Correlation between the Positive Effect of Vitamin D Supplementation and Physical Performance in Young Male Soccer Players
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Approach
2.2. Characteristics of the Study Group
2.3. Vitamin D supplementation and Analysis
- Group I (n = 12)—subjected to training and supplemented with vitamin D (cholecalciferol in a dose of 20,000 IU (Decristol, Sun-Farm Sp. z o.o., Lomianki, Poland), which was taken twice a week for 8 weeks). Supplementation was carried out under the supervision of a sports dietitian during the preparation for the annual training cycle (from January to March).
- Group II (n = 13)—subjected to training only, without vitamin D supplementation.
2.4. Serum Collection and Evaluation of Serum 25-Hydroxyvitamin D Concentration
2.5. Sprint Tests
2.6. Jump Tests
2.7. Multistage Shuttle Run Test
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, G. Metabolism and biomarkers of vitamin D. Scand. J. Clin. Lab. Investig. Suppl. 2012, 243, 7–13. [Google Scholar]
- Jones, G.; Prosser, D.E.; Kaufmann, M. Cytochrome P450-mediated metabolism of vitamin D. J. Lipid Res. 2014, 55, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.J.; Allison, R.; Close, G.L. Vitamin D and the Athlete: Current Perspectives and New Challenges. Sports Med. 2018, 48, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-T.; Tavera-Mendoza, L.E.; Laperriere, D.; Libby, E.; MacLeod, N.B.; Nagai, Y.; Bourdeau, V.; Konstorum, A.; Lallemant, B.; Zhang, R.; et al. Large-Scale in Silico and Microarray-Based Identification of Direct 1,25-Dihydroxyvitamin D3 Target Genes. Mol. Endocrinol. 2005, 19, 2685–2695. [Google Scholar] [CrossRef] [PubMed]
- Hii, C.S.; Ferrante, A. The Non-Genomic Actions of Vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A. Relevance of vitamin D in muscle health. Rev. Endocr. Metab. Disord. 2012, 13, 71–77. [Google Scholar] [CrossRef]
- Gallagher, J.C. Vitamin D and bone density, fractures, and falls: The end of the story? Lancet Diabetes Endocrinol. 2018, 6, 834–835. [Google Scholar] [CrossRef]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef]
- Boland, R.; De Boland, A.R.; Buitrago, C.; Morelli, S.; Santillán, G.; Vazquez, G.; Capiati, D.; Baldi, C. Non-genomic stimulation of tyrosine phosphorylation cascades by 1,25(OH)2D3 by VDR-dependent and -independent mechanisms in muscle cells. Steroids 2002, 67, 477–482. [Google Scholar] [CrossRef]
- Wali, R.K.; Kong, J.; Sitrin, M.D.; Bissonnette, M.; Li, Y.C. Vitamin D receptor is not required for the rapid actions of 1,25-dihydroxyvitamin D3 to increase intracellular calcium and activate protein kinase C in mouse osteoblasts. J. Cell. Biochem. 2003, 88, 794–801. [Google Scholar] [CrossRef]
- Buitrago, C.G.; Arango, N.S.; Boland, R.L. 1α,25(OH)2D3-dependent modulation of Akt in proliferating and differentiating C2C12 skeletal muscle cells. J. Cell. Biochem. 2012, 113, 1170–1181. [Google Scholar] [CrossRef] [PubMed]
- Stratos, I.; Li, Z.; Herlyn, P.; Rotter, R.; Behrendt, A.-K.; Mittlmeier, T.; Vollmar, B. Vitamin D Increases Cellular Turnover and Functionally Restores the Skeletal Muscle after Crush Injury in Rats. Am. J. Pathol. 2013, 182, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Trovato, F.M.; Castrogiovanni, P.; Szychlinska, M.A.; Purrello, F.; Musumeci, G. Impact of Western and Mediterranean Diets and Vitamin D on Muscle Fibers of Sedentary Rats. Nutrients 2018, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Oku, Y.; Tanabe, R.; Nakaoka, K.; Yamada, A.; Noda, S.; Hoshino, A.; Haraikawa, M.; Goseki-Sone, M. Influences of dietary vitamin D restriction on bone strength, body composition and muscle in rats fed a high-fat diet: Involvement of mRNA expression of MyoD in skeletal muscle. J. Nutr. Biochem. 2016, 32, 85–90. [Google Scholar] [CrossRef]
- Polly, P.; Tan, T.C. The role of vitamin D in skeletal and cardiac muscle function. Front. Physiol. 2014, 5, 145. [Google Scholar] [CrossRef] [PubMed]
- Ogan, D.; Pritchett, K. Vitamin D and the Athlete: Risks, Recommendations, and Benefits. Nutrients 2013, 5, 1856–1868. [Google Scholar] [CrossRef]
- Sugimoto, H.; Shiro, Y. Diversity and Substrate Specificity in the Structures of Steroidogenic Cytochrome P450 Enzymes. Biol. Pharm. Bull. 2012, 35, 818–823. [Google Scholar] [CrossRef][Green Version]
