelings, B.W.; Brookes, J.D. The interaction between climate warming and eutrophication to promote cyanobacteria is dependent on trophic state and varies among taxa. Limnol. Oceanogr. 2014, 59, 99–114. [Google Scholar] [CrossRef] [Green Version]
- Carey, C.C.; Ibelings, B.W.; Hoffmann, E.P.; Hamilton, D.P.; Brookes, J.D. Eco-physiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res. 2012, 46, 1394–1407. [Google Scholar] [CrossRef]
- Lürling, M.; Eshetu, F.; Faassen, E.J.; Kosten, S.; Huszar, V.L.M. Comparison of cyanobacterial and green algal growth rates at different temperatures. Freshw. Biol. 2013, 58, 552–559. [Google Scholar] [CrossRef]
- Paerl, H.W.; Xu, H.; McCarthy, M.J.; Zhu, G.; Qin, B.; Li, Y.; Gardner, W.S. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient (N & P) management strategy. Water Res. 2011, 45, 1973–1983. [Google Scholar] [PubMed]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.Q.; Paerl, H.W.; Brookes, J.D.; Liu, J.G.; Jeppesen, E. Why Lake Taihu continues to be plagued with cyanobacterial blooms through 10 years (2007–2017) efforts. Sci. Bull. 2019, 64, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Wang, X.Q.; Jiang, H.B.; Qiu, B.S. Effects of iron availability on competition between Microcystis and Pseudanabaena or Chlorella species. Eur. J. Phycol. 2015, 50, 260–270. [Google Scholar] [CrossRef]
- Ren, L.X.; Huang, J.; Wang, B.; Wang, H.Y.; Gong, R.; Hu, Z.X. Effects of temperature on the growth and competition between Microcystis aeruginosa and Chlorella pyrenoidosa with different phosphorus availabilities. Desalination Water Treat. 2021, 241, 87–111. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, H.B.; Gao, K.S.; Qiu, B.S. Acclimation to low ultraviolet-B radiation increases photosystem I abundance and cyclic electron transfer with enhanced photosynthesis and growth in the cyanobacterium Nostoc sphaeroides. Environ. Microbiol. 2020, 22, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Zhang, D.F.; Duan, Z.P.; Parajuli, K.; Hu, J.Y. Effects of light color on interspecific competition between Microcystis aeruginosa and Chlorella pyrenoidosa in batch experiment. Environ. Sci. Pollut. Res. 2019, 27, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Häder, D.P.; Williamson, C.E.; Wängberg, S.Å.; Rautio, M.; Rose, K.C.; Gao, K.S.; Helbling, E.W.; Sinha, R.P.; Worrest, R. Effects of UV radiation on aquatic ecosystems and interactions with other environmental factors. Photochem. Photobiol. Sci. B Biol. 2015, 14, 108–126. [Google Scholar] [CrossRef] [Green Version]
- Carrillo, P.; Medina-Sánchez, J.M.; Herrera, G.; Durán, C.; Segovia, M.; Cortés, D.; Salles, S.; Korbee, N.; Figueroa, F.L.; Mercado, J.M. Interactive effect of UVR and phosphorus on the coastal phytoplankton community of the Western Mediterranean Sea: Unravelling eco-physiological mechanisms. PLoS ONE 2015, 10, e0142987. [Google Scholar] [CrossRef] [PubMed]
- Beardall, J.; Stojkovic, S.; Gao, K.S. Interactive effects of nutrient supply and other environmental factors on the sensitivity of marine primary producers to ultraviolet radiation: Implications for the impacts of global change. Aquat. Biol. 2014, 22, 5–23. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.G.; Gao, G.; Tu, B.; Qiao, H.J.; Ge, H.M.; Wu, H.Y. Physiological response of the toxic and non-toxic strains of a bloom-forming cyanobacterium Microcystis aeruginosa to changing ultraviolet radiation regimes. Hydrobiologia 2019, 833, 143–156. [Google Scholar] [CrossRef]
- Ma, Z.L.; Fang, T.X.; Thring, R.W.; Li, Y.B.; Yu, H.G.; Zhou, Q.; Zhao, M. Toxic and non-toxic strains of Microcystis aeruginosa induce temperature dependent allelopathy toward growth and photosynthesis of Chlorella vulgaris. Harmful Algae 2015, 48, 21–29. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Yin, Y.; Zhang, E.L.; Zhu, G.W.; Liu, M.L.; Feng, L.Q.; Qin, B.Q.; Liu, X.H. Spectral attenuation of ultraviolet and visible radiation in lakes in the Yunnan Plateau, and the middle and lower reaches of the Yangtze River, China. Photochem. Photobiol. Sci. 2011, 10, 469–482. [Google Scholar] [CrossRef]
- Cai, L.L.; Zhu, G.W.; Zhu, M.Y.; Yang, G.J. Succession of phytoplankton structure and its relationship with algae bloom in littoral zone of Meiliang Bay, Taihu Lake. Ecol. Sci. 2012, 31, 345–351. (In Chinese) [Google Scholar]
- Xu, H.; McCarthy, M.J.; Paerl, H.W.; Brookes, J.D.; Zhu, G.; Hall, N.S.; Qin, B.; Zhang, Y.; Zhu, M.; Hampel, J.J.; et al. Contributions of external nutrient loading and internal cycling to cyanobacterial bloom dynamics in Lake Taihu, China: Implications for nutrient management. Limnol. Oceanogr. 2021, 9999, 1492–1509. [Google Scholar] [CrossRef]
- Chen, Y.W.; Qin, B.Q.; Teubner, K.; Dokulil, M.T. Long-term dynamics of phytoplankton assemblages: Microcystis-domination in Lake Taihu, a large shallow lake in China. J. Plankton Res. 2003, 25, 445–453. [Google Scholar] [CrossRef]
- Bittencourt-Oliveira, M.C.; Chia, M.A.; Oliveira, H.S.B.; Araujo, M.K.C.; Molica, R.J.R.; Dias, C.T.S. Allelopathic interactions between microcystin-producing and non-microcystin-producing cyanobacteria and green microalgae: Implications for microcystins production. J. Appl. Phycol. 2015, 27, 275–284. [Google Scholar] [CrossRef]
- Yang, J.; Deng, X.; Xian, Q.; Qian, X.; Li, A. Allelopathic effect of Microcystis aeruginosa on Microcystis wesenbergii: Microcystin-LR as a potential allelochemical. Hydrobiologia 2014, 727, 65–73. [Google Scholar] [CrossRef]
- Ren, L.X.; Wang, P.F.; Wang, C.; Paerl, H.W.; Wang, H.Y. Effects of phosphorus availability and the phosphorus utilization behavior by Microcystis aeruginosa on its adaptation capability to ultraviolet radiation. Environ. Pollut. 2020, 256, 113441. [Google Scholar] [CrossRef]
- Li, Z.K.; Dai, G.Z.; Juneau, P.; Qiu, B.S.; Posewitz, M. Different physiological responses of cyanobacteria to ultraviolet-b radiation under iron-replete and iron-deficient conditions: Implications for underestimating the negative effects of UV-B radiation. J. Phycol. 2017, 53, 425–436. [Google Scholar] [CrossRef]
- Yang, Z.; Kong, F.X.; Shi, X.L.; Yu, Y.; Zhang, M. UV-B radiation and phosphorus limitation interact to affect the growth, pigment content, and photosynthesis of the toxic cyanobacterium Microcystis aeruginosa. J. Appl. Phycol. 2014, 26, 1669–1674. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Gao, K.S. Impacts of solar UV radiation on the photosynthesis, growth, and UV-absorbing compounds in Gracilaria lemaneiformis (Rhodophyta) grown at different nitrate concentrations. J. Phycol. 2009, 45, 314–323. [Google Scholar] [CrossRef]
