The Use of Patient-Reported Outcome Measures in Daily Clinical Practice of a Pediatric Nephrology Department
Abstract
:1. Introduction
2. Materials and Methods
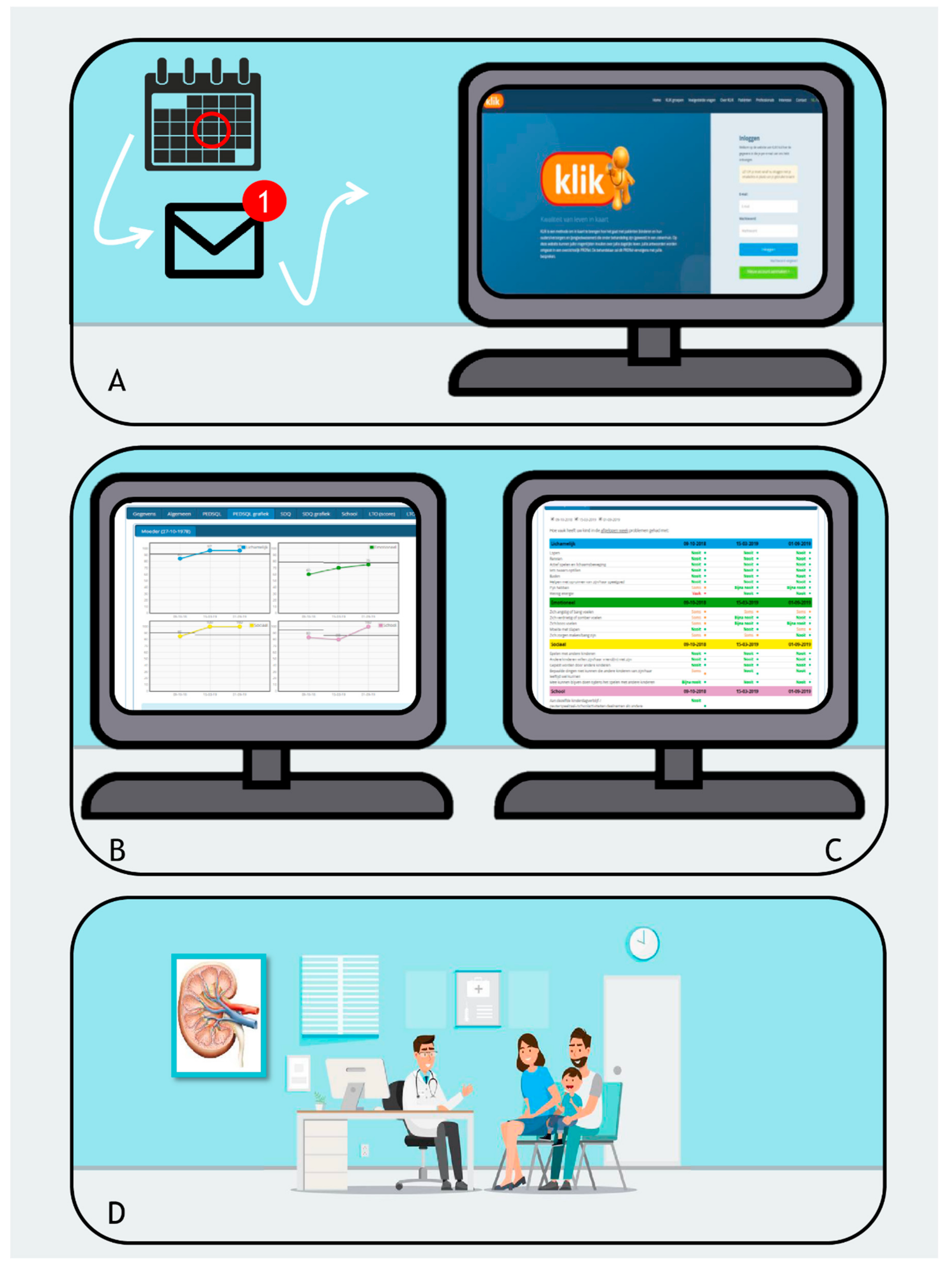
2.1. The KLIK PROM Portal and Patient Selection
2.2. Feasibility
2.3. Measures
2.3.1. TNO-AZL Preschool Children Quality of Life (TAPQOL)
2.3.2. Pediatric Quality of Life Inventory (PedsQL) Generic Scale 4.0
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
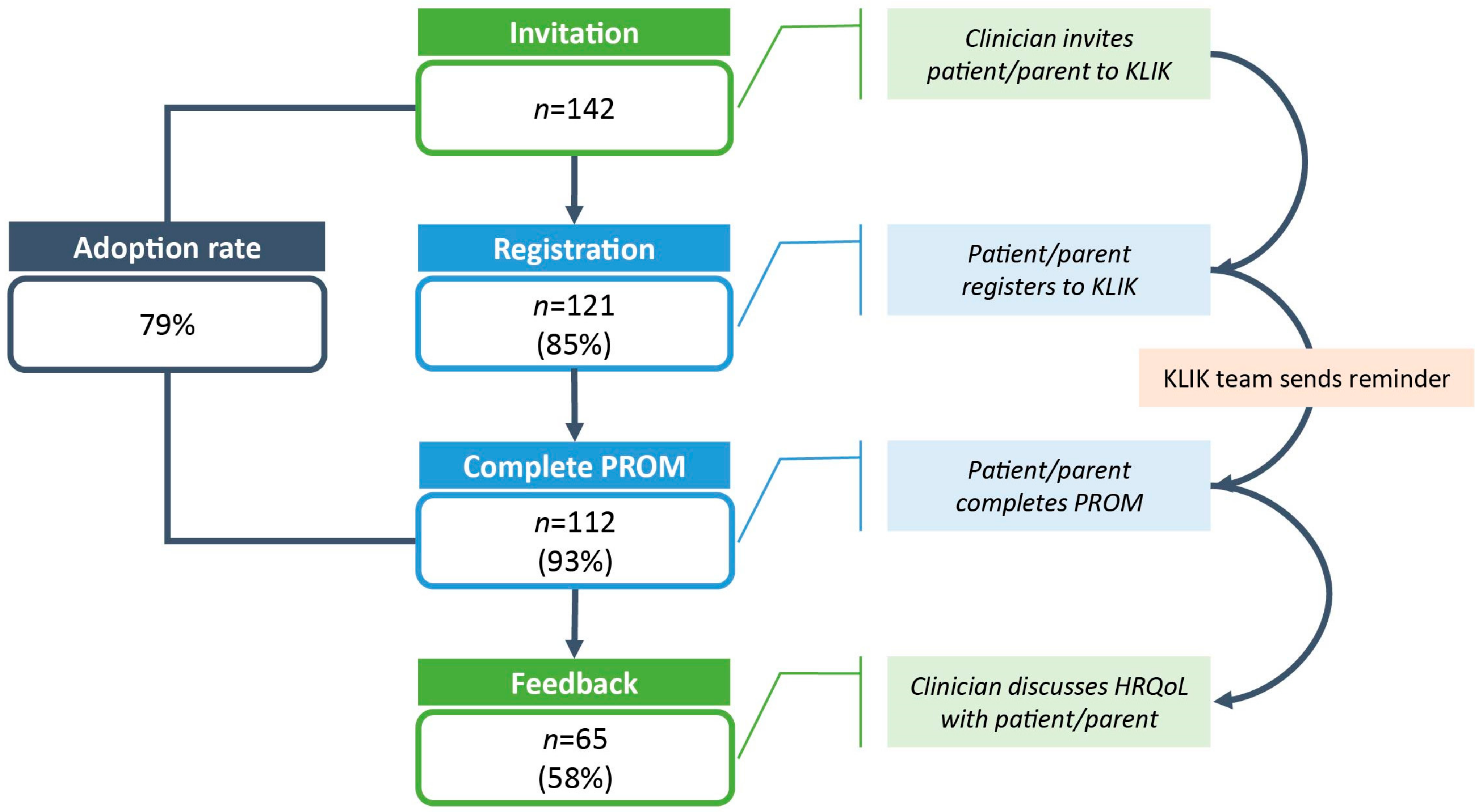
3.1. Feasibility
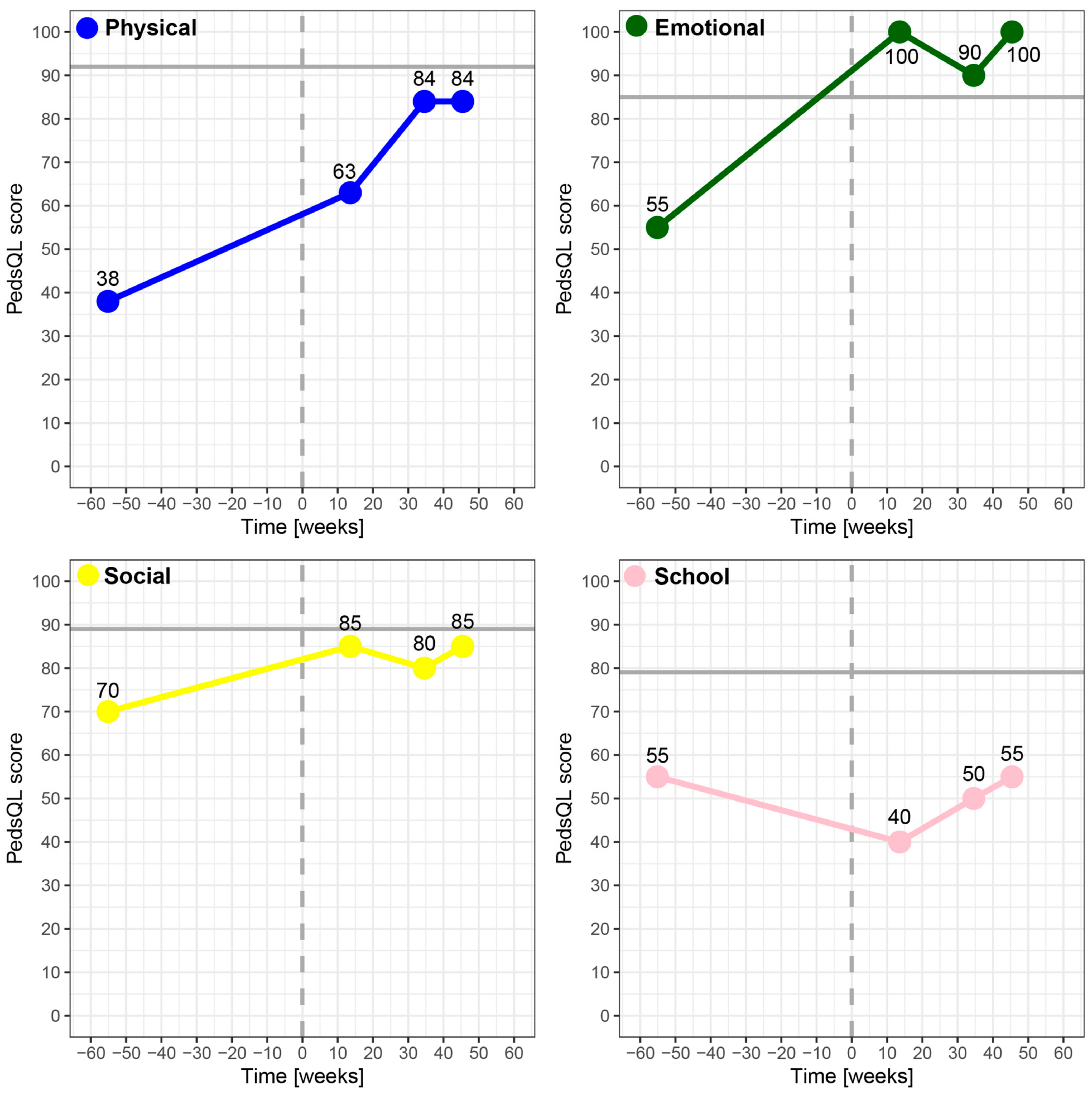
3.2. HRQoL Differences

| Registered | Not Registered | p-Value | |
|---|---|---|---|
| Registration, n | 121 | 21 | |
| Age at invitation (years), mean ± SD | 9.7 ± 4.8 | 11.5 ± 4.3 | 0.09 1 |
| Male, n (%) | 68 (56) | 14 (67) | 0.48 2 |
| ≥1 PROM Completed | No PROM Completed | ||
| Completion, n | 112 | 9 | |
| Age at registration (years), median (IQR) | 10.2 (6.1–13.4) | 15.6 (9.0–16.3) | 0.06 3 |
| Male, n (%) | 64 (57) | 4 (44) | 0.50 2 |
| CKD 0–5 y (n = 22) | Reference 1–5 y (n = 378) | p-Value | |
|---|---|---|---|
| Age (months), range | 4–66 | 10–60 | - |
| Male, n (%) | 14 (63.6) | NA 2 | - |
| Sleeping | 68.2 ± 31.6 | 80.9 ± 18.1 | 0.07 |
| Appetite | 70.8 ± 25.2 | 84.3 ± 13.7 | 0.02 |
| Lung problems | 92.4 ± 18.3 | 92.0 ± 15.8 | 0.92 |
| Stomach problems | 75.8 ± 18.0 | 90.3 ± 15.3 | 0.001 |
| Skin problems | 83.0 ± 24.7 | 91.6 ± 11.5 | 0.12 |
| Motor functioning 1 | 88.5 ± 17.5 | 97.2 ± 6.6 | 0.03 |
| Social functioning 1 | 90.4 ± 17.0 | 89.8 ± 16.9 | 0.89 |
| Problem behavior | 76.6 ± 20.0 | 66.9 ± 16.3 | 0.03 |
| Communication 1 | 83.2 ± 26.1 | 89.9 ± 11.2 | 0.24 |
| Anxiety | 76.5 ± 19.0 | 76.5 ± 18.3 | >0.99 |
| Positive mood | 96.2 ± 17.8 | 98.6 ± 6.6 | 0.53 |
| Liveliness | 90.9 ± 16.0 | 97.6 ± 9.1 | 0.06 |
| CKD 5–7 (n = 10) | Reference 5–7 (n = 274) | p-Value | CKD 8–18 (n = 54) | Reference 8–18 (n = 1012) | p-Value | |
|---|---|---|---|---|---|---|