- Bacchetta, J.; Zaritsky, J.J.; Sea, J.L.; Chun, R.; Lisse, T.S.; Zavala, K.; Nayak, A.; Wesseling-Perry, K.; Westerman, M.; Hollis, B.W.; et al. Suppression of Iron-Regulatory Hepcidin by Vitamin D. J. Am. Soc. Nephrol. 2014, 25, 564–572. [Google Scholar] [CrossRef]
- Sinha, A.; Hollingsworth, K.G.; Ball, S.; Cheetham, T. Improving the Vitamin D Status of Vitamin D Deficient Adults Is Associated with Improved Mitochondrial Oxidative Function in Skeletal Muscle. J. Clin. Endocrinol. Metab. 2013, 98, E509–E513. [Google Scholar] [CrossRef]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Rusińska, A.; Płudowski, P.; Walczak, M.; Borszewska-Kornacka, M.K.; Bossowski, A.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dobrzańska, A.; Franek, E.; Helwich, E.; et al. Vitamin D Supplementation Guidelines for General Population and Groups at Risk of Vitamin D Deficiency in Poland—Recommendations of the Polish Society of Pediatric Endocrinology and Diabetes and the Expert Panel with Participation of National Specialist Consultants and Representatives of Scientific Societies—2018 Update. Front. Endocrinol. 2018, 9, 246. [Google Scholar] [CrossRef]
- Pludowski, P.; Holick, M.F.; Grant, W.B.; Konstantynowicz, J.; Mascarenhas, M.R.; Haq, A.; Povoroznyuk, V.; Balatska, N.; Barbosa, A.P.; Karonova, T.; et al. Vitamin D supplementation guidelines. J. Steroid Biochem. Mol. Biol. 2018, 175, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Close, G.; Russell, J.; Cobley, J.; Owens, D.; Wilson, G.; Gregson, W.; Fraser, W.; Morton, J. Assessment of vitamin D concentration in non-supplemented professional athletes and healthy adults during the winter months in the UK: Implications for skeletal muscle function. J. Sports Sci. 2013, 31, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Książek, A.; Zagrodna, A.; Słowińska-Lisowska, M. Vitamin D, Skeletal Muscle Function and Athletic Performance in Athletes—A Narrative Review. Nutrients 2019, 11, 1800. [Google Scholar] [CrossRef]
- Hamilton, B.; Whiteley, R.; Farooq, A.; Chalabi, H. Vitamin D concentration in 342 professional football players and association with lower limb isokinetic function. J. Sci. Med. Sport 2014, 17, 139–143. [Google Scholar] [CrossRef]
- Fishman, M.P.; Lombardo, S.J.; Kharrazi, F.D. Vitamin D Deficiency Among Professional Basketball Players. Orthop. J. Sports Med. 2016, 4, 2325967116655742. [Google Scholar] [CrossRef]
- Cannell, J.J.; Hollis, B.W.; Sorenson, M.B.; Taft, T.N.; Anderson, J.J.B. Athletic Performance and Vitamin D. Med. Sci. Sports Exerc. 2009, 41, 1102–1110. [Google Scholar] [CrossRef]
- Farrokhyar, F.; Tabasinejad, R.; Dao, D.; Peterson, D.; Ayeni, O.R.; Hadioonzadeh, R.; Bhandari, M. Prevalence of Vitamin D Inadequacy in Athletes: A Systematic-Review and Meta-Analysis. Sports Med. 2015, 45, 365–378. [Google Scholar] [CrossRef]
- Holick, M.F. The Vitamin D Epidemic and its Health Consequences. J. Nutr. 2005, 135, 2739S–2748S. [Google Scholar] [CrossRef]
- Owens, D.J.; Fraser, W.D.; Close, G.L. Vitamin D and the athlete: Emerging insights. Eur. J. Sport Sci. 2015, 15, 73–84. [Google Scholar] [CrossRef]
- Krzywanski, J.; Mikulski, T.; Krysztofiak, H.; Mlynczak, M.; Gaczynska, E.; Ziemba, A. Seasonal Vitamin D Status in Polish Elite Athletes in Relation to Sun Exposure and Oral Supplementation. PLoS ONE 2016, 11, e0164395. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Li, X.; Tan, Q.; Shao, J.; Yi, M. Effects of vitamin D3 supplementation on serum 25(OH)D concentration and strength in athletes: A systematic review and meta-analysis of randomized controlled trials. J. Int. Soc. Sports Nutr. 2019, 16, 55. [Google Scholar] [CrossRef] [PubMed]
- Żebrowska, A.; Sadowska-Krępa, E.; Stanula, A.; Waśkiewicz, Z.; Łakomy, O.; Bezuglov, E.; Nikolaidis, P.T.; Rosemann, T.; Knechtle, B. The effect of vitamin D supplementation on serum total 25(OH) levels and biochemical markers of skeletal muscles in runners. J. Int. Soc. Sports Nutr. 2020, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Quan, M.; Cao, Z.-B. Effect of vitamin D supplementation on upper and lower limb muscle strength and muscle power in athletes: A meta-analysis. PLoS ONE 2019, 14, e0215826. [Google Scholar] [CrossRef] [PubMed]