- Jiang, H.B.; Qiu, B.S. Inhibition of photosynthesis by UV-B exposure and its repair in the bloom-forming cyanobacterium Microcystis aeruginosa. J. Appl. Phycol. 2011, 23, 691–696. [Google Scholar] [CrossRef]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohenbazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.J.; Liu, X.L.; Tan, J.; Li, D.T.; Yang, H. Diversity and dynamics of microcystin-producing cyanobacteria in China’s third largest lake, Lake Taihu. Harmful Algae 2009, 8, 637–644. [Google Scholar] [CrossRef]
- Duan, Z.P.; Tan, X.; Li, N.G. Ultrasonic selectivity on depressing photosynthesis of cyanobacteria and green algae probed by chlorophyll-a fluorescence transient. Water Sci. Technol. 2017, 76, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Song, L.R. Comparative studies on physiological responses to phosphorus in two phenotypes of bloom-forming Microcystis. Hydrobiologia 2007, 592, 475–486. [Google Scholar] [CrossRef]
- Helbling, E.W.; Carrillo, P.; Medinasánchez, J.M.; Durán, C.; Herrera, G.; Villarargaiz, M.; Villafa, V.E. Interactive effects of vertical mixing, nutrients and ultraviolet radiation: In situ photosynthetic responses of phytoplankton from high mountain lakes in Southern Europe. Biogeosciences 2013, 10, 1037–1050. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.C.; Li, D.H.; Cao, X.Y.; Song, C.L.; Zhou, Y.Y. Photosynthesis regulates succession of toxic and nontoxic strains in blooms of Microcystis (Cyanobacteria). Phycologia 2015, 54, 640–648. [Google Scholar] [CrossRef]
- Chen, J.J.; Yeh, H.H. The mechanisms of potassium permanganate on algae removal. Water Res. 2005, 39, 4420–4428. [Google Scholar] [CrossRef]
- Hou, J.; Yang, Z.J.; Wang, P.F.; Wang, C.; Yang, Y.; Wang, X. Changes in Microcystis aeruginosa cell integrity and variation in microcystin-LR and proteins during Tanfloc flocculation and floc storage. Sci. Total Environ. 2018, 626, 264–273. [Google Scholar] [CrossRef]
- Gao, L.; Pan, X.L.; Zhang, D.Y.; Mu, S.Y.; Lee, D.J.; Halik, U. Extracellular polymeric substances buffer against the biocidal effect of H2O2 on the bloom-forming cyanobacterium Microcystis aeruginosa. Water Res. 2015, 69, 51–58. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Hou, J.; Wang, P.F.; Wang, C.; Wang, X.; You, G.X. Influence of extracellular polymeric substances on cell-NPs heteroaggregation process and toxicity of cerium dioxide NPs to Microcystis aeruginosa. Environ. Pollut. 2018, 242, 1206–1216. [Google Scholar] [CrossRef]
- Xu, H.; Cai, H.; Yu, G.; Jiang, H. Insights into extracellular polymeric substances of cyanobacterium Microcystis aeruginosa using fractionation procedure and parallel factor analysis. Water Res. 2013, 47, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, W.; Gao, L.; Lu, L. Changes in extracellular polysaccharide content and morphology of Microcystis aeruginosa at different specific growth rates. J. Appl. Phycol. 2013, 25, 1023–1030. [Google Scholar] [CrossRef]
- Cai, X.; Hutchins, D.A.; Fu, F.X.; Gao, K.S. Effects of ultraviolet radiation on photosynthetic performance and N2 fixation in Trichodesmium erythraeum IMS 101. Biogeosciences Discuss. 2017, 14, 4455–4466. [Google Scholar] [CrossRef] [Green Version]
- Qu, F.; Liang, H.; He, J.; Ma, J.; Wang, Z.; Yu, H.; Li, G. Characterization of dissolved extracellular organic matter (dEOM) and bound extracellular organic matter (bEOM) of Microcystis aeruginosa and their impacts on UF membrane fouling. Water Res. 2012, 46, 2881–2890. [Google Scholar] [CrossRef]
- Six, C.; Thomas, J.C.; Garczarek, L.; Ostrowski, M.; Dufresne, A.; Blot, N.; Scanlan, D.J.; Partensky, F. Diversity and evolution of phycobilisomes in marine Synechococcus spp.: A comparative genomics study. Genome Biol. 2007, 8, R259. [Google Scholar] [CrossRef]
- Häder, D.P.; Kumar, H.D.; Smith, R.C.; Worrest, R.C. Effects of solar UV radiation on aquatic ecosystems and interactions with climate change. Photochem. Photobiol. Sci. 2007, 6, 267–285. [Google Scholar] [CrossRef]
- Hessen, D.O.; Frigstad, H.; Færøvig, P.J.; Wojewodzic, M.W.; Leu, E. UV radiation and its effects on P-uptake in arctic diatoms. J. Exp. Mar. Biol. Ecol. 2012, 411, 45–51. [Google Scholar] [CrossRef]
- Wang, G.H.; Chen, K.; Chen, L.Z.; Hu, C.X.; Zhang, D.L.; Liu, Y.D. The involvement of the antioxidant system in protection of desert cyanobacterium Nostoc sp. against UV-B radiation and the effects of exogenous antioxidants. Ecotoxicol. Environ. Saf. 2008, 69, 150–157. [Google Scholar] [CrossRef]
- Zhu, Z.; Fu, F.X.; Qu, P.P.; Mak, E.W.K.; Jiang, H.B.; Zhang, R.F.; Zhu, Z.Y.; Gao, K.S.; Hutchins, D.A. Interactions between ultraviolet radiation exposure and phosphorus limitation in the marine nitrogen-fixing cyanobacteria Trichodesmium and Crocosphaera. Limnol. Oceanogr. 2020, 65, 363–376. [Google Scholar] [CrossRef]
- Jiang, H.B.; Qiu, H.S. Photosynthetic adaption of a bloom-forming cyanobacterium Microcystis aeruginosa (Cyanophyceae) to prolonged UV-B exposure. J. Phycol. 2005, 41, 983–992. [Google Scholar] [CrossRef]
- Helbling, E.W.; Buma, A.G.J.; Boelen, P.; Strate, H.J.; Giordanino, M.V.F.; Villafane, V.E. Increase in Rubisco activity and gene expression due to elevated temperature partially counteracts ultraviolet radiation–induced photoinhibition in the marine diatom Thalassiosira weissflogii. Limnol. Oceanogr. 2011, 56, 1330–1342. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.T.; Gao, K.S. UV-A enhanced growth and UV-B induced positive effects in the recovery of photochemical yield in Gracilaria lemaneiformis (Rhodophyta). J. Photochem. Photobiol. B 2010, 100, 117–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, H.; Oguma, K.; Katayama, H.; Ohgaki, S. Effects of low- or medium-pressure ultraviolet lamp irradiation on Microcystis aeruginosa and Anabaena variabilis. Water Res. 2007, 41, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, W.H.; Gao, K.S. Effects of temperature, pH, and UV radiation on alkaline phosphatase activity in the terrestrial cyanobacterium Nostoc flagelliforme. J. Appl. Phycol. 2013, 25, 1031–1038. [Google Scholar] [CrossRef]
- Shelly, K.; Heraud, P.; Beardall, J. Interactive effect of PAR and UV-B radiation on PSII electron transport in the marine alga Dunaliella tertiolecta (Chlorophyceae). J. Phycol. 2010, 39, 509–512. [Google Scholar] [CrossRef]
- Villafañe, V.E.; Cabrerizo, M.J.; Erzinger, G.S.; Bermejo, P.; Strauch, S.M.; Valiñas, M.S.; Helbling, E.W. Photosynthesis and growth of temperate and sub-tropical estuarine phytoplankton in a scenario of nutrient enrichment under solar ultraviolet radiation exposure. Estuaries Coasts 2017, 40, 842–855. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.B.; Qiu, B.S. Effects of UVB Radiation on competition between the bloom-forming cyanobacterium Microcystis aeruginosa and the chlorophyceae Chlamydomonas microsphaera. J. Phycol. 2013, 49, 318–328. [Google Scholar] [CrossRef]
- Ling, F.; Hamzeh, M.; Dodard, S.; Zhao, Y.H.; Sunahara, G.I. Effects of TiO2 nanoparticles on ROS production and growth inhibition using freshwater green algae pre-exposed to UV irradiation. Environ. Toxicol. Pharmacol. 2015, 39, 1074–1080. [Google Scholar]
- Latifi, A.; Ruiz, M.; Zhang, C.C. Oxidative stress in cyanobacteria. FEMS Microbiol Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.K.; Dai, G.Z.; Juneau, P.; Qiu, B.S. Capsular polysaccharides facilitate enhanced iron acquisition by the colonial cyanobacterium Mcrocystis sp. isolated from a freshwater lake. J. Phycol. 2016, 52, 105–115. [Google Scholar]
- He, Y.Y.; Häder, D.P. Reactive oxygen species and UV-B: Effect on cyanobacteria. Photochem. Photobiol. Sci. 2002, 1, 729–736. [Google Scholar] [CrossRef]
- Paerl, H.W.; Jane, T.; Bl, P.T. Carotenoid enhancement and its role in maintaining blue-green algal (Microcystis aeruginosa) surface blooms. Limnol. Oceanogr. 1983, 28, 847–857. [Google Scholar] [CrossRef]
- Qin, H.J.; Li, S.S.; Li, D.H. Differential responses of different phenotypes of Microcystis (Cyanophyceae) to UV-B radiation. Phycologia 2015, 54, 118–129. [Google Scholar] [CrossRef]
- Briand, E.; Bormans, M.; Quiblier, C.; Salençon, M.J.; Humbert, J.F. Evidence of the cost of the production of microcystins by Microcystis aeruginosa under differing light and nitrate environmental conditions. PLoS ONE 2012, 7, e29981. [Google Scholar] [CrossRef]
- Janssen, E.M.L.; Erickson, P.R.; Mcneill, K. Dual roles of dissolved organic matter as sensitizer and quencher in the photooxidation of tryptophan. Environ. Sci. Technol. 2014, 48, 4916–4924. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Sheng, G.P.; Luo, H.W.; Zhang, F.; Yuan, S.J.; Juan, X.U.; Zeng, R.J.; Wu, J.G.; Yu, H.Q. Contribution of extracellular polymeric substances (EPS) to the sludge aggregation. Environ. Sci. Technol. 2010, 44, 4355–4360. [Google Scholar] [CrossRef]
- Zhang, M.; Kong, F.X.; Tan, X.; Yang, Z.; Cao, H.S.; Xing, P. Biochemical, morphological, and genetic variations in Microcystis aeruginosa due to colony disaggregation. World J. Microbiol. Biotechnol. 2007, 23, 663–670. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Y.; Wei, J.; Zhang, X.; Zhang, L.; Yang, Z.; Huang, Y. Ultraviolet-B radiation stress alters the competitive outcome of algae: Based on analyzing population dynamics and photosynthesis. Chemosphere 2021, 272, 129645. [Google Scholar] [CrossRef]
- García-Gómez, C.; Parages, M.L.; Jiménez, C.; Palma, A.; Mata, M.T.; Segovia, M. Cell survival after UV radiation stress in the unicellular chlorophyte Dunaliella tertiolecta is mediated by DNA repair and MAPK phosphorylation. J. Exp. Bot. 2012, 63, 5259–5274. [Google Scholar] [CrossRef] [Green Version]
- Zilliges, Y.; Kehr, J.C.; Meissner, S.; Ishida, K.; Mikkat, S.; Hagemann, M.; Kaplan, A.; Börner, T.; Dittmann, E. The cyanobacterial hepatotoxin microcystin binds to proteins and increases the fitness of Microcystis under oxidative stress conditions. PLoS ONE 2011, 6, e17615. [Google Scholar] [CrossRef] [Green Version]
- Shelly, K.; Roberts, S.; Heraud, P.; Beardall, J. Interactions between UV-B exposure and phosphorus nutrition: I. Effects on growth, phosphate uptake, and chlorophyll fluorescence. J. Phycol. 2005, 41, 1204–1211. [Google Scholar] [CrossRef]
- Boucar, M.; Shen, L.Q.; Wang, K.; Zhang, Z.C.; Qiu, B.S. UV-B irradiation enhances the production of unique mycosporine-like amino acids and carotenoids in the subaerial cyanobacterium Pseudanabaena sp. CCNU1. Eur. J. Phycol. 2021, 56, 316–323. [Google Scholar] [CrossRef]
- Häder, D.P.; Sinha, R.P. Solar ultraviolet radiation-induced DNA damage in aquatic organisms: Potential environmental impact. Mutat. Res. 2005, 571, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.X.; Wang, P.F.; Wang, C.; Chen, J.; Hou, J.; Qian, J. Algal growth and utilization of phosphorus studied by combined mono-culture and co-culture experiments. Environ. Pollut. 2017, 220, 274–285. [Google Scholar] [CrossRef]
- Rzymski, P.; Poniedzialek, B.; Kokocinski, M.; Jurczak, T.; Lipski, D.; Wiktorowicz, K. Interspecific allelopathy in cyanobacteria: Cylindrospermopsin and Cylindrospermopsis raciborskii effect on the growth and metabolism of Microcystis aeruginosa. Harmful Algae 2014, 35, 1–8. [Google Scholar] [CrossRef]
- Mário, U.G.B.; Alan, E.W.; João, I.R.L.; Silvano, P.P.; Riley, P.B.; Edna, G.F.; José, C. Environmental factors associated with toxic cyanobacterial blooms across 20 drinking water reservoirs in a semi-arid region of Brazil. Harmful Algae 2019, 86, 128–137. [Google Scholar]
- Sun, Y.; Zhang, X.; Zhang, L.; Huang, Y.; Montagnes, D. UVB radiation suppresses antigrazer morphological defense in Scenedesmus obliquus by inhibiting algal growth and carbohydrate-regulated gene expression. Environ. Sci. Technol. 2020, 54, 4495–4503. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Y.; Häder, D.P. Involvement of reactive oxygen species in the UV-B damage to the cyanobacterium Anabaena sp. J. Photoch. Photobiol. B 2002, 66, 73–80. [Google Scholar] [CrossRef]
- Takaichi, S.; Mochimaru, M. Carotenoids and carotenogenesis in cyanobacteria: Unique ketocarotenoids and carotenoid glycosides. Cell. Mol. Life Sci. 2007, 64, 2607–2619. [Google Scholar] [CrossRef]
- Lüder, U.H.; Knoetzel, J.; Wiencke, C. Acclimation of photosynthesis and pigments to seasonally changing light conditions in the endemic Antarctic red macroalga Palmaria decipiens. Polar Biol. 2001, 24, 598–603. [Google Scholar] [CrossRef]

| Contents of Piments (pg/cell) | C. pyrenoidosa | Non-Toxic M. aeruginosa | Toxic M. aeruginosa | ||||
|---|---|---|---|---|---|---|---|
| PAR | UV-B | PAR | UV-B | PAR | UV-B | ||
| Day 1 | Chl-a | 0.14 ± 0.02 | 0.15 ± 0.02 | 0.18 ± 0.03 | 0.16 ± 0.01 | 0.17 ± 0.02 | 0.17 ± 0.03 |
| CAR | 0.12 ± 0.02 | 0.19 ± 0.02 * | 0.07 ± 0.01 | 0.11 ± 0.01 * | 0.06 ± 0.02 | 0.12 ± 0.02 * | |
| PC | \ | \ | 0.62 ± 0.07 | 0.84 ± 0.06 * | 0.55 ± 0.03 | 0.75 ± 0.04 * | |
| CAR/Chl-a | 0.86 ± 0.02 | 1.25 ± 0.06 * | 0.41 ± 0.02 | 0.68 ± 0.05 * | 0.37 ± 0.05 | 0.72 ± 0.01 * | |
| PC/Chl-a | \ | \ | 3.46 ± 0.15 | 5.15 ± 0.35 * | 3.26 ± 0.16 | 4.64 ± 0.28 * | |
| Day 8 | Chl-a | 0.36 ± 0.04 | 0.24 ± 0.03 * | 0.27 ± 0.01 | 0.11 ± 0.01 * | 0.25 ± 0.01 | 0.12 ± 0.02 * |
| CAR | 0.18 ± 0.01 | 0.09 ± 0.01 * | 0.11 ± 0.01 | 0.07 ± 0.01 * | 0.10 ± 0.02 | 0.05 ± 0.01 | |
| PC | \ | \ | 0.70 ± 0.01 | 0.54 ± 0.04 * | 0.67 ± 0.03 | 0.46 ± 0.03 * | |
| CAR/Chl-a | 0.50 ± 0.02 | 0.39 ± 0.03 * | 0.42 ± 0.05 | 0.68 ± 0.02 * | 0.42 ± 0.06 | 0.63 ± 0.03 * | |
| PC/Chl-a | \ | \ | 2.61 ± 0.03 | 5.07 ± 0.38 * | 2.68 ± 0.05 | 4.12 ± 0.24 * | |
| Contents of Piments (pg/cell) | C. pyrenoidosa | Non-Toxic M. aeruginosa | Toxic M. aeruginosa | ||||
|---|---|---|---|---|---|---|---|
| PAR | UV-B | PAR | UV-B | PAR | UV-B | ||
| Day 1 | Chl-a | 0.14 ± 0.01 | 0.15 ± 0.02 | 0.18 ± 0.02 | 0.16 ± 0.02 | 0.17 ± 0.02 | 0.17 ± 0.02 |
| CAR | 0.12 ± 0.02 | 0.23 ± 0.02 * | 0.09 ± 0.01 | 0.14 ± 0.01 * | 0.08 ± 0.01 | 0.15 ± 0.01 * | |
| PC | \ | \ | 0.67 ± 0.02 | 0.97 ± 0.04 * | 0.58 ± 0.02 | 0.94 ± 0.02 * | |
| CAR/Chl-a | 0.84 ± 0.05 | 1.52 ± 0.08 * | 0.41 ± 0.01 | 0.83 ± 0.02 * | 0.37 ± 0.05 | 0.88 ± 0.09 * | |
| PC/Chl-a | \ | \ | 3.57 ± 0.22 | 6.01 ± 0.48 * | 3.17 ± 0.18 | 5.58 ± 0.42 * | |
| Day 8 | Chl-a | 0.50 ± 0.02 | 0.35 ± 0.04 * | 0.41 ± 0.02 | 0.30 ± 0.02 * | 0.39 ± 0.01 | 0.29 ± 0.01 * |
| CAR | 0.23 ± 0.02 | 0.27 ± 0.01 * | 0.18 ± 0.02 | 0.26 ± 0.01 * | 0.15 ± 0.01 | 0.19 ± 0.01 | |
| PC | \ | \ | 0.70 ± 0.02 | 0.83 ± 0.03 * | 0.69 ± 0.01 | 0.79 ± 0.03 * | |
| CAR/Chl-a | 0.46 ± 0.03 | 0.78 ± 0.06 * | 0.45 ± 0.05 | 0.86 ± 0.06 * | 0.38 ± 0.02 | 0.68 ± 0.02 * | |
| PC/Chl-a | \ | \ | 1.72 ± 0.02 | 3.83 ± 0.21 * | 1.78 ± 0.09 | 2.84 ± 0.25 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, L.; Huang, J.; Ding, K.; Wang, Y.; Yang, Y.; Zhang, L.; Wu, H. Comparative Study of Algal Responses and Adaptation Capability to Ultraviolet Radiation with Different Nutrient Regimes. Int. J. Environ. Res. Public Health 2022, 19, 5485. https://doi.org/10.3390/ijerph19095485
Ren L, Huang J, Ding K, Wang Y, Yang Y, Zhang L, Wu H. Comparative Study of Algal Responses and Adaptation Capability to Ultraviolet Radiation with Different Nutrient Regimes. International Journal of Environmental Research and Public Health. 2022; 19(9):5485. https://doi.org/10.3390/ijerph19095485
Chicago/Turabian StyleRen, Lingxiao, Jing Huang, Keqiang Ding, Yi Wang, Yangyang Yang, Lijuan Zhang, and Haoyu Wu. 2022. "Comparative Study of Algal Responses and Adaptation Capability to Ultraviolet Radiation with Different Nutrient Regimes" International Journal of Environmental Research and Public Health 19, no. 9: 5485. https://doi.org/10.3390/ijerph19095485
APA StyleRen, L., Huang, J., Ding, K., Wang, Y., Yang, Y., Zhang, L., & Wu, H. (2022). Comparative Study of Algal Responses and Adaptation Capability to Ultraviolet Radiation with Different Nutrient Regimes. International Journal of Environmental Research and Public Health, 19(9), 5485. https://doi.org/10.3390/ijerph19095485