| Age (years), mean ± SD | 7.2 ± 0.6 | 6.5 ± 0.9 | 0.076 | 12.8 ± 3.1 | 13.4 ± 3.0 | 0.557 |
| Male, n (%) | 4 (40) | 152 (55.5) | 0.33 | 33 (61.1) | 521 (51.5) | 0.17 |
| Total score | 79.1 ± 12.2 | 86.0 ± 11.6 | 0.065 | 79.1 ± 12.1 | 84.9 ± 12.6 | 0.001 |
| Physical functioning | 82.5 ± 15.1 | 91.1 ± 12.6 | 0.038 | 83.0 ± 14.9 | 91.5 ± 12.4 | <0.001 |
| Emotional functioning | 70.5 ± 4.3 | 77.9 ± 16.5 | 0.162 | 75.4 ± 17.9 | 79.2 ± 18.3 | 0.133 |
| Social functioning | 85.5 ± 13.8 | 86.4 ± 16.7 | 0.868 | 82.7 ± 17.0 | 84.5 ± 16.9 | 0.451 |
| School functioning | 76.0 ± 20.1 | 85.8 ± 15.4 | 0.051 | 73.1 ± 16.9 | 80.5 ± 16.7 | 0.002 |
| Psychosocial functioning | 77.3 ± 13.5 | 83.4 ± 13.7 | 0.170 | 77.0 ± 12.6 | 81.4 ± 14.6 | 0.033 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rovin, B.H.; Adler, S.G.; Barratt, J.; Bridoux, F.; Burdge, K.A.; Chan, T.M.; Cook, H.T.; Fervenza, F.C.; Gibson, K.L.; Glassock, R.J.; et al. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276. [Google Scholar] [CrossRef] [PubMed]
- Tjaden, L.A.; Grootenhuis, M.A.; Noordzij, M.; Groothoff, J.W. Health-related quality of life in patients with pediatric onset of end-stage renal disease: State of the art and recommendations for clinical practice. Pediatr. Nephrol. 2016, 31, 1579–1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, A.; Wong, G.; McTaggart, S.; Henning, P.; Mackie, F.; Carroll, R.P.; Howard, K.; Craig, J.C. Quality of life of young adults and adolescents with chronic kidney disease. J. Pediatr. 2013, 163, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- El Shafei, A.M.; Soliman Hegazy, I.; Fadel, F.I.; Nagy, E.M. Assessment of Quality of Life among Children with End-Stage Renal Disease: A Cross-Sectional Study. J. Environ. Public Health 2018, 2018, 8565498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilicoglu, A.G.; Bahali, K.; Canpolat, N.; Bilgic, A.; Mutlu, C.; Yalçın, Ö.; Pehlivan, G.; Sever, L. Impact of end-stage renal disease on psychological status and quality of life. Pediatr. Int. 2016, 58, 1316–1321. [Google Scholar] [CrossRef]
- Dotis, J.; Pavlaki, A.; Printza, N.; Stabouli, S.; Antoniou, S.; Gkogka, C.; Kontodimopoulos, N.; Papachristou, F. Quality of life in children with chronic kidney disease. Pediatr. Nephrol. 2016, 31, 2309–2316. [Google Scholar] [CrossRef]
- Gerson, A.C.; Wentz, A.; Abraham, A.G.; Mendley, S.R.; Hooper, S.R.; Butler, R.W.; Gipson, D.S.; Lande, M.B.; Shinnar, S.; Moxey-Mims, M.M.; et al. Health-related quality of life of children with mild to moderate chronic kidney disease. Pediatrics 2010, 125, e349–e357. [Google Scholar] [CrossRef] [Green Version]
- Baek, H.S.; Kang, H.G.; Choi, H.J.; Cheong, H.I.; Ha, I.S.; Han, K.H.; Kim, S.H.; Cho, H.Y.; Shin, J.I.; Park, Y.S.; et al. Health-related quality of life of children with pre-dialysis chronic kidney disease. Pediatr. Nephrol. 2017, 32, 2097–2105. [Google Scholar] [CrossRef]
- Aggarwal, H.K.; Jain, D.; Pawar, S.; Yadav, R.K. Health-related quality of life in different stages of chronic kidney disease. QJM 2016, 109, 711–716. [Google Scholar] [CrossRef] [Green Version]
- Splinter, A.; Tjaden, L.A.; Haverman, L.; Adams, B.; Collard, L.; Cransberg, K.; van Dyck, M.; Van Hoeck, K.J.; Hoppe, B.; Koster-Kamphuis, L.; et al. Children on dialysis as well as renal transplanted children report severely impaired health-related quality of life. Qual. Life Res. 2018, 27, 1445–1454. [Google Scholar] [CrossRef] [Green Version]
- Wightman, A.; Bradford, M.C.; Smith, J. Health-related quality of life changes following renal transplantation in children. Pediatr. Transplant. 2019, 23, e13333. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Division of Mental Health and Prevention of Substance Abuse. WHOQOL: Measuring quality of life. World Health Organ. 1997, 11, 160–165. [Google Scholar]