- Farrokhyar, F.; Sivakumar, G.K.; Savage, K.; Koziarz, A.; Jamshidi, S.; Ayeni, O.R.; Peterson, D.; Bhandari, M. Effects of Vitamin D Supplementation on Serum 25-Hydroxyvitamin D Concentrations and Physical Performance in Athletes: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Sports Med. 2017, 47, 2323–2339. [Google Scholar] [CrossRef]
- Wyon, M.A.; Koutedakis, Y.; Wolman, R.; Nevill, A.M.; Allen, N. The influence of winter vitamin D supplementation on muscle function and injury occurrence in elite ballet dancers: A controlled study. J. Sci. Med. Sport 2014, 17, 8–12. [Google Scholar] [CrossRef]
- Stockton, K.A.; Mengersen, K.; Paratz, J.D.; Kandiah, D.; Bennell, K.L. Effect of vitamin D supplementation on muscle strength: A systematic review and meta-analysis. Osteoporos. Int. 2011, 22, 859–871. [Google Scholar] [CrossRef]
- Jung, H.C.; Seo, M.W.; Lee, S.; Jung, S.W.; Song, J.K. Correcting Vitamin D Insufficiency Improves Some but Not All Aspects of Physical Performance During Winter Training in Taekwondo Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 635–643. [Google Scholar] [CrossRef]
- Bezuglov, E.; Tikhonova, A.; Zueva, A.; Khaitin, V.; Lyubushkina, A.; Achkasov, E.; Waśkiewicz, Z.; Gerasimuk, D.; Żebrowska, A.; Nikolaidis, P.T.; et al. The Dependence of Running Speed and Muscle Strength on the Serum Concentration of Vitamin D in Young Male Professional Football Players Residing in the Russian Federation. Nutrients 2019, 11, 1960. [Google Scholar] [CrossRef]
- Brännström, A.; Yu, J.-G.; Jonsson, P.; Åkerfeldt, T.; Stridsberg, M.; Svensson, M. Vitamin D in relation to bone health and muscle function in young female soccer players. Eur. J. Sport Sci. 2017, 17, 249–256. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Shanely, R.A.; Dew, D.; Meaney, M.P.; Luo, B. Vitamin D2 Supplementation Amplifies Eccentric Exercise-Induced Muscle Damage in NASCAR Pit Crew Athletes. Nutrients 2013, 6, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D. Vitamin D Deficiency and Energy Metabolism. Endocrinology 2015, 156, 1933–1935. [Google Scholar] [CrossRef] [PubMed]
- Close, G.L.; Leckey, J.; Patterson, M.; Bradley, W.; Owens, D.J.; Fraser, W.D.; Morton, J.P. The effects of vitamin D3supplementation on serum total 25[OH]D concentration and physical performance: A randomised dose–response study. Br. J. Sports Med. 2013, 47, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Byers, A.W.; Connolly, G.; Campbell, W.W. Vitamin D status and supplementation impacts on skeletal muscle function: Comparisons between young athletes and older adults. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Górski, J. Fizjologiczne Podstawy Wysiłku Fizycznego; PZWL: Warszawa, Poland, 2006. [Google Scholar]
- Helgerud, J.; Engen, L.C.; Wisløff, U.; Hoff, J. Aerobic endurance training improves soccer performance. Med. Sci. Sports Exerc. 2001, 33, 1925–1931. [Google Scholar] [CrossRef]
- Yagüe, M.D.L.P.; Yurrita, L.C.; Cabañas, M.C.; Cenzual, M.C. Role of Vitamin D in Athletes and Their Performance: Current Concepts and New Trends. Nutrients 2020, 12, 579. [Google Scholar] [CrossRef]
- Fitzgerald, J.S.; Peterson, B.J.; Warpeha, J.M.; Wilson, P.B.; Rhodes, G.S.; Ingraham, S.J. Vitamin D Status and V[Combining Dot Above]O2peak During a Skate Treadmill Graded Exercise Test in Competitive Ice Hockey Players. J. Strength Cond. Res. 2014, 28, 3200–3205. [Google Scholar] [CrossRef]
- Von Hurst, P.R.; Beck, K.L. Vitamin D and skeletal muscle function in athletes. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 539–545. [Google Scholar] [CrossRef]
- Jastrzebski, Z.; Wnorowski, K.; Mikolajewski, R.; Jaskulska, E.; Radziminski, L. The Effect of a 6-Week Plyometric Training on Explosive Power in Volleyball Players. Balt. J. Heal. Phys. Act. 2014, 6, 79–89. [Google Scholar] [CrossRef]
- Jastrzębska, M.; Kaczmarczyk, M.; Michalczyk, M.; Radzimiński, Ł.; Stępień, P.; Jastrzębska, J.; Wakuluk, D.; Suárez, A.D.; Lopez Sánchez, G.F.; Cieszczyk, P.; et al. Can Supplementation of Vitamin D Improve Aerobic Capacity in Well Trained Youth Soccer Players? J. Hum. Kinet. 2018, 61, 63–72. [Google Scholar] [CrossRef]
- Skalska, M.; Nikolaidis, P.T.; Knechtle, B.; Rosemann, T.J.; Radzimiński, