Abstract
Malaria and helminthic co-infection during pregnancy causes fetomaternal haemorrhage and foetal growth retardation. This study determined the pooled burden of pregnancy malaria and helminthic co-infection in sub-Saharan Africa. CINAHL, EMBASE, Google Scholar, Scopus, PubMed, and Web of Science databases were used to retrieve data from the literature, without restricting language and publication year. The Joanna Briggs Institute’s critical appraisal tool for prevalence studies was used for quality assessment. STATA Version 14.0 was used to conduct the meta-analysis. The I2 statistics and Egger’s test were used to test heterogeneity and publication bias. The random-effects model was used to estimate the pooled prevalence at a 95% confidence interval (CI). The review protocol has been registered in PROSPERO, with the number CRD42019144812. In total, 24 studies (n = 14,087 participants) were identified in this study. The pooled analysis revealed that 20% of pregnant women were co-infected by malaria and helminths in sub-Saharan Africa. The pooled prevalence of malaria and helminths were 33% and 35%, respectively. The most prevalent helminths were Hookworm (48%), Ascaris lumbricoides (37%), and Trichuris trichiura (15%). Significantly higher malaria and helminthic co-infection during pregnancy were observed. Health systems in sub-Saharan Africa must implement home-grown innovative solutions to underpin context-specific policies for the early initiation of effective intermittent preventive therapy.
1. Introduction
Globally, approximately 1.5 billion cases of infection from malaria and helminths pose a significant risk of mortality and morbidity to the population at risk including pregnant women and the foetus [1,2]. Recently, a total of 12 million incidences of gestational malaria were reported out of 33 million pregnancies in sub-Saharan Africa (SSA) [2].
Ten countries in SSA—Burkina Faso, Cameroon, The Democratic Republic of the Congo, Ghana, Mali, Mozambique, Niger, Nigeria, Uganda, and The United Republic of Tanzania—that were hard hit by malaria endorsed the “High Burden to High Impact Approach (HBHI)” [3]; this sets out four response mechanisms to malaria elimination—namely, a political will to reduce death associated with malaria, strategic information to deliver impact, better guidance and policies, and a coordinated national malaria response strategy [4,5,6,7,8]. However, the already fragile healthcare delivery in SSA has faced the doubled burden of malaria and the novel coronavirus (nCoV-2) pandemic, which has stalled the hard-won gains in the fight against malaria [9,10,11].
The burden of helminthic infection during pregnancy in SSA ranges from 11% to 31% [12]. The most common helminths associated with unintended pregnancy outcomes in SSA include Hookworm (32%) [13], Ascaris lumbricoides (52%) [14], Trichuris trichiura (2.9%) [15], and Schistosomiasis (13%) [16]. Concurrent infection for more than one helminthic species during pregnancy shows negative health consequences on birth and maternal outcomes similar to malaria parasitaemia [17,18].
The World Health Organisation (WHO) 2030 road map aims to establish an efficient helminths control program specifically for women of reproductive age. Nevertheless, helminths continue to constitute major public health problems for pregnant women in SSA [19,20,21]. Co-infection from malaria and helminths is a major indicator of global health inequality, and failure to tackle this health disparity slows down the race to realising universal health coverage and attainment of the Sustainable Development Goal (SDG)-3 [22,23,24,25,26,27,28,29].
1.1. Clinical Implications of Concurrent Malaria and Helminthic Infection in Pregnancy
Malaria during pregnancy increases the risk of miscarriage and stillbirth by 3 to 4 times, compared with pregnant women with no clinically confirmed malaria [30]. Helminths cause alterations in immune response and physiological changes that affect fecundity, due to induced immunological states, with resultant adverse effects on conception and pregnancy [31]. Anemia during pregnancy is the most common adverse health outcome caused by Ascaris lumbricoides [32,33], and Hookworm [34]. In addition, Schistosoma mansoni is also associated with anemia and undernutrition during pregnancy [34], while A. lumbricoides is implicated with gallbladder perforation [35]. Pregnancy malaria co-infection with A. lumbricoides and Hookworm has been associated with increased odds of P. falciparum infection [36,37], and the pathophysiology of pregnant women simultaneously infected with Plasmodium species and helminths revealed negative pregnancy outcomes such as anemia, fetomaternal haemorrhage, antepartum stillbirth syndrome, and low birth weight [38,39,40]. Malaria and helminths co-infection causes elevated and unregulated inflammatory biomarkers such as C-reactive protein and serum level Hepcidin, which results in reduced iron absorption during pregnancy [41,42,43,44,45,46,47,48,49,50,51]. In addition, comorbidity of Plasmodium falciparum and helminthiasis elucidates the incidence of cervical cancer among pregnant women [52,53,54]. Moreover, during pregnancy, malaria co-infection with A. lumbricoides has been associated with an increased odds of P. falciparum infection [55,56], and malaria–Hookworm co-infection is associated with risks of increased Plasmodium parasitaemia [57,58].
Currently, evidence on the burden of intestinal helminths and malaria co-infection, the nature of their interaction, and their impact on pregnancy is not well established in endemic countries [59,60,61]. Most of the studies conducted in SSA emphasized the negative health outcomes of infection from malaria and helminths among pre- and schoolchildren [62,63,64,65,66], while very limited attention has been given to the dire impact of concurrent maternal gestational nematode and Plasmodium species infection [67,68,69,70]. Therefore, this systematic review and meta-analysis synthesised the available data on the burden of malaria and helminthic co-infections and their interaction among pregnant women living in SSA. It will further highlight evidence-informed planning and implementation for the comprehensive elimination of co-endemic malaria and helminthic infections during pregnancy in SSA [71,72].
1.2. Operational Definitions
Malaria in pregnancy: This is an adverse clinical condition developed by pregnant women after being infected by Plasmodium species, which increases the risk of anemia, stillbirth, spontaneous abortion, low birth weight, and neonatal death [73]. Infants born to mothers living in endemic areas are vulnerable to malaria from approximately 3 months of age, which is when immunity acquired from the mother starts to wane [74,75,76,77].
Co-Infection: This is a clinical condition of particular human health importance caused by the simultaneous infection of a host (human being) by multiple pathogen species, for instance, multiple parasite infections [78,79,80,81,82].
Helminths: These are worms that infect the gastrointestinal tract of humans upon accidental ingestion of their infective eggs [83].
2. Materials and Methods
2.1. Reporting
The Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statement guidelines were used to fully record and report the search results and the reasons for exclusion of studies [84] (Figure 1) (Supplementary File S1). The review protocol has been registered in PROSPERO with registration code CRD42019144812 [85]. An updated guideline for reporting systematic reviews (PRISMA checklist 2020) was used to report the corresponding section of the manuscript with its detailed contents and items [86,87] (Supplementary File S3).
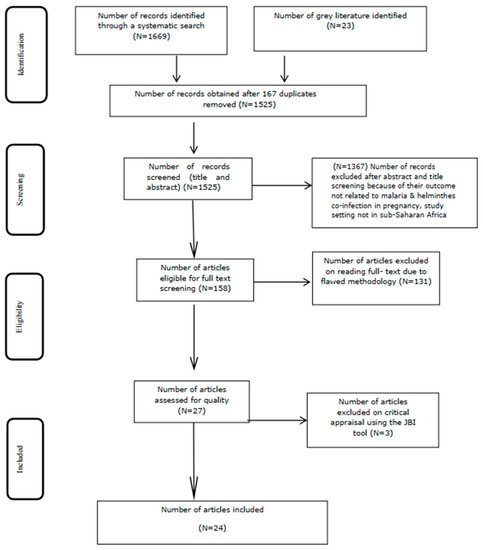
Figure 1.
Flow diagram of the included studies. Moher, D. et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Medicine, 2009, 6(7).
2.2. Search Strategy and Information Sources
A robust search was performed on CINAHL, EMBASE, Google Scholar, Scopus, PubMed, and Web of Science databases to retrieve published and unpublished data from the literature. (Supplementary File S1). No restrictions were made regarding the language and years of all publications. The Boolean operators “AND” and “OR” were used to combine the MeSH terms ““Hookworm Infections”[Mesh] OR “Ascaris”[Mesh] OR “Ascaris lumbricoides”[Mesh] OR “Ascariasis”[Mesh] OR “Trichuris”[Mesh] OR “Trichuriasis”[Mesh] OR “Schistosoma”[Mesh] OR “Schistosoma mansoni”[Mesh] OR “Schistosoma haematobium”[Mesh] OR “Schistosomiasis mansoni”[Mesh] OR “Schistosomiasis haematobia”[Mesh] OR “Intestinal helminthiasis” [Supplementary Concept] OR “Anemia”[Mesh] AND “Co-infection”[Mesh] OR “Comorbidity”[Mesh] OR “Malaria”[Mesh] OR “Malaria, Vivax”[Mesh] OR “Malaria, Falciparum”[Mesh] OR “Acute malaria” [Supplementary Concept] AND “Pregnancy”[Mesh] OR “Pregnant Women”[Mesh]” and text words “Hookworm Infections*[tw] OR Soil-transmitted helminthiasis OR Ascaris*[tw] OR Ascaris lumbricoides*[tw] OR Ascariasis*[tw] OR Trichuris*[tw] OR Trichuriasis*[tw] OR Schistosoma*[tw] OR Schistosoma mansoni*[tw] OR Schistosoma haematobium*[tw] OR Schistosomiasis mansoni*[tw] OR Schistosomiasis haematobia*[tw] OR Intestinal helminthiasis*[tw] OR Anemia*[tw] AND Co-infection*[tw] OR Comorbidity*[tw] OR Malaria*[tw] OR Malaria, Vivax*[tw] OR Plasmodium vivax*[tw] OR Malaria, Falciparum*[tw] OR Plasmodium falciparum*[tw] OR Acute malaria*[tw] AND Pregnancy*[tw] OR Pregnant Women*[tw]” to run key search topics. Potentially relevant studies were fully retrieved, including their citation details, and additional data were obtained from the reference lists of some of the articles selected for critical appraisal.
2.3. Study Selection
All the identified citations were exported into the EndNote version 15.0 reference manager. Two independent reviewers (M.T.B. and E.H.) rigorously screened the titles, abstracts, and the full text of selected literature against the inclusion criteria. The double-check of the included studies was performed by a third reviewer (H.T.A.). Discussions were made among the reviewers to resolve disagreements that arose at each stage of the study selection process.
2.4. Eligibility Criteria
Inclusion Criteria: Observational studies published in SSA, which reported the co-infection of malaria in pregnancy with helminths as their main outcome were eligible for inclusion. Studies published in all languages of SSA until 20 January 2022 were included.
Exclusion Criteria: Systematic reviews, studies with poor methodological quality after, and reports of studies conducted outside SSA were excluded. Studies that employed inappropriate sampling frames, inadequate sample sizes, and poor data analysis were excluded. Studies that reported malaria or helminthic infection alone during pregnancy were also excluded.
2.5. Quality Assessment
The Joanna Briggs Institute’s (JBI) standardised critical appraisal instrument for prevalence studies was used to assess the methodological quality of included studies [88]. The JBI checklist contains nine quality measurement items (Supplementary File S2). Studies scoring 6 and above out of the 9 criteria were considered to have high quality to be included in the meta-analysis (Table 1). Two reviewers (M.T.B. and H.T.A.) independently screened the eligible studies, and a third reviewer (E.H.) was involved to resolve the disagreement. The observed risk of bias in this study is low (93%) (Table 1). Studies that employed appropriate way of sampling procedures, had a clear description of settings and target population, appropriateness and adequacy of subject recruitment, reliability, and validity of methods used for the identification of outcomes of interest that included no co-infected cases (numerator), and a clear description of the study population (denominator) were deemed quality articles for final meta-analysis (Table 2).