- Haverman, L.; Limperg, P.F.; Young, N.L.; Grootenhuis, M.A.; Klaassen, R.J. Paediatric health-related quality of life: What is it and why should we measure it? Arch. Dis. Child. 2017, 102, 393–400. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services FDA Center for Drug Evaluation and Research; US Department of Health and Human Services FDA Center for Biologics Evaluation and Research; US Department of Health and Human Services FDA Center for Devices and Radiological Health Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims: Draft guidance. Health Qual. Life Outcomes 2006, 4, 79. [CrossRef] [Green Version]
- Black, N.; Burke, L.; Forrest, C.B.; Sieberer, U.H.R.; Ahmed, S.; Valderas, J.M.; Bartlett, S.J.; Alonso, J. Patient-reported outcomes: Pathways to better health, better services, and better societies. Qual. Life Res. 2016, 25, 1103–1112. [Google Scholar] [CrossRef] [Green Version]
- Boyce, M.B.; Browne, J.P.; Greenhalgh, J. The experiences of professionals with using information from patient-reported outcome measures to improve the quality of healthcare: A systematic review of qualitative research. BMJ Qual. Saf. 2014, 23, 508–518. [Google Scholar] [CrossRef]
- Valderas, J.M.; Kotzeva, A.; Espallargues, M.; Guyatt, G.; Ferrans, C.E.; Halyard, M.Y.; Revicki, D.A.; Symonds, T.; Parada, A.; Alonso, J. The impact of measuring patient-reported outcomes in clinical practice: A systematic review of the literature. Qual. Life Res. 2008, 17, 179–193. [Google Scholar] [CrossRef]
- Marshall, S.; Haywood, K.; Fitzpatrick, R. Impact of patient-reported outcome measures on routine practice: A structured review. J. Eval. Clin. Pract. 2006, 12, 559–568. [Google Scholar] [CrossRef]
- Basch, E.; Deal, A.M.; Dueck, A.C.; Scher, H.I.; Kris, M.G.; Hudis, C.; Schrag, D. Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. J. Am. Med. Assoc. 2017, 318, 197–198. [Google Scholar] [CrossRef] [Green Version]
- Haverman, L.; Van Oers, H.A.; Limperg, P.F.; Hijmans, C.T.; Schepers, S.A.; Sint Nicolaas, S.M.; Verhaak, C.M.; Bouts, A.H.M.; Fijnvandraat, K.; Peters, M.; et al. Implementation of electronic patient reported outcomes in pediatric daily clinical practice: The KLIK experience. Clin. Pract. Pediatr. Psychol. 2014, 2, 50–67. [Google Scholar] [CrossRef]
- Engelen, V.; Haverman, L.; Koopman, H.; Schouten-van Meeteren, N.; Meijer-van den Bergh, E.; Vrijmoet-Wiersma, J.; van Dijk, E.M.; Last, B.; Detmar, S.; Grootenhuis, M. Development and implementation of a patient reported outcome intervention (QLIC-ON PROfile) in clinical paediatric oncology practice. Patient Educ. Couns. 2010, 81, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Brundage, M.D.; Smith, K.C.; Little, E.A.; Bantug, E.T.; Snyder, C.F. Communicating patient-reported outcome scores using graphic formats: Results from a mixed-methods evaluation. Qual. Life Res. 2015, 24, 2457–2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snyder, C.; Smith, K.; Holzner, B.; Rivera, Y.M.; Bantug, E.; Brundage, M. Making a picture worth a thousand numbers: Recommendations for graphically displaying patient-reported outcomes data. Qual. Life Res. 2019, 28, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Haverman, L.; van Oers, H.A.; van Muilekom, M.M.; Grootenhuis, M.A. Options for the Interpretation of and Recommendations for Acting on Different PROMs in Daily Clinical Practice Using KLIK. Med. Care 2019, 57, S52–S58. [Google Scholar] [CrossRef] [PubMed]
- Haverman, L.; van Rossum, M.A.J.; van Veenendaal, M.; van den Berg, J.M.; Dolman, K.M.; Swart, J.; Kuijpers, T.W.; Grootenhuis, M.A. Effectiveness of a Web-Based Application to Monitor Health-Related Quality of Life. Pediatrics 2013, 131, e533–e543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana, M.J.; Haverman, L.; Absolom, K.; Takeuchi, E.; Feeny, D.; Grootenhuis, M.; Velikova, G. Training clinicians in how to use patient-reported outcome measures in routine clinical practice. Qual. Life Res. 2015, 24, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Haverman, L.; Engelen, V.; van Rossum, M.A.J.A.; Heymans, H.S.A.S.; Grootenhuis, M.A. Monitoring health-related quality of life in paediatric practice: Development of an innovative web-based application. BMC Pediatr. 2011, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Fekkes, A.M.; Theunissen, N.C.M.; Brugman, E.; Veen, S.; Verrips, E.G.H.; Vogels, T.; Wit, J.M.; Verloove-Vanhorick, S.P. Development and Psychometric Evaluation of the TAPQOL: A Health-Related Quality of Life Instrument for 1-5-Year-Old Children. Qual. Life Res. 2000, 9, 961–972. [Google Scholar] [CrossRef]
- Varni, J.W.; Seid, M.; Kurtin, P.S. PedsQLTM 4.0: Reliability and Validity of the Pediatric Quality of Life InventoryTM Version 4.0 Generic Core Scales in Healthy and Patient Populations. Med. Care 2001, 39, 800–812. [Google Scholar] [CrossRef]
- Engelen, V.; Haentjens, M.M.; Detmar, S.B.; Koopman, H.M.; Grootenhuis, M.A. Health related quality of life of Dutch children: Psychometric properties of the PedsQL in the Netherlands. BMC Pediatr. 2009, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- Muilekom, M.M.; van Luijten, M.A.J.; van Oers, H.A.; Conijn, T.; Maurice-Stam, H.; van Goudoever, J.B.; Grootenhuis, M.A.; Haverman, L. Group, on behalf of the K. collaborator Pediatric patients report lower Health-Related Quality of Life in daily clinical practice compared to new normative PedsQL data. Acta Paediatr. 2021, 110, 2267–2279. [Google Scholar] [CrossRef] [PubMed]
- Schepers, S.A.; van Oers, H.A.; Maurice-Stam, H.; Huisman, J.; Verhaak, C.M.; Grootenhuis, M.A.; Haverman, L. Health related quality of life in Dutch infants, toddlers, and young children. Health Qual. Life Outcomes 2017, 15, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Damschroder, L.J.; Aron, D.C.; Keith, R.E.; Kirsh, S.R.; Alexander, J.A.; Lowery, J.C. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement. Sci. 2009, 4, 50. [Google Scholar] [CrossRef] [Green Version]
- Teela, L.; van Muilekom, M.M.; Kooij, L.H.; Gathier, A.W.; van Goudoever, J.B.; Grootenhuis, M.A.; Haverman, L.; van Oers, H.A. Clinicians’ perspective on the implemented KLIK PROM portal in clinical practice. Qual. Life Res. 2020, 11, 3267–3277. [Google Scholar] [CrossRef] [PubMed]
- Schepers, S.A.; Sint Nicolaas, S.M.; Haverman, L.; Wensing, M.; Schouten van Meeteren, A.Y.N.; Veening, M.A.; Caron, H.N.; Hoogerbrugge, P.M.; Kaspers, G.J.L.; Verhaak, C.M.; et al. Real-world implementation of electronic patient-reported outcomes in outpatient pediatric cancer care. Psychooncology 2017, 26, 951–959. [Google Scholar] [CrossRef]
- Van Muilekom, M.M.; Teela, L.; van Oers, H.A.; van Goudoever, J.B.; Grootenhuis, M.A.; Haverman, L. Patients’ and parents’ perspective on the implementation of Patient Reported Outcome Measures in pediatric clinical practice using the KLIK PROM portal. Qual. Life Res. 2021, 1, 241–254. [Google Scholar] [CrossRef]
- Schoenmaker, N.J.; Haverman, L.; Tromp, W.F.; Van Der Lee, J.H.; Offringa, M.; Adams, B.; Bouts, A.H.M.; Collard, L.; Cransberg, K.; Van Dyck, M.; et al. Children of non-Western origin with end-stage renal disease in the Netherlands, Belgium and a part of Germany have impaired health-related quality of life compared with Western children. Nephrol. Dial. Transplant. 2014, 29, 448–457. [Google Scholar] [CrossRef] [Green Version]
- Tjaden, L.A.; Vogelzang, J.; Jager, K.J.; Van Stralen, K.J.; Maurice-Stam, H.; Grootenhuis, M.A.; Groothoff, J.W. Long-term quality of life and social outcome of childhood end-stage renal disease. J. Pediatr. 2014, 165, 336–342. [Google Scholar] [CrossRef]
- Schick-Makaroff, K.; Levay, A.; Thompson, S.; Flynn, R.; Sawatzky, R.; Thummapol, O.; Klarenbach, S.; Karimi-Dehkordi, M.; Greenhalgh, J. An Evidence-Based Theory About PRO Use in Kidney Care: A Realist Synthesis. Patient 2022, 15, 21–38. [Google Scholar] [CrossRef]
- International Society for Quality of Life Research. User’s Guide to Implementing Patient-Reported Outcomes Assessment in Clinical Practice; Aaronson, N., Elliot, T., Greenhalgh, J., Halyard, M., Hess, R., Miller, D., Reeve, B.B., Santana, M.J., Snyder, C., Eds.; International Society for Quality of Life Research: Milwaukee, WI, USA, 2015. [Google Scholar]
- Weissberg-Benchell, J.; Zielinski, T.E.; Rodgers, S.; Greenley, R.N.; Askenazi, D.; Goldstein, S.L.; Fredericks, E.M.; McDiarmid, S.; Williams, L.; Limbers, C.A.; et al. Pediatric health-related quality of life: Feasibility, reliability and validity of the PedsQLTM transplant module. Am. J. Transplant. 2010, 10, 1686–1694. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veltkamp, F.; Teela, L.; van Oers, H.A.; Haverman, L.; Bouts, A.H.M. The Use of Patient-Reported Outcome Measures in Daily Clinical Practice of a Pediatric Nephrology Department. Int. J. Environ. Res. Public Health 2022, 19, 5338. https://doi.org/10.3390/ijerph19095338
Veltkamp F, Teela L, van Oers HA, Haverman L, Bouts AHM. The Use of Patient-Reported Outcome Measures in Daily Clinical Practice of a Pediatric Nephrology Department. International Journal of Environmental Research and Public Health. 2022; 19(9):5338. https://doi.org/10.3390/ijerph19095338
Chicago/Turabian StyleVeltkamp, Floor, Lorynn Teela, Hedy A. van Oers, Lotte Haverman, and Antonia H. M. Bouts. 2022. "The Use of Patient-Reported Outcome Measures in Daily Clinical Practice of a Pediatric Nephrology Department" International Journal of Environmental Research and Public Health 19, no. 9: 5338. https://doi.org/10.3390/ijerph19095338
APA StyleVeltkamp, F., Teela, L., van Oers, H. A., Haverman, L., & Bouts, A. H. M. (2022). The Use of Patient-Reported Outcome Measures in Daily Clinical Practice of a Pediatric Nephrology Department. International Journal of Environmental Research and Public Health, 19(9), 5338. https://doi.org/10.3390/ijerph19095338