Table 1.
Quality assessment of the eligible studies.

Table 2.
Descriptive summary of the eligible studies.
2.6. Data Extraction
Data extraction was principally carried out by two reviewers (M.T.B. and E.H.). The validity and eligibility of the extracted data for the meta-analysis were cross-checked by a third reviewer (H.T.A.). Variables such as the name of the corresponding author and publication year, study design and data collection period, sample size and study setting, the test approaches for the diagnosis of malaria, and helminths were extracted (Table 2). In addition, data extraction tools were used to extract the percentage of infection from Plasmodium falciparum, Hookworm, Ascaris lumbricoides, Trichuris trichiura, Schistosomiasis, the burden of helminths, prevalence of malaria, and malaria–helminthic co-infections, respectively.
2.7. Outcome Measurement
Malaria and helminthic co-infection during the gestation period were considered to occur when a laboratory-confirmed case of at least one Plasmodium and helminth species identified from blood and faecal bio-specimens was obtained from pregnant women [63].
2.8. Statistical Analysis
A quantitative meta-analysis of eligible studies was performed to estimate the event rate (prevalence of malaria–helminthic co-infection during pregnancy) [89]. Based on the random distribution assumption, the prevalence of each disease condition was obtained from the individual study estimate (ES), which includes a standard error (seES) and lower and upper confidence intervals. The pooled estimates were calculated and reported with respect to the relative weight given for each study [90,91]. Egger’s regression test analyses were used to check the publication bias [92]. The standard chi-squared I2 test was used to test heterogeneity [93]. A random-effects model using the double arcsine transformation approach was applied [94]. Decisions made regarding the included studies were checked by sensitivity analyses test. Funnel plot asymmetry visual examination and Egger’s regression tests were used to check for publication bias [95]. The pooled magnitude of co-infection of pregnancy malaria and helminths in SSA were estimated by computing a forest plot with 95%CI. Microsoft Excel 2019 workbook was used for data collection. The meta-analysis was performed using STATA version 14.0.3.
3. Results
3.1. Literature Search
A total of 1525 publications (Figure 1) were obtained from PubMed, CINAHL, EMBASE, Google Scholar, Scopus, and Web of Science databases, after removing 167 duplicates (Supplementary File S1). Following title and abstract screening, a total of 1367 articles were excluded. Furthermore, 27 studies were eligible for quality assessment, out of which 24 studies were included in the meta-analysis (Figure 1).
3.2. Characteristics of Included Studies
A total of 14,087 pregnant women from 24 eligible studies from SSA participated in this systematic review. Studies with the highest (n = 2,507) and lowest (n = 87) sample sizes were reported from Uganda and Nigeria, respectively (Table 2). Only six studies reported data on parity rate with primigravida (n = 1159) and multigravida (n = 1803). Ten studies were reported from Nigeria [96,97,98,99,100,101,102,103,104,105,106], and three studies were from Kenya [69,107,108] and Uganda [109,110,111]. Two studies were reported from Ethiopia [112,113], Gabon [114,115], and Ghana [116,117], respectively. The remaining studies were reported from Malawi [118] and Cameroon [119]. All of the studies included in the review were conducted using cross-sectional study designs (Table 2). The majority of the studies employed the Kato–Katz thick smear, followed by formalin-ether and MacMaster concentration techniques for the detection of helminthic infection from faecal specimens, while the conventional microscopic method was used for the detection of malaria parasites (Table 2). Funnel plot asymmetry visual examination indicated no publication bias (Figure 2).
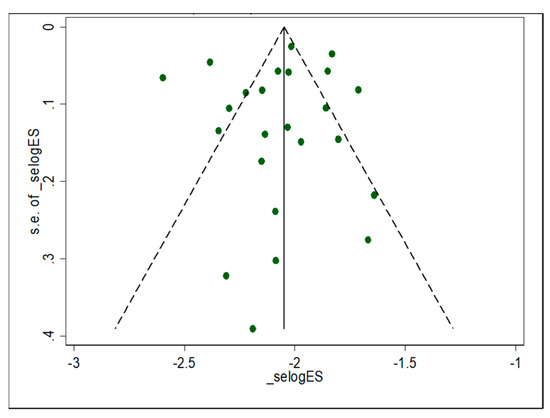
Figure 2.
Funnel plot with pseudo 95% confidence limit of individual study estimates attributed with prevalence of malaria and helminthic co-infection among pregnant women in sub-Saharan Africa.
3.3. Meta-Analysis
3.3.1. The Burden of Malaria Infection
The prevalence of malaria ranges from 4.6% to 36.2% (Table 2). The lowest and the highest pooled prevalence of malaria were 15% (95%CI: 12%, 17%) and 42% (95%CI: 39%, 45%) (Figure 3). The overall pooled prevalence of malaria was 33% (95%CI: 25%, 41%) (Figure 3).
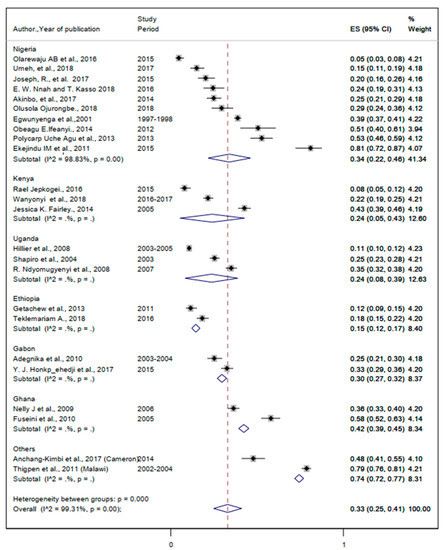
Figure 3.
Forest plot for the overall and country—specific pooled prevalence of malaria among pregnant women in sub—Saharan Africa.
3.3.2. The Burden of Helminthic Infection
The pooled prevalence of helminthiasis was 35% (95%CI: 25%, 45%) (Figure 4). The prevalence of Hookworm infection ranged from 2% to 69% (Table 2). The pooled prevalence of Hookworm infection was 48% (95%CI: 36%, 61%) (Figure 5). The lowest and the highest prevalence of infection from Ascaris lumbricoides were 2% and 75%, respectively (Table 2). The pooled prevalence of Ascaris lumbricoides were 37% (95%CI: 30%, 44%) (Figure 6). The prevalence of Trichuriasis ranged from 1% to 21.4% (Table 2). The pooled prevalence of Trichuris trichiura was 35% (95%CI: 25%, 45%) (Figure 7). Only six studies have descriptively reported the burden of Schistosoma mansoni with the lowest (1.3%) and highest (46.8%) levels (Table 2).
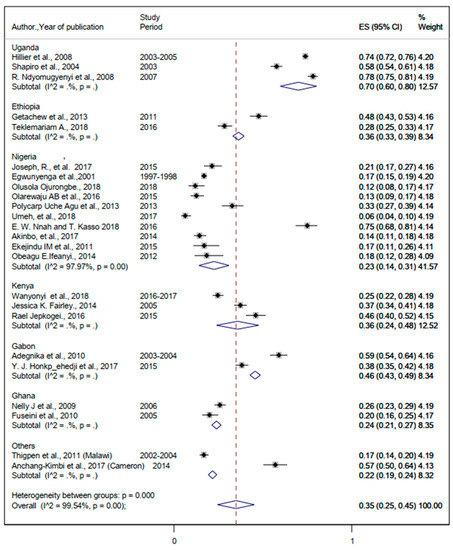
Figure 4.
Forest plot for the overall and country-specific pooled prevalence of helminthic infection among pregnant women in sub-Saharan Africa.
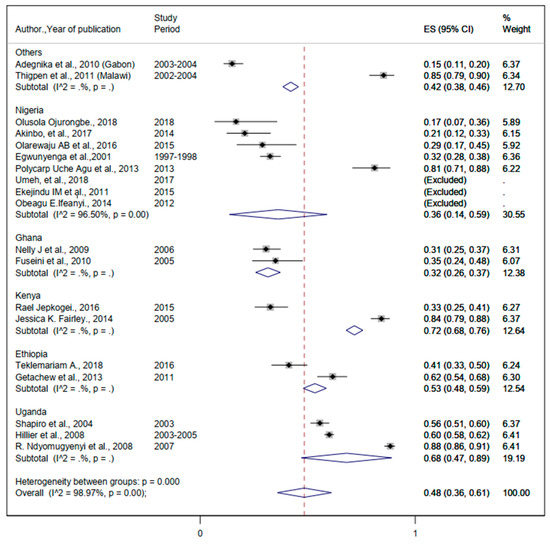
Figure 5.
The proportion of Hookworm estimated from the overall helminthic infection among pregnant women in sub-Saharan Africa.
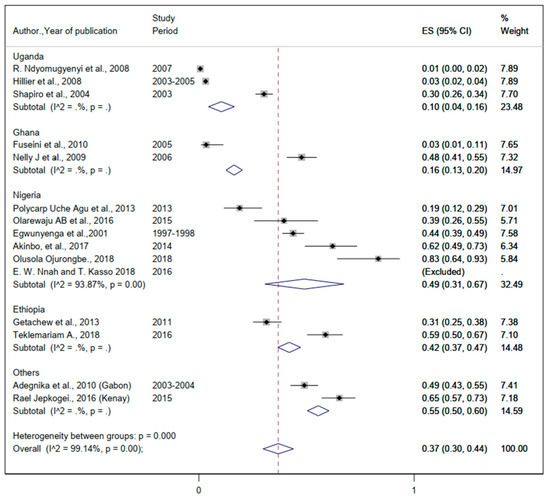
Figure 6.
The proportion of Ascaris lumbricoides estimated from the overall helminthic infection among pregnant women in sub-Saharan Africa.
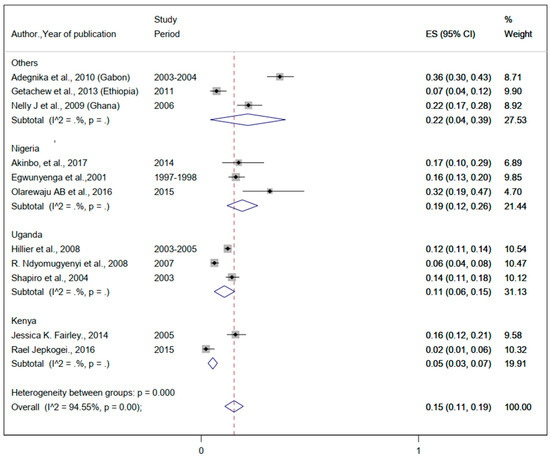
Figure 7.
The proportion of Trichuris trichiura estimated from the overall helminthic infection among pregnant women in sub-Saharan Africa.
3.3.3. The Burden of Malaria and Helminthic Co-Infection
The lowest and the highest prevalence rates of comorbidity with malaria and helminths were 3% and 69% (Table 2). The pooled prevalence of malaria and helminthic co-infection was 20% (95%CI: 15%, 26%) (Figure 8).
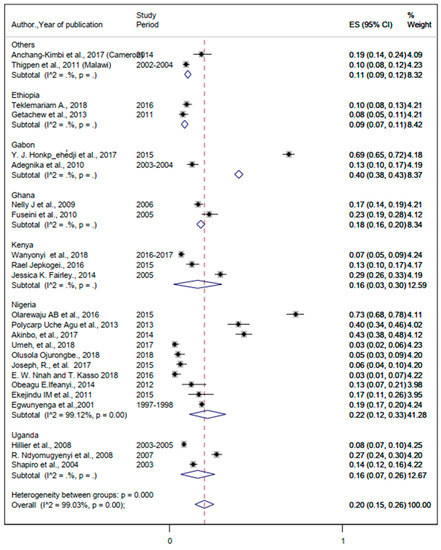
Figure 8.
The overall pooled estimate and country-specific prevalence of malaria and helminthic co-infection among pregnant women in sub-Saharan Africa.
4. Discussion
This study estimated the pooled prevalence of co-infection of malaria and helminths during pregnancy from a total of 24 eligible studies and 14,087 pregnant women in SSA. The pooled prevalence of comorbidity of malaria and helminths among pregnant women in SSA was 20%, ranging from 9% in Ethiopia to 40% in Ghana. The burden of simultaneous infection from Plasmodium and helminthic species among pregnant mothers living in Uganda and Kenya was similar (16%). This could be attributed to the poor implementation of the intermittent preventive treatment of malaria during pregnancy, barriers to access to clean water, and inadequate sanitation in these three countries [120,121,122,123]. To tackle the impact of malaria and helminthic comorbidity on pregnant mothers, the WHO Africa region must establish a malaria data-sharing hub that can serve as a shared evidence-informing centre [124]. This will be a game changer by enabling the health systems in SSA to allocate scarce resources by applying a combination of updated tools for intervention and elimination strategies [125,126,127].
The burden of malaria in the gestational period among women immune-compromised by helminthic infection in SSA was 33%. This finding was higher than those of studies in Colombia (3.4%) [128] and Ethiopia (12.72%) [129]. This implicates the challenges to global malaria elimination efforts and calls for a collective concerted effort from countries in SSA to implement context-specific and tailored, evidence-based malaria elimination interventions [128,129]. Pregnant women’s poor adherence to the use of prescribed prophylactic antimalarial drugs and preventive measures puts strain on the malaria elimination goal [130,131,132,133,134,135,136].
This implies a concerted need to intensify malaria vaccine coverage in SSA to save the lives of pregnant mothers, in addition to having preventive, therapeutic, and control strategies in place to end malaria during pregnancy [137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165]. Countries in SSA must make changes in their malaria elimination strategies by adopting context-specific, home-grown innovative solutions, learning from grassroots experience, and strengthening public-private partnerships [142,143,144,145,146,147,148,149,150,151].
Our review revealed that the pooled prevalence of helminthiasis among pregnant mothers in SSA whose immunity is weakened by malaria was 35%. Uganda had a burden of helminthic infection in pregnancy (70%), which was higher than Cameron and Malawi combined (22%). Hookworm (48%), Ascaris lumbricoides (37%), and Trichuris trichiura (15%), respectively, were the pooled estimates of the most prevalent helminths associated with unintended pregnancy complications in SSA. The findings of our study were higher than those reported as global burden of helminthic infection during pregnancy in terms of the aggregate (3.6%) and species-specific Hookworm (19%), Ascaris lumbricoides (17%), and Trichuris trichiura (11%) [152]. This could be attributed to the inadequate availability of water, sanitation, and hygiene services in SSA, which remains below the global target of 80 % [153,154,155,156]. The prevalence of Schistosoma mansoni and malaria was determined by narrative synthesis because only 6 studies from the eligible 24 articles were reported with (n = 692) pregnant women from five countries in SSA who were co-infected by malaria and Schistosoma mansoni. Only five countries in SSA have (n = 1159) and (n = 1803) pregnant women in primigravid and multigravida who were co-infected by malaria and helminths.
4.1. Optimisation of Anti-Malarial and Anti-Helminthic Infections in Endemic Areas
Although there are universal malaria interventions such as bed nets and access to prompt diagnosis and treatment for pregnant women in malaria-endemic settings, universal access to sanitation and hygiene should be implemented to prevent malaria and helminths co-infection in women of reproductive age and schoolchildren in endemic settings [157]. Moreover, improved diagnostic tools are required to better quantify the burden of malaria–helminth co-infection, as this might help understand the burden of these infections for evidence-based planning and implementation of integrated control and elimination of both malaria and helminthic infections in co-endemic areas [158]. Future malaria vaccine development efforts might also need to understand the immune modulation in malaria–helminth co-infection for better consideration of the effect of the helminth–malaria infection in vaccine immunogenicity [159].
4.2. Ending Preventable Maternal Mortality due to Malarial and Helminthic Co-Infection
The global effort to end the preventable death of the mother caused by the comorbidity of Plasmodium parasitaemia and helminthiasis requires a concerted global health leadership and commitment [160,161]. Sustainable implementation of the water, sanitation, and hygiene (WASH) programs, combined with improving the practice of early initiation of effective intermittent preventive therapy, can avert unintended health consequences as a result of malaria in pregnancy [162,163,164,165,166,167]. Unavailability of a platform for sharing real-time data, poor financing, and inadequate political commitment, coupled with the lack of an enabling and empowering environment to use state-of-the-art technology for the development of anti-malarial and anti-helminthic vaccines in the clinical and biomedical research and innovations in SSA, continue to hinder efforts to bring context-based solutions to achieve SDG3 [168,169,170,171,172,173,174,175,176,177].
4.3. Implications for Practice, Policy, and Future Research and Innovation
Ensuring adequate access and enforcing adherence to safety and hygiene practices among pregnant women and safeguarding gestational mothers from economically disadvantaged households by creating sustainable access to economic opportunities will be essential to meet the global effort to control, prevent, and eliminate helminthic infections in sub-Saharan Africa [178,179,180,181,182,183,184,185,186]. To meet the 2030 target of successful elimination of helminthic infection, health systems in SSA and their international development partners must enhance the capacity and uptake of promising vaccine technology and innovation to improve maternal outcomes following gestational treatment of intestinal nematodes to help guide clinical decision making [187,188,189,190,191,192,193,194,195,196]. Sustainable and inclusive financing must be in place for the cutting-edge research and prudent innovation to deeply investigate the clinical outcomes of immunogenicity of comorbidity of malaria and helminths among gestational mothers in SSA [197,198,199,200,201,202,203,204,205,206]. Given the presence of sub-patent asymptomatic malaria burden that cannot be detected by microscopy [207], and P. falciparum parasites with histidine-rich protein 2 (pfhrp2)and histidine-rich protein 3 (pfhrp3) gene deletions that can escape the current HRP2 based-RDTs detection [208], the estimated burden of malaria- helminth co-infection might be underestimated in these 24 articles. Therefore, future studies that investigate the public health impact of asymptomatic malaria in pregnant women living in helminth co-endemic settings should be undertaken for better policy decision making.
5. Conclusions
Significantly higher levels of malaria and helminthic co-infection during pregnancy were observed. Existing interventions, such as deworming, prioritisation, and distribution of insecticide-treated bed nets and other control measures addressing pregnant women need to be highly encouraged. In addition, health systems strengthening gatekeepers and health policy framers in sub-Saharan Africa must implement home-grown, innovative solutions to underpin context-specific policies and practice for early initiation of effective intermittent preventive therapy for the prevention of malaria in pregnancy. Investments in reverse vaccinology to augment cutting-edge research and innovations in the comorbidity of gestational malaria and helminths through public–private partnerships must be implemented by sub-Saharan African countries and their international development partners. Tailored advocacy on focused antenatal care must be in place to inform and raise awareness among pregnant women regarding the health benefits of universal sanitation and hygiene coverage, together with the effective establishment of integrated community-level early detection and treatment of malaria and helminthic co-infection in sub-Saharan Africa.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph19095444/s1.
Author Contributions
M.T.B., E.H., M.B. and H.T.A. took the principal role in the conception of the idea and research question, developing methodologies, and writing the article. M.T.B., E.H. and H.T.A. were involved in data extraction. B.O.A., M.T.B., M.B., H.T.A., E.H., A.S.C.Y. and K.K. were involved in the analysis and interpretation of the findings. A.T.B., P.C.A., A.S.K., B.O.A., M.T.B. and Z.E.-K. participated in analysis, interpretation, and writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The Armauer Hansen Research Institute for enabling us and granting access to the databases and The Joanna Briggs Institute (JBI), for helping us with access to the databases and development of the review protocol. The Evidence-Based Healthcare Centre at Jimma University in Ethiopia provided us with a comprehensive systematic review training opportunity.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. World Malaria Report 2020—20 Years of Global Progress & Challenges; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Malaria Report. 2020. Available online: https://www.wipo.int/amc/en/ (accessed on 11 March 2022).
- Lubinda, J.; Bi, Y.; Hamainza, B.; Haque, U.; Moore, A.J. Modelling of malaria risk, rates, and trends: A spatiotemporal approach for identifying and targeting sub-national areas of high and low burden. PLoS Comput. Biol. 2021, 17, e1008669. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Report of the WHO Strategic Advisory Group on Malaria Eradication i Malaria Eradication: Benefits, Future Scenarios & Feasibility A Report of the Strategic Advisory Group on Malaria Eradication; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. Guideline WHO Guidelines for Malaria—16 February 2021. Available online: http://apps.who.int/bookorders (accessed on 11 March 2022).
- Kumari, R.; Jayswar, H.; Dhingra, N. High Burden to High Impact (HBHI) approaches—Country perspective for adoption and adaptation in India. J. Commun. Dis. 2020, 52, 5–16. [Google Scholar] [CrossRef]
- World Health Organization. A Strategic Framework for Malaria Prevention and Control During Pregnancy in the African Region; WHO Regional Office for Africa: Brazzaville, Congo, 2004. [Google Scholar]
- World Health Organization. UHC in Africa: A Framework for Action. 2016. Available online: https://apps.who.int/iris/handle/10665/341157 (accessed on 11 March 2022).
- Rogerson, S.J.; Beeson, J.G.; Laman, M.; Poespoprodjo, J.R.; William, T.; Simpson, J.A.; Price, R.N.; Anstey, N.; Fowkes, F.; McCarthy, J.; et al. Identifying and combating the impacts of COVID-19 on malaria. BMC Med. 2020, 18, 239. [Google Scholar] [CrossRef] [PubMed]
- Feleke, S.M.; Reichert, E.N.; Mohammed, H.; Brhane, B.G.; Mekete, K.; Mamo, H.; Petros, B.; Solomon, H.; Abate, E.; Hennelly, C.; et al. Plasmodium falciparum is evolving to escape malaria rapid diagnostic tests in Ethiopia. Nat. Microbiol. 2021, 6, 1289–1299. [Google Scholar] [CrossRef]
- World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021.
- Montresor, A.; Mupfasoni, D.; Mikhailov, A.; Mwinzi, P.; Lucianez, A.; Mekasha, S.; Woyessa, A.; Shafi, O.; Vercruysse, J.; Grimes, J.E.T.; et al. The global progress of soil-transmitted helminthiases control in 2020 and world health organization targets for 2030. PLoS Negl. Trop. Dis. 2020, 14, e0008505. [Google Scholar] [CrossRef]
- Leta, G.T.; Mekete, K.; Wuletaw, Y.; Gebretsadik, A.; Sime, H.; Mekasha, S.; Woyessa, A.; Shafi, O.; Vercruysse, J.; Grimes, J.E.T.; et al. National mapping of soil-transmitted helminth and schistosome infections in Ethiopia. Parasites Vectors 2020, 13, 437. [Google Scholar] [CrossRef]
- van Eijk, A.M.; Lindblade, K.A.; Odhiambo, F.; Peterson, E.; Rosen, D.H.; Karanja, D.; Ayisi, J.G.; Shi, Y.P.; Adazu, K.; Slutsker, L. Geohelminth infections among pregnant women in rural western Kenya: A cross-sectional study. PLoS Negl. Trop. Dis. 2009, 3, e370. [Google Scholar] [CrossRef]
- Bolka, A.; Gebremedhin, S. Prevalence of intestinal parasitic infection and its association with anemia among pregnant women in Wondo Genet district, Southern Ethiopia: A cross-sectional study. BMC Infect. Dis. 2019, 19, 483. [Google Scholar] [CrossRef]
- Adam, I.; ALhabardi, N.A.; Al-Wutayd, O.; Khamis, A.H. Prevalence of schistosomiasis and its association with anemia among pregnant women: A systematic review and meta-analysis. Parasites Vectors 2021, 14, 133. [Google Scholar] [CrossRef]
- Kamau, A.; Mogeni, P.; Okiro, E.A.; Snow, R.W.; Bejon, P. A systematic review of changing malaria disease burden in sub-Saharan Africa since 2000: Comparing model predictions and empirical observations. BMC Med. 2020, 18, 94. [Google Scholar] [CrossRef]
- Moore, K.A.; Fowkes, F.J.I.; Wiladphaingern, J.; Wai, N.S.; Paw, M.K.; Pimanpanarak, M.; Carrara, V.I.; Raksuansak, J.; Simpson, J.A.; White, N.J.; et al. Mediation of the effect of malaria in pregnancy on stillbirth and neonatal death in an area of low transmission: Observational data analysis. BMC Med. 2017, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Singh, S.; Mewara, A. Diagnostic Techniques for Soil-Transmitted Helminths—Recent Advances. Res. Rep. Trop. Med. 2021, 12, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Bangert, M.; Bancalari, P.; Mupfasoni, D.; Mikhailov, A.; Gabrielli, A.F.; Montresor, A. Provision of deworming intervention to pregnant women by antenatal services in countries endemic for soil-transmitted helminthiasis. PLoS Negl. Trop. Dis. 2019, 13, e0007406. [Google Scholar] [CrossRef] [PubMed]
- Makaula, P.; Sadalaki, J.R.; Muula, A.S.; Kayuni, S.; Jemu, S.; Bloch, P. Schistosomiasis in Malawi: A systematic review. Parasites Vectors 2014, 7, 570. [Google Scholar] [CrossRef]
- Mwinnyaa, G.; Hazel, E.; Maïga, A.; Amouzou, A. Estimating population-based coverage of reproductive, maternal, newborn, and child health (RMNCH) interventions from health management information systems: A comprehensive review. BMC Health Serv. Res. 2021, 21, 1083. [Google Scholar] [CrossRef]
- Ferreira, L.Z.; Blumenberg, C.; Utazi, C.E.; Nilsen, K.; Hartwig, F.P.; Tatem, A.J.; Barros, A.J.D. Geospatial estimation of reproductive, maternal, newborn and child health indicators: A systematic review of methodological aspects of studies based on household surveys. Int. J. Health Geogr. 2020, 19, 41. [Google Scholar] [CrossRef]
- Rios Quituizaca, P.; Gatica-Domínguez, G.; Nambiar, D.; Ferreira Santos, J.L.; Brück, S.; Ruas, L.V.; Barros, A.J.D. National and subnational coverage and inequalities in reproductive, maternal, newborn, child, and sanitary health interventions in Ecuador: A comparative study between 1994 and 2012. Int. J. Equity Health 2021, 20, 48. [Google Scholar] [CrossRef]
- Boerma, T.; Requejo, J.; Victora, C.G.; Amouzou, A.; George, A.; Taylor, C.M.; Amouzou, A.; Jiwani, S.S.; da Silva, I.C.M.; Sidze, E.M.; et al. Countdown to 2030: Tracking progress towards universal coverage for reproductive, maternal, newborn, and child health. Lancet 2018, 391, 1538–1548. [Google Scholar] [CrossRef]
- Faye, C.M.; Wehrmeister, F.C.; Melesse, D.Y.; Mutua, M.K.K.; Maïga, A.; Taylor, C.M.; Amouzou, A.; Jiwani, S.S.; da Silva, I.C.M.; Sidze, E.M.; et al. Large and persistent subnational inequalities in reproductive, maternal, newborn and child health intervention coverage in sub-Saharan Africa. BMJ Glob. Health 2020, 5, e002232. [Google Scholar] [CrossRef]
- Barros, A.J.D.; Wehrmeister, F.C.; Ferreira, L.Z.; Vidaletti, L.P.; Hosseinpoor, A.R.; Victora, C.G. Are the poorest poor being left behind? Estimating global inequalities in reproductive, maternal, newborn and child health. BMJ Glob. Health 2020, 5, e002229. [Google Scholar] [CrossRef]
- Molyneux, D.H.; Lindsay, S.W.; Fitzpatrick, C.; Engels, D. The cross-cutting contribution of the end of neglected tropical diseases to the sustainable development goals. Infect. Dis. Poverty 2017, 6, 73. [Google Scholar]
- WHO. Monitoring the Health-Related Sustainable Development Goals (SDGs); WHO: Geneva, Switzerland, 2017; pp. 9–10. [Google Scholar]
- Bakken, L.; Iversen, P.O. The impact of malaria during pregnancy on low birth weight in East-Africa: A topical review. Malar. J. 2021, 20, 348. [Google Scholar] [CrossRef] [PubMed]
- Chetty, A.; Omondi, M.A.; Butters, C.; Smith, K.A.; Katawa, G.; Ritter, M.; Layland, L.; Horsnell, W. Impact of Helminth Infections on Female Reproductive Health and Associated Diseases. Front. Immunol. 2020, 11, 577516. [Google Scholar] [CrossRef] [PubMed]
- Animaw, Z.; Melese, A.; Demelash, H.; Seyoum, G.; Abebe, A. Intestinal parasitic infections and associated factors among pregnant women in Ethiopia: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2021, 21, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Demeke, G.; Mengistu, G.; Abebaw, A.; Toru, M.; Yigzaw, M.; Shiferaw, A.; Mengist, H.M.; Dilnessa, T. Effects of intestinal parasite infection on hematological profiles of pregnant women attending antenatal care at Debre Markos Referral Hospital, Northwest Ethiopia: Institution based prospective cohort study. PLoS ONE 2021, 16, e0250990. [Google Scholar] [CrossRef]
- Brooker, S.; Hotez, P.J.; Bundy, D.A.P. Hookworm-related anaemia among pregnant women: A systematic review. PLoS Negl. Trop. Dis. 2008, 2, e291. [Google Scholar] [CrossRef]
- Mosawi, S.H.; Dalimi, A.; Charkhi, M.A.; Baarae, O.; Darman, A.; Mosavi, M.; Baryal, M.W.; Stanikzai, H. Gallbladder perforation due to Ascaris lumbricoides in a pregnant woman and 6 year old girl from afghanistan: Case report. Iran. J. Parasitol. 2019, 14, 477–481. [Google Scholar]
- Righetti, A.A.; Glinz, D.; Adiossan, L.G.; Koua, A.Y.G.; Niamké, S.; Hurrell, R.F.; Wegmuller, R.; N’Goran, E.K.; Utzinger, J. Interactions and Potential Implications of Plasmodium falciparum-Hookworm Coinfection in Different Age Groups in South-Central Côte d’Ivoire. PLoS Negl. Trop. Dis. 2012, 6, e1889. [Google Scholar] [CrossRef]
- Tuasha, N.; Hailemeskel, E.; Erko, B.; Petros, B. Comorbidity of intestinal helminthiases among malaria outpatients of Wondo Genet health centers, southern Ethiopia: Implications for integrated control. BMC Infect. Dis. 2019, 19, 659. [Google Scholar] [CrossRef]
- Hirst, J.; Villar, J.; Victora, C.; Papageorghiou, A.; Finkton, D.; Barros, F.; Gravett, M.; Giuliani, F.; Purwar, M.; Frederick, I.; et al. The antepartum stillbirth syndrome: Risk factors and pregnancy conditions identified from the INTERGROWTH-21 st Project. BJOG Int. J. Obstet. Gynaecol. 2018, 125, 1145–1153. [Google Scholar] [CrossRef]
- Boel, M.; Carrara, V.I.; Rijken, M.; Proux, S.; Nacher, M.; Pimanpanarak, M.; Paw, M.K.; Moo, O.; Gay, H.; Bailey, W.; et al. Complex interactions between soil-transmitted helminths and malaria in pregnant women on the thai-burmese border. PLoS Negl. Trop. Dis. 2010, 4, e887. [Google Scholar] [CrossRef] [PubMed]
- Adam, I.; Salih, M.M.; Mohmmed, A.A.; Rayis, D.A.; Elbashir, M.I. Pregnant women carrying female fetuses are at higher risk of placental malaria infection. PLoS ONE 2017, 12, e0182394. [Google Scholar] [CrossRef] [PubMed]
- Mpairwe, H.; Tweyongyere, R.; Elliott, A. Pregnancy and helminth infections. Parasite Immunol. 2014, 36, 328–337. [Google Scholar] [CrossRef]
- Ndibazza, J.; Webb, E.L.; Lule, S.; Mpairwe, H.; Akello, M.; Oduru, G.; Kizza, M.; Akurut, H.; Muhangi, L.; Magnussen, P.; et al. Associations between maternal helminth and malaria infections in pregnancy and clinical malaria in the offspring: A birth cohort in Entebbe, Uganda. J. Infect. Dis. 2013, 208, 2007–2016. [Google Scholar] [CrossRef] [PubMed]
- Alarcón de Noya, B.; Ruiz Guevara, R. Pregnancy as a risk factor to disease and the vertical transmission to the fetus, of a host of parasitic ailments. CientMed 2020, 1, 1–16. [Google Scholar] [CrossRef]
- Maestre, A.; Carmona-Fonseca, J. Immune responses during gestational malaria: A review of the current knowledge and future trend of research. J. Infect. Dev. Ctries. 2014, 8, 391–402. [Google Scholar] [CrossRef]
- Wilairatana, P.; Mahannop, P.; Tussato, T.; Hayeedoloh, I.M.; Boonhok, R.; Klangbud, W.K.; Mala, W.; Kotepui, K.U.; Kotepui, M. C-reactive protein as an early biomarker for malaria infection and monitoring of malaria severity: A meta-analysis. Sci. Rep. 2021, 11, 22033. [Google Scholar] [CrossRef]
- Sarfo, B.O.; Hahn, A.; Schwarz, N.G.; Jaeger, A.; Sarpong, N.; Marks, F.; Adu-Sarkodie, Y.; Tamminga, T.; May, J. The usefulness of c-reactive protein in predicting malaria parasitemia in a sub-saharan african region. PLoS ONE 2018, 13, e0201693. [Google Scholar] [CrossRef]
- Abrams, E.T.; Kwiek, J.J.; Mwapasa, V.; Kamwendo, D.D.; Tadesse, E.; Lema, V.M.; Molyneux, M.E.; Rogerson, S.J.; Meshnick, S.R. Malaria during pregnancy and foetal haematological status in Blantyre, Malawi. Malar. J. 2005, 4, 39. [Google Scholar] [CrossRef]
- Sharma, L.; Shukla, G. Placental Malaria: A new insight into the pathophysiology. Front. Med. 2017, 4, 117. [Google Scholar] [CrossRef]
- Brabin, B.; Tinto, H.; Roberts, S.A. Testing an infection model to explain excess risk of preterm birth with long-term iron supplementation in a malaria endemic area. Malar. J. 2019, 18, 374. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Koski, K.G.; Scott, M.E. Maternal Gastrointestinal Nematode Infection Up-regulates Expression of Genes Associated with Long-Term Potentiation in Perinatal Brains of Uninfected Developing Pups. Sci. Rep. 2019, 9, 4165. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Baisley, K.; Kavishe, B.; Changalucha, J.; Andreasen, A.; Mayaud, P.; Gumodoka, B.; Kapiga, S.; Hayes, R.; Watson-Jones, D. Impact of malaria and helminth infections on immunogenicity of the human papillomavirus-16/18 AS04-adjuvanted vaccine in Tanzania. Vaccine 2014, 32, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Mabbott, N.A. The Influence of Parasite Infections on Host Immunity to Co-infection with Other Pathogens. Front. Immunol. 2018, 9, 2579. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Rodolfo, R.; Akudibillah, G.; Dusabimana, A.; Harmon, S.; Mabeya, H. Effects of malaria/helminthic coinfections on cervical cancer progression among sub Saharan African women on highly active antiretroviral therapy: A scoping review. Gynecol. Oncol. Rep. 2019, 29, 64–69. [Google Scholar] [CrossRef]
- Mwangi, T.W.; Bethony, J.M.; Brooker, S. Malaria and helminth interactions in humans: An epidemiological viewpoint. Ann. Trop. Med. Parasitol. 2006, 100, 551–570. [Google Scholar] [CrossRef]
- Boltena, M.T.; El-Khatib, Z.; Kebede, A.S.; Asamoah, B.O.; Boltena, A.T.; Yeshambaw, M.; Biru, M. Comorbidity of geo-helminthes among malaria outpatients of the health facilities in Ethiopia: Systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 862. [Google Scholar] [CrossRef]
- Degarege, A.; Erko, B. Epidemiology of Plasmodium and helminth coinfection and possible reasons for heterogeneity. BioMed Res. Int. 2016, 2016, 3083568. [Google Scholar] [CrossRef]
- Makenga, G.; Menon, S.; Baraka, V.; Minja, D.T.R.; Nakato, S.; Delgado-Ratto, C.; Francis, F.; Lusingu, J.P.; Van Geertruyden, J.-P. Prevalence of malaria parasitaemia in school-aged children and pregnant women in endemic settings of sub-Saharan Africa: A systematic review and meta-analysis. Parasite Epidemiol. Control 2020, 11, e00188. [Google Scholar] [CrossRef]
- Karshima, S.N. Prevalence and distribution of soil-transmitted helminth infections in Nigerian children: A systematic review and meta-analysis. Infect. Dis. Poverty 2018, 7, 1118–1132. [Google Scholar] [CrossRef]
- Bahati, Y.L.; Delanghe, J.; Balaluka, G.B.; Kishabongo, A.S.; Philippé, J. Asymptomatic submicroscopic Plasmodium infection is highly prevalent and is associated with anemia in children younger than 5 years in South Kivu/Democratic Republic of Congo. Am. J. Trop. Med. Hyg. 2020, 102, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Degarege, A.; Veledar, E.; Degarege, D.; Erko, B.; Nacher, M.; Madhivanan, P. Plasmodium falciparum and soil-transmitted helminth co-infections among children in sub-Saharan Africa: A systematic review and meta-analysis. Parasites Vectors 2016, 9, 344. [Google Scholar] [CrossRef] [PubMed]
- Osakunor, D.N.M.; Sengeh, D.M.; Mutapi, F. Coinfections and comorbidities in African health systems: At the interface of infectious and noninfectious diseases. PLoS Negl. Trop. Dis. 2018, 12, e0006711. [Google Scholar] [CrossRef] [PubMed]
- Snow, R.W. Global malaria eradication and the importance of Plasmodium falciparum epidemiology in Africa. BMC Med. 2015, 13, 23. [Google Scholar] [CrossRef]
- Eisele, T.P.; Larsen, D.A.; Anglewicz, P.A.; Keating, J.; Yukich, J.; Bennett, A.; Hutchinson, P.; Steketee, R.W. Malaria prevention in pregnancy, birthweight, and neonatal mortality: A meta-analysis of 32 national cross-sectional datasets in Africa. Lancet Infect. Dis. 2012, 12, 942–949. [Google Scholar] [CrossRef]
- Carrasco-Escobar, G.; Fornace, K.; Benmarhnia, T. Mapping socioeconomic inequalities in malaria in Sub-Sahara African countries. Sci. Rep. 2021, 11, 15121. [Google Scholar] [CrossRef]
- Osungbade, K.O.; Oladunjoye, O.O. Prevention of congenital transmission of malaria in sub-Saharan African Countries: Challenges and implications for health system strengthening. J. Trop. Med. 2012, 2012, 648456. [Google Scholar] [CrossRef][Green Version]
- Zeukeng, F.; Tchinda, V.H.M.; Bigoga, J.D.; Seumen, C.H.T.; Ndzi, E.S.; Abonweh, G.; Makoge, V.; Motsebo, A.; Moyou, R.S. Co-infections of Malaria and Geohelminthiasis in Two Rural Communities of Nkassomo and Vian in the Mfou Health District, Cameroon. PLoS Negl. Trop. Dis. 2014, 8, e3236. [Google Scholar] [CrossRef]
- Mahande, A.M.; Mahande, M.J. Prevalence of parasitic infections and associations with pregnancy complications and outcomes in northern Tanzania: A registry-based cross-sectional study. BMC Infect. Dis. 2016, 16, 78. [Google Scholar] [CrossRef]
- Fourie, C. The trouble with inequalities in global health partnerships. Med. Anthr. Theory 2018, 5, 142–155. [Google Scholar] [CrossRef]
- Accrombessi, M.; Issifou, S. Malaria control and elimination in sub-Saharan Africa: Data from antenatal care centres. Lancet Glob. Health 2019, 7, e1595–e1596. [Google Scholar] [CrossRef]
- Zerbo, A.; Castro Delgado, R.; Arcos González, P. Water sanitation and hygiene in Sub-Saharan Africa: Coverage, risks of diarrheal diseases, and urbanization. J. Biosaf. Biosecur. 2021, 3, 41–45. [Google Scholar] [CrossRef]
- European Union. Tackling Infectious Disease in Sub-Saharan Africa; European Union: Maastricht, The Netherlands, 2018. [Google Scholar]
- Gosling, R.; Chimumbwa, J.; Uusiku, P.; Rossi, S.; Ntuku, H.; Harvard, K.; White, C.; Tatarsky, A.; Chandramohan, D.; Chen, I. District-level approach for tailoring and targeting interventions: A new path for malaria control and elimination. Malar. J. 2020, 19, 125. [Google Scholar] [CrossRef] [PubMed]
- Kayentao, K.; Garner, P.; van Eijk, A.M.; Naidoo, I.; Roper, C.; Mulokozi, A.; MacArthur, J.R.; Luntamo, M.; Ashorn, P.; Doumbo, O.K.; et al. Intermittent preventive therapy for malaria during pregnancy using 2 vs. 3 or more doses of sulfadoxine-pyrimethamine and risk of low birth weight in Africa: Systematic review and meta-analysis. JAMA J. Am. Med. Assoc. 2013, 309, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, J.; Arku, R.E.; Baumgartner, J.; Brown, J.; Clasen, T.; Eisenberg, J.N.; Hovmand, P.; Jagger, P.; Luke, D.A.; Quinn, A.; et al. Systems science approaches for global environmental health research: Enhancing intervention design and implementation for household air pollution (hap) and water, sanitation, and hygiene (wash) programs. Environ. Health Perspect. 2020, 128, 105001. [Google Scholar] [CrossRef]
- Haque, S.S.; Freeman, M.C. Erratum: ‘The Applications of Implementation Science in Water, Sanitation, and Hygiene (WASH) Research and Practice’. Environ. Health Perspect. 2021, 129, 89001. [Google Scholar] [CrossRef]
- Walker, M.; Freitas, L.T.; Halder, J.B.; Brack, M.; Keiser, J.; King, C.H.; Levecke, B.; Lim, Y.A.-L.; Pieri, O.; Sow, D.; et al. Improving anthelmintic treatment for schistosomiasis and soil-transmitted helminthiases through sharing and reuse of individual participant.-Lata [version 1; peer review: Awaiting peer review]. Wellcome Open Res. 2022, 7, 5. [Google Scholar] [CrossRef]
- Sanders, D.M.; Todd, C.; Chopra, M. Confronting Africa’s health crisis: More of the same will not be enough. Br. Med. J. 2005, 331, 755–758. [Google Scholar] [CrossRef][Green Version]
- Juma, P.A.; Jones, C.M.; Mijumbi-Deve, R.; Wenham, C.; Masupe, T.; Sobngwi-Tambekou, J.; Biemba, G.; Mtombo, N.; Parkhurst, J. Governance of health research in four eastern and southern African countries. Health Res. Policy Syst. 2021, 19, 132. [Google Scholar] [CrossRef]
- Azevedo, M.J. Historical Perspectives on the State of Health and Health Systems in Africa; Palgrave Macmillan: London, UK, 2017; Volume II. [Google Scholar] [CrossRef]
- Oleribe, O.O.; Momoh, J.; Uzochukwu, B.S.C.; Mbofana, F.; Adebiyi, A.; Barbera, T.; Williams, R.; Robinson, S.D.T. Identifying key challenges facing healthcare systems in Africa and potential solutions. Int. J. Gen. Med. 2019, 12, 395–403. [Google Scholar] [CrossRef]
- Nabyonga-Orem, J.; Asamani, J.A.; Makanga, M. The state of health research governance in Africa: What do we know and how can we improve? Health Res. Policy Syst. 2021, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Kasprowicz, V.O.; Chopera, D.; Waddilove, K.D.; Brockman, M.A.; Gilmour, J.; Hunter, E.; Kilembe, W.; Karita, E.; Gaseitsiwe, S.; Sanders, E.J.; et al. African-led health research and capacity building—Is it working? BMC Public Health 2020, 20, 1104. [Google Scholar] [CrossRef] [PubMed]
- Bakibinga, P.; Kamande, E.; Kisia, L.; Omuya, M.; Matanda, D.J.; Kyobutungi, C. Challenges and prospects for implementation of community health volunteers’ digital health solutions in Kenya: A qualitative study. BMC Health Serv. Res. 2020, 20, 888. [Google Scholar] [CrossRef] [PubMed]
- Manyazewal, T.; Woldeamanuel, Y.; Blumberg, H.M.; Fekadu, A.; Marconi, V.C. The potential use of digital health technologies in the African context: A systematic review of evidence from Ethiopia. NPJ Digit. Med. 2021, 4, 125. [Google Scholar] [CrossRef]
- Beyene, J.; Harrar, S.W.; Altaye, M.; Astatkie, T.; Awoke, T.; Shkedy, Z.; Mersha, T.B. A Roadmap for Building Data Science Capacity for Health Discovery and Innovation in Africa. Front. Public Health 2021, 9, 1435. [Google Scholar] [CrossRef]
- Bader, E.; Alhaj, A.M.; Hussan, A.A.; Adam, I. Malaria and stillbirth in Omdurman Maternity Hospital, Sudan. Int. J. Gynecol. Obstet. 2010, 109, 144–146. [Google Scholar] [CrossRef]
- Moore, K.A.; Simpson, J.A.; Scoullar, M.J.L.; McGready, R.; Fowkes, F.J.I. Quantification of the association between malaria in pregnancy and stillbirth: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1101–e1112. [Google Scholar] [CrossRef]
- Say, L.; Donner, A.; Gülmezoglu, A.M.; Taljaard, M.; Piaggio, G. The prevalence of stillbirths: A systematic review. Reprod. Health 2006, 3, 1. [Google Scholar] [CrossRef]
- Global Technical Strategy for Malaria 2016–2030; 2021 update; World Health Organization: Geneva, Switzerland, 2021.
- Buxton, M.; Machekano, H.; Gotcha, N.; Nyamukondiwa, C.; Wasserman, R.J. Are vulnerable communities thoroughly informed on mosquito bio-ecology and burden? Int. J. Environ. Res. Public Health 2020, 17, 8196. [Google Scholar] [CrossRef]
- Tegegne, Y.; Worede, A.; Derso, A.; Ambachew, S. The Prevalence of Malaria among Children in Ethiopia: A Systematic Review and Meta-Analysis. J. Parasitol. Res. 2021, 2021, 6697294. [Google Scholar] [CrossRef]
- Imhoff-Kunsch, B.; Briggs, V. Antihelminthics in pregnancy and maternal, newborn and child health. Paediatr. Perinat. Epidemiol. 2012, 26, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Barua, P.; Beeson, J.G.; Maleta, K.; Ashorn, P.; Rogerson, S.J. The impact of early life exposure to Plasmodium falciparum on the development of naturally acquired immunity to malaria in young Malawian children. Malar. J. 2019, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Hartgers, F.C.; Yazdanbakhsh, M. Co-infection of helminths and malaria: Modulation of the immune responses to malaria. Parasite Immunol. 2006, 28, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Fairley, J.K.; Bisanzio, N.; King, C.H.; Kitron, U.; Mungai, P.; Muchiri, E.; King, C.L.; Malhotra, I. Birthweight in offspring of mothers with high prevalence of helminth and malaria infection in coastal Kenya. Am. J. Trop. Med. Hyg. 2013, 88, 48–53. [Google Scholar] [CrossRef]
- Woolhouse, M.E.J.; Thumbi, S.M.; Jennings, A.; Chase-Topping, M.; Callaby, R.; Kiara, H.; Oosthuizen, M.C.; Mbole-Kariuki, M.N.; Conradie, I.; Handel, I.G.; et al. Co-infections determine patterns of mortality in a population exposed to parasite infection. Sci. Adv. 2015, 1, e1400026. [Google Scholar] [CrossRef]
- Singer, M. Development, coinfection, and the syndemics of pregnancy in sub-Saharan Africa. Infect. Dis. Poverty 2013, 2, 26. [Google Scholar] [CrossRef]
- Wolday, D.; Gebrecherkos, T.; Arefaine, Z.G.; Kiros, Y.K.; Gebreegzabher, A.; Tasew, G.; Abdulkader, M.; Abraha, H.E.; Desta, A.A.; Hailu, A.; et al. Effect of co-infection with intestinal parasites on COVID-19 severity: A prospective observational cohort study. EClinicalMedicine 2021, 39, 101054. [Google Scholar] [CrossRef]
- Candela, E.; Goizueta, C.; Periago, M.V.; Muñoz-Antoli, C. Prevalence of intestinal parasites and molecular characterization of Giardia intestinalis, Blastocystis spp. and Entamoeba histolytica in the village of Fortín Mbororé (Puerto Iguazú, Misiones, Argentina). Parasites Vectors 2021, 14, 510. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 51, 264–269. [Google Scholar] [CrossRef]
- NIHR. International Prospective Register of Systematic Reviews Registering a Systematic Review on PROSPERO What Does Registration on PROSPERO Involve? Inclusion Criteria When to Register Your Review PROSPERO International Prospective Register of Systematic Rev; NIHR: Southampton, UK, 2019; pp. 1–12. [Google Scholar]
- Tawfik, G.M.; Dila, K.A.S.; Mohamed, M.Y.F.; Tam, D.N.H.; Kien, N.D.; Ahmed, A.M.; Huy, N.T. A step by step guide for conducting a systematic review and meta-analysis with simulation data. Trop. Med. Health 2019, 47, 46. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 2020–2021. [Google Scholar]
- Joanna Briggs Institute. Checklist for Prevalence Studies; Joanna Briggs Institute: Adelaide, Australia, 2016; p. 7. [Google Scholar]
- Naing, C.; Whittaker, M.; Nyunt-Wai, V.; Reid, S.; Wong, S.F.; Mak, J.W.; Tanner, M. Malaria and soil-transmitted intestinal helminth co-infection and its effect on anemia: A meta-analysis. Trans. R. Soc. Trop. Med. Hyg. 2013, 107, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Pei, Q.; Qiao, H.; Zhang, M.; Wang, G.; Feng, H.; Pan, J.; Shi, Y. Pocket-creation method versus conventional method of endoscopic submucosal dissection for superficial colorectal neoplasms: A meta-analysis. Gastrointest. Endosc. 2021, 93, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.Y.; Dahm, P.; Poolman, R.W.; Preminger, G.M.; Canales, B.J.; Montori, V.M. How to Use a Systematic Literature Review and Meta-Analysis. J. Urol. 2008, 180, 1249–1256. [Google Scholar] [CrossRef]
- Ahn, E.; Kang, H. Introduction to systematic review and meta-analysis. Korean J. Anesthesiol. 2018, 71, 103–112. [Google Scholar] [CrossRef]
- Phillips, C.V. Publication bias in situ. BMC Med. Res. Methodol. 2004, 4, 20. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. Interpretation of tests of heterogeneity and bias in meta-analysis. J. Eval. Clin. Pract. 2008, 14, 951–957. [Google Scholar] [CrossRef]
- Lin, L.; Xu, C. Arcsine-based transformations for meta-analysis of proportions: Pros, cons, and alternatives. Health Sci. Rep. 2020, 3, e178. [Google Scholar] [CrossRef]
- Copas, J.B.; Shi, J.Q. A sensitivity analysis for publication bias in systematic reviews. Stat. Methods Med. Res. 2001, 10, 251–265. [Google Scholar] [CrossRef]
- Joseph, R.; Chessed, G.; Daniel, L.; Haruna, Y.; Demshemino, M.; Bagula, R. Prevalence of malaria and geohelminth co-infection among antenatal women at the Federal Medical Centre and Specialist Hospital, Yola, Adamawa Sate, Nigeria. J. Appl. Sci. Environ. Manag. 2017, 21, 469. [Google Scholar] [CrossRef][Green Version]
- Egwunyenga, A.; Ajayi, J.; Nmorsi, O.; Duhlinska-Popova, D. Plasmodium/intestinal Helminth Co-infections among Pregnant Nigerian Women. Memórias Do Inst. Oswaldo Cruz. 2001, 96, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Ojurongbe, O.; Okorie, P.N.; Opatokun, R.L.; Ojurongbe, T.A.; Mabayoje, V.O.; Olowe, O.A.; Adeyeba, O.A. Prevalence and associated factors of Plasmodium falciparum and soil transmitted helminth infections among pregnant women in Osun state, Nigeria. Afr. Health Sci. 2018, 18, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Babamale, O.A.; Shittu, O.; Danladi, Y.K.; Abdulraheem, J.Y.; Ugbomoiko, U.S. Pattern of Plasmodium-intestinal helminth co-infection among pregnant women in a high transmission zone of malaria in Nigeria. Asian Pac. J. Trop. Dis. 2016, 6, 424–428. [Google Scholar] [CrossRef]
- Agu, P.U.; Ogboi, J.S.; Akpoigbe, K.; Okeke, T.; Ezugwu, E. Impact of plasmodium falciparum and hookworm infections on the frequency of anaemia in pregnant women of rural communities in Enugu, South East Nigeria. Pan Afr. Med. J. 2013, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Umeh, J.C.; Inyang-Etoh, P.C.; Okpokam, D.C.; Otu-Bassey, I.B. Malaria and hookworm co-infection and their effects on anaemia of pregnant women attending ante-natal clinic in University Teaching Hospital, Calabar, Nigeria. Asian J. Med. Sci. 2018, 9, 27–35. [Google Scholar] [CrossRef]
- Nnah, E.; Kasso, T. The Prevalence of Malaria and Helminth Infection in Pregnancy at Booking and Their Relationship to Anaemia at the University of Port Harcourt Teaching Hospital, Southern Nigeria. Int. J. Trop. Dis. Health 2018, 28, 1–9. [Google Scholar] [CrossRef]
- Oyefabi, A.; Adetiba, E.; Leeshak, E.A.O. Origina l Artic l e Co-infection of malaria and intestinal parasites among pregnant women in Edo State, Nigeria ABSTRACT. J. Med. Trop. 2019, 19, 116–122. [Google Scholar] [CrossRef]
- Ekejindu, I.M.; Akah, B.; Okpala, E.C.; Onwurah, O. Malaria and Hookworm Co-Infection among Pregnant and Non-Pregnant Women in a Semi-Urban Area in Anambra State, Nigeria. J. Med. Sci. 2011, 6, 33–35. [Google Scholar]
- Ifeanyi, O.E.; Chibunna, O.M.; Braxton, N.A.Q.; Uche, E.C. Impact of Plasmodium falciparum malaria and hookworm infection on anaemia among pregnant women of ikwuano local government area, Abia state, Nigeria. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 104–111. [Google Scholar]
- Wanyonyi, W.A.; Mulambalah, C.S.; Mulama, D.H.; Omukunda, E.; Siteti, D.I. Malaria and Geohelminthiasis Coinfections in Expectant Women: Effect on Maternal Health and Birth Outcomes in a Malaria Endemic Region in Kenya. J. Parasitol. Res. 2018, 2018, 2613484. [Google Scholar] [CrossRef]
- Jepkogei, M.R.; Moses, N.; Judith, M. Socio-Demographic Factors Associated with Malaria-Geohelminth Co-Infection and Syndemics in Pregnancy: A Cross Sectional Study of Pregnant Women Attending Ante Natal Care at Nandi Hills Sub County Hospital, Kenya. Available online: www.iiste.org (accessed on 9 March 2022).
- Hillier, S.D.; Booth, M.; Muhangi, L.; Nkurunziza, P.; Khihembo, M.; Kakande, M.; Sewankambo, M.; Kizindo, R.; Kizza, M.; Muwanga, M.; et al. Plasmodium falciparum and helminth coinfection in a semiurban population of pregnant women in Uganda. J. Infect. Dis. 2008, 198, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Ndyomugyenyi, R.; Kabatereine, N.; Olsen, A.; Magnussen, P. Malaria and hookworm infections in relation to haemoglobin and serum ferritin levels in pregnancy in Masindi district, western Uganda. Trans. R Soc. Trop. Med. Hyg. 2008, 102, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.E.; Tukahebwa, E.M.; Kasten, J.; Clarke, S.E.; Magnussen, P.; Olsen, A.; Kabatereine, N.B.; Ndyomugyenyi, R.; Brooker, S. Epidemiology of helminth infections and their relationship to clinical malaria in southwest Uganda. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Getachew, M.; Tafess, K.; Zeynudin, A.; Yewhalaw, D. Prevalence Soil Transmitted Helminthiasis and malaria co-infection among pregnant women and risk factors in Gilgel Gibe dam Area, Southwest Ethiopia. BMC Res. Note 2013, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Teklemariam, A.; Alemseged, M.; Adugna, S. Malaria-intestinal helminthes co-infection among patients in Wolkite Health Center and Attat Hospital, Gurage Zone, Southern Ethiopia. J. Parasitol. Vector Biol. 2018, 10, 26–32. [Google Scholar]
- Adegnika, A.A.; Ramharter, M.; Agnandji, S.T.; Ngoa, U.A.; Issifou, S.; Yazdanbahksh, M.; Kremsner, P.G. Epidemiology of parasitic co-infections during pregnancy in Lambaréné, Gabon. Trop. Med. Int. Health 2010, 15, 1204–1209. [Google Scholar] [CrossRef]
- Honkpéhèdji, Y.J.; Adegbite, B.R.; Zinsou, J.F.; Dejon-Agobé, J.C.; Edoa, J.-R.; Manego, R.Z.; McCall, M.; Ngwese, M.M.; Mougeni, F.L.; Mombo-Ngoma, G.; et al. Association of low birth weight and polyparasitic infection during pregnancy in Lambaréné, Gabon. Trop. Med. Int. Health 2021, 26, 973–981. [Google Scholar] [CrossRef]
- Yatich, N.J.; Rayner, J.C.; Turpin, A.; Jolly, P.E.; Ellis, W.O.; Stiles, J.; Agbenyega, T.; Ehiri, J.E.; Funkhouser, E.; Williams, J.H.; et al. Malaria and Intestinal Helminth Co-infection Among Pregnant Women in Ghana: Prevalence and Risk Factors. Am. J. Trop. Med. Hyg. 2009, 80, 896–901. [Google Scholar] [CrossRef]
- Tay, S.C.K.; Nani, E.A.; Walana, W. Parasitic infections and maternal anaemia among expectant mothers in the Dangme East District of Ghana. BMC Res. Notes 2017, 10, 3. [Google Scholar] [CrossRef]
- Thigpen, M.C.; Filler, S.J.; Kazembe, P.N.; Parise, M.E.; Macheso, A.; Campbell, C.H.; Newman, R.D.; Steketee, R.W.; Hamel, M. Associations between peripheral Plasmodium falciparum malaria parasitemia, human immunodeficiency virus, and concurrent helminthic infection among pregnant women in Malawi. Am. J. Trop. Med. Hyg. 2011, 84, 379–385. [Google Scholar] [CrossRef][Green Version]
- Anchang-Kimbi, J.K.; Elad, D.M.; Sotoing, G.T.; Achidi, E.A. Coinfection with Schistosoma haematobium and Plasmodium falciparum and Anaemia Severity among Pregnant Women in Munyenge, Mount Cameroon Area: A Cross-Sectional Study. J. Parasitol. Res. 2017, 2017, 61734650. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S. Are malaria elimination efforts on right track? An analysis of gains achieved and challenges ahead. Infect. Dis. Poverty 2019, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Alemu, F.; Kumie, A.; Medhin, G.; Gebre, T.; Godfrey, P. A socio-ecological analysis of barriers to the adoption, sustainablity and consistent use of sanitation facilities in rural Ethiopia. BMC Public Health 2017, 17, 706. [Google Scholar] [CrossRef] [PubMed]
- Mwendera, C.A.; De Jager, C.; Longwe, H.; Phiri, K.; Hongoro, C.; Mutero, C.M. Changing the policy for intermittent preventive treatment with sulfadoxine-pyrimethamine during pregnancy in Malawi. Malar. J. 2017, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, F.M.; Nedjat, S.; Sajadi, H.S.; Parsaeian, M.; Assan, A.; Majdzadeh, R. Malaria intermittent preventive treatment in Nigeria: A qualitative study to explore barriers. BMC Infect. Dis. 2021, 21, 438. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Regional Office for Europe. Framework for Control and Prevention of Soil-Transmitted Helminthiases in the WHO European Region. Available online: http://www.euro.who.int/ (accessed on 9 March 2022).
- Maskin, E.; Monga, C.; Thuilliez, J.; Berthélemy, J.C. The economics of malaria control in an age of declining aid. Nat. Commun. 2019, 10, 2269. [Google Scholar] [CrossRef]
- Walker, P.G.T.; Griffin, J.T.; Ferguson, N.M.; Ghani, A.C. Estimating the most efficient allocation of interventions to achieve reductions in Plasmodium falciparum malaria burden and transmission in Africa: A modelling study. Lancet Glob. Health 2016, 4, e474–e484. [Google Scholar] [CrossRef]
- Head, M.G.; Goss, S.; Gelister, Y.; Alegana, V.; Brown, R.J.; Clarke, S.C.; Fitchett, J.R.A.; Atun, R.; Scott, J.A.G.; Newell, M.-L.; et al. Global funding trends for malaria research in sub-Saharan Africa: A systematic analysis. Lancet Glob. Health 2017, 5, e772–e781. [Google Scholar] [CrossRef]
- Cardona-Arias, J.A.; Carmona-Fonseca, J. Meta-analysis of the prevalence of malaria associated with pregnancy in Colombia 2000–2020. PLoS ONE 2021, 16, e0255028. [Google Scholar] [CrossRef]
- Tegegne, Y.; Asmelash, D.; Ambachew, S.; Eshetie, S.; Addisu, A.; Zeleke, A.J. The Prevalence of Malaria among Pregnant Women in Ethiopia: A Systematic Review and Meta-Analysis. J. Parasitol. Res. 2019, 2019, 8396091. [Google Scholar] [CrossRef]
- Bilal, J.A.; Malik, E.E.; Al-Nafeesah, A.; Adam, I. Global prevalence of congenital malaria: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Tediosi, F.; Penny, M. Evidence for optimal allocation of malaria interventions in Africa. Lancet Glob. Health 2016, 4, e432–e433. [Google Scholar] [CrossRef][Green Version]
- The malERA Refresh Consultative Panel on Combination Interventions and Modelling. malERA: An updated research agenda for combination interventions and modelling in malaria elimination and eradication. PLoS Med. 2017, 14, e1002453. [Google Scholar]
- Blas, E.; Multisectoral Action Framework for Malaria. Roll Back Malar Partnership/UNDP. Available online: http://www.rollbackmalaria.org/files/files/resources/Multisectoral-Action-Framework-for-Malaria.pdf (accessed on 9 March 2022).
- WHO. The Global Global Malaria Malaria Action Plan For a malaria free world. In Guidel Malar Treat; WHO: Geneva, Switzerland, 2015; pp. 12–74. [Google Scholar]
- Danwang, C.; Bigna, J.J.; Nzalie, R.N.T.; Robert, A. Epidemiology of clinical congenital and neonatal malaria in endemic settings: A systematic review and meta-analysis. Malar. J. 2020, 19, 312. [Google Scholar] [CrossRef] [PubMed]
- Esopo, K.; Derby, L.; Haushofer, J. Interventions to improve adherence to antenatal and postnatal care regimens among pregnant women in sub-Saharan Africa: A systematic review. BMC Pregnancy Childbirth 2020, 20, 316. [Google Scholar] [CrossRef]
- Ippolito, M.M.; Moser, K.A.; Kabuya, J.-B.B.; Cunningham, C.; Juliano, J.J. Antimalarial Drug Resistance and Implications for the WHO Global Technical Strategy. Curr. Epidemiol. Rep. 2021, 8, 46–62. [Google Scholar] [CrossRef]
- Muanda, F.T.; Chaabane, S.; Boukhris, T.; Santos, F.; Sheehy, O.; Perreault, S.; Blais, L.; Bérard, A. Antimalarial drugs for preventing malaria during pregnancy and the risk of low birth weight: A systematic review and meta-analysis of randomized and quasi-randomized trials. BMC Med. 2015, 13, 193. [Google Scholar] [CrossRef]
- Blasco, B.; Leroy Di Fidock, D.A. Antimalarial drug resistance: Linking Plasmodium falciparum parasite biology to the clinic. Nat. Med. 2017, 23, 917–928. [Google Scholar] [CrossRef]
- Shibeshi, M.A.; Kifle, Z.D.; Atnafie, S.A. Antimalarial drug resistance and novel targets for antimalarial drug discovery. Infect. Drug Resist. 2020, 13, 4047–4060. [Google Scholar] [CrossRef]
- Ehrlich, H.Y.; Jones, J.; Parikh, S. Molecular surveillance of antimalarial partner drug resistance in sub-Saharan Africa: A spatial-temporal evidence mapping study. Lancet Microbe 2020, 1, e209–e217. [Google Scholar] [CrossRef]
- McMahon, A.; Mihretie, A.; Ahmed, A.A.; Lake, M.; Awoke, W.; Wimberly, M.C. Remote sensing of environmental risk factors for malaria in different geographic contexts. Int. J. Health Geogr. 2021, 20, 28. [Google Scholar] [CrossRef] [PubMed]
- Rogerson, S.J.; Aitken, E.H. Progress towards vaccines to protect pregnant women from malaria. EBioMedicine 2019, 42, 12–13. [Google Scholar] [CrossRef] [PubMed]
- Mordmüller, B.; Sulyok, M.; Egger-Adam, D.; Resende, M.; De Jongh, W.A.; Jensen, M.H.; Smedegaard, H.H.; Ditlev, S.B.; Soegaard, M.; Poulsen, L.; et al. First-in-human, Randomized, Double-blind Clinical Trial of Differentially Adjuvanted PAMVAC, A Vaccine Candidate to Prevent Pregnancy-associated Malaria. Clin. Infect. Dis. 2019, 69, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Chico, R.M.; Cano, J. Devising a strategy for prevention of malaria in pregnant women in the Asia Pacific. Lancet Infect. Dis. 2019, 19, 919–920. [Google Scholar] [CrossRef]
- World Health Organization. Zeroing in on Malaria Elimination: Final Report of the E-2020 Initiative; WHO: Geneva, Switzerland, 2021; pp. 1–20. [Google Scholar]
- Russell, T.L.; Beebe, N.W.; Cooper, R.D.; Lobo, N.F.; Burkot, T.R. Successful malaria elimination strategies require interventions that target changing vector behaviours. Malar. J. 2013, 12, 56. [Google Scholar] [CrossRef]
- Lal, A.A.; Rajvanshi, H.; Jayswar, H.; Das, A.; Bharti, P. Malaria elimination: Using past and present experience to make malaria-free India by 2030. J. Vector Borne Dis. 2019, 56, 60–65. [Google Scholar] [CrossRef]
- World Health Organization. Eliminating Malaria: Learning from the Past, Looking Ahead; Progress and Impact Series; WHO: Geneva, Switzerland, 2011; Volume 8, pp. 1–85. [Google Scholar]
- Maat, H.; Balabanova, D.; Mokuwa, E.; Richards, P.; Mohan, V.; Ssengooba, F.; Twinomuhangi, R.; Woldie, M.; Mayhew, S. Towards sustainable community-based systems for infectious disease and disaster response; lessons from local initiatives in four African countries. Sustainability 2021, 13, 10083. [Google Scholar] [CrossRef]
- Ajayi, I.O.O.; Ajumobi, O.; Ogunwale, A.; Adewole, A.; Odeyinka, O.T.; Balogun, M.S.; Nguku, P.; Bamiselu, O.; Fellows, F.N. Is the malaria short course for program managers, a priority for malaria control effort in Nigeria? Evidence from a qualitative study. PLoS ONE 2020, 15, e0236576. [Google Scholar] [CrossRef]
- Orok, A.B.; Ajibaye, O.; Aina, O.O.; Iboma, G.; Oboshi, S.A.; Iwalokun, B. Malaria interventions and control programes in Sub-Saharan Africa: A narrative review. Cogent. Med. 2021, 8, 1940639. [Google Scholar] [CrossRef]
- Fernando, D.; Wijeyaratne, P.; Wickremasinghe, R.; Abeyasinghe, R.R.; Galappaththy, G.N.L.; Wickremasinghe, R.; Hapugoda, M.; Abeywickrema, W.A.; Rodrigo, C. Use of a public-private partnership in malaria elimination efforts in Sri Lanka; A case study. BMC Health Serv. Res. 2018, 18, 202. [Google Scholar] [CrossRef]
- Jones, R.T.; Tusting, L.S.; Smith, H.M.P.; Segbaya, S.; Macdonald, M.B.; Bangs, M.J.; Logan, J.G. The role of the private sector in supporting malaria control in resource development settings. J. Infect. Dis. 2020, 222, S701–S708. [Google Scholar] [CrossRef] [PubMed]
- Tizifa, T.A.; Kabaghe, A.N.; McCann, R.S.; Berg, H.V.D.; Van Vugt, M.; Phiri, K.S. Prevention Efforts for Malaria. Curr. Trop. Med. Rep. 2018, 5, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Badmos, A.O.; Alaran, A.J.; Adebisi, Y.A.; Bouaddi, O.; Onibon, Z.; Dada, A.; Lin, X.; Lucero-Prisno, D.E. What sub-Saharan African countries can learn from malaria elimination in China. Trop. Med. Health 2021, 49, 86. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, A.; Ghodsian, S.; Jabbari, M.; Olfatifar, M.; Abdoli, A.; Ghaffarifar, F. Global prevalence of intestinal parasitic infections and associated risk factors in pregnant women: A systematic review and meta-analysis. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 457–470. [Google Scholar] [CrossRef]
- Chami, G.F.; Kontoleon, A.A.; Bulte, E.; Fenwick, A.; Kabatereine, N.B.; Tukahebwa, E.M.; Dunne, D.W. Profiling Nonrecipients of Mass Drug Administration for Schistosomiasis and Hookworm Infections: A Comprehensive Analysis of Praziquantel and Albendazole Coverage in Community-Directed Treatment in Uganda. Clin. Infect. Dis. 2016, 62, 200–207. [Google Scholar] [CrossRef]
- Mutapi, F.; Maizels, R.; Fenwick, A.; Woolhouse, M. Human schistosomiasis in the post mass drug administration era. Lancet Infect. Dis. 2017, 17, e42–e48. [Google Scholar] [CrossRef]
- Torres-Vitolas, C.A.; Dhanani, N.; Fleming, F.M. Factors affecting the uptake of preventive chemotherapy treatment for schistosomiasis in sub-saharan africa: A systematic review. PLoS Negl. Trop. Dis. 2021, 15, e0009017. [Google Scholar] [CrossRef]
- Kanyangarara, M.; Allen, S.; Jiwani, S.S.; Fuente, D. Access to water, sanitation and hygiene services in health facilities in sub-Saharan Africa 2013-2018: Results of health facility surveys and implications for COVID-19 transmission. BMC Health Serv. Res. 2021, 21, 601. [Google Scholar] [CrossRef]
- WHO Evidence Review Group on Malaria in Pregnancy. Malaria Policy Advisory Committee Meeting Meeting Report of the WHO Evidence Review Group on Malaria in Pregnancy; WHO/HTM/GMP/MPAC/2017.18; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- de Neve, J.-W.; Andriantavison, R.L.; Croke, K.; Krisam, J.; Rajoela, V.H.; Rakotoarivony, R.A.; Rambeloson, V.; Schultz, L.; Qamruddin, J.; Verguet, S. Health, financial, and education gains of investing in preventive chemotherapy for schistosomiasis, soil-transmitted helminthiases, and lymphatic filariasis in Madagascar: A modeling study. PLoS Negl. Trop. Dis. 2018, 12, e0007002. [Google Scholar] [CrossRef]
- Salari, P.; Fürst, T.; Knopp, S.; Utzinger, J.; Tediosi, F. Cost of interventions to control schistosomiasis: A systematic review of the literature. PLoS Negl. Trop. Dis. 2020, 14, e0008098. [Google Scholar] [CrossRef]
- Price, A.; Verma, A.; Welfare, W. Are health education interventions effective for the control and prevention of urogenital schistosomiasis in sub-Saharan Africa? A systematic review. Trans. R. Soc. Trop. Med. Hyg. 2015, 109, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Zegeye, B.; Ahinkorah, B.O.; Ameyaw, E.K.; Seidu, A.A.; Yaya, S. Utilization of Deworming Drugs and Its Individual and Community Level Predictors among Pregnant Married Women in Cameroon: A Multilevel Modeling. BioMed Res. Int. 2021, 2021, 6645336. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, A.; Zhang, Y.; Stoever, K. Control of the Neglected Tropical Diseases in sub-Saharan Africa: The unmet needs. Int. Health 2009, 1, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Zegeye, B.; Keetile, M.; Ahinkorah, B.O.; Ameyaw, E.K.; Seidu, A.A.; Yaya, S. Utilization of deworming medication and its associated factors among pregnant married women in 26 sub-Saharan African countries: A multi-country analysis. Trop. Med. Health 2021, 49, 53. [Google Scholar] [CrossRef]
- Sustainable Development Goal (SDG) 6. Synthesis Report 2018 on Water and Sanitation; United Nations: Geneva, Switzerland, 2018. [CrossRef]
- WHO. Global Progress Report on Wash in Health Care Facilities; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Moser, W.; Schindler, C.; Keiser, J. Efficacy of recommended drugs against soil transmitted helminths: Systematic review and network meta-analysis. BMJ 2017, 358, j4307. [Google Scholar] [CrossRef]
- Kabore, A.; Ibikounle, M.; Tougoue, J.J.; Mupoyi, S.; Ndombe, M.; Shannon, S.; Ottesen, E.A.; Mukunda, F.; Awaca, N. Initiating NTD programs targeting schistosomiasis and soil-transmitted helminthiasis in two provinces of the Democratic Republic of the Congo: Establishment of baseline prevalence for mass drug administration. Acta Trop. 2017, 166, 177–185. [Google Scholar] [CrossRef]
- Craig, P.; Dieppe, P.; Macintyre, S.; Mitchie, S.; Nazareth, I.; Petticrew, M. Developing and evaluating complex interventions: The new Medical Research Council guidance. BMJ 2008, 337, 979–983. [Google Scholar] [CrossRef]
- Hawadak, J.; Dongang Nana, R.R.; Singh, V. Global trend of Plasmodium malariae and Plasmodium ovale spp. malaria infections in the last two decades (2000–2020): A systematic review and meta-analysis. Parasites Vectors 2021, 14, 297. [Google Scholar] [CrossRef]
- Turner, H.C.; Stolk, W.A.; Solomon, A.W.; King, J.D.; Montresor, A.; Molyneux, D.H.; Toor, J. Are current preventive chemotherapy strategies for controlling and eliminating neglected tropical diseases cost-effective? BMJ Glob. Health 2021, 6, e005456. [Google Scholar] [CrossRef]
- Jacobsen, A.; Schmiegelow, C.; Sørensen, B.; Msemo, O.A.; Nielsen, K.; Nielsen, B.; Møller, S.; Lusingu, J.; Minja, D.; Hedegaard, M.; et al. Biosensor for Detecting Fetal Growth Restriction in a Low-Resource Setting. Reprod. Med. 2021, 2, 57–67. [Google Scholar] [CrossRef]
- Lau, R.; Chris, R.B.; Phuong, M.S.; Khatib, A.; Kopalakrishnan, S.; Bhasker, S.; Raheel, H.; Lecce, C.; Yegorov, S.; Mishra, S.; et al. Treatment of soil-transmitted helminth infections in pregnancy: A systematic review and meta-analysis of maternal outcomes. J. Travel Med. 2020, 27, taz079. [Google Scholar] [CrossRef] [PubMed]
- Zawawi, A.; Else, K.J. Soil-Transmitted Helminth Vaccines: Are We Getting Closer? Front. Immunol. 2020, 11, 2426. [Google Scholar] [CrossRef] [PubMed]
- Perera, D.J.; Ndao, M. Promising Technologies in the Field of Helminth Vaccines. Front. Immunol. 2021, 12, 711650. [Google Scholar] [CrossRef] [PubMed]
- Filbey, K.J.; Finney, C.A.M.; Giacomin, P.R.; Siracusa, M.C. Editorial: Recent Advances in the Immunology of Helminth Infection—Protection, Pathogenesis and Panaceas. Front. Immunol. 2021, 12, 10–12. [Google Scholar] [CrossRef]
- Tran, V.T.; Ravaud, P. Frugal innovation in medicine for low resource settings. BMC Med. 2016, 14, 102–104. [Google Scholar] [CrossRef]
- Pacifico Silva, H.; Lehoux, P.; Miller, F.A.; Denis, J.L. Introducing responsible innovation in health: A policy-oriented framework. Health Res. Policy Syst. 2018, 16, 90. [Google Scholar] [CrossRef]
- Mundle, D.; Davis Pluess, J. Innovative Finance to Expand access to Healthcare: Opportunities for Business; BSR: New York, NY, USA, 2017; pp. 1–54. [Google Scholar]
- Bloom, D.E.; Michael, K.; Klaus, P. Modern Infectious Diseases: Macroeconomic Impacts and Policy Responses; IZA Discussion Paper No. 13625; NBE: Cambridge, MA, USA, 2020. [Google Scholar] [CrossRef]
- Nkengasong, J.N.; Tessema, S.K. Africa Needs a New Public Health Order to Tackle Infectious Disease Threats. Cell 2020, 183, 296–300. [Google Scholar] [CrossRef]
- Quinn, S.C.; Kumar, S. Health inequalities and infectious disease epidemics: A challenge for global health security. Biosecurity Bioterrorism 2014, 12, 263–273. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Malani, A. Economics of Infectious Diseases. In The Oxford Handbook of Health; Oxford University Press: Oxford, UK, 2012; pp. 1–20. [Google Scholar]
- Co-infections and co-morbidities. In Proceedings of the EDCTP Stakeholder Meeting, The Hague, the Netherlands, 13 September 2017.
- Lonnie, W. Sustaining Workforce Engagement: How to Ensure Your Employees Are Healthy, Happy, and Productive, 1st ed.; Productivity Press: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Mugabe, J.O. Science, Technology and Innovation in Africa’s Regional Integration: From Rhetoric to Practice; ACODE Policy Research Series; ACODE: Kampala, Uganda, 2011. [Google Scholar]
- Agaba, B.B.; Yeka, A.; Nsobya, S.; Arinaitwe, E.; Nankabirwa, J.; Opigo, J.; Mbaka, P.; Lim, C.S.; Kalyango, J.N.; Karamagi, C. Systematic review of the status of pfhrp2 and pfhrp3 gene deletion, approaches and methods used for its estimation and reporting in Plasmodium falciparum populations in Africa: Review of published studies 2010–2019. Malar. J. 2019, 18, 355. [Google Scholar] [CrossRef]
- Slater, H.C.; Ross, A.; Felger, I.; Hofmann, N.E.; Robinson, L.; Cook, J.; Gonçalves, B.P.; Björkman, A.; Ouedraogo, A.L.; Morris, U. The temporal dynamics and infectiousness of subpatent Plasmodium falciparum infections in relation to parasite density. Nat. Commun. 2019, 10, 1433. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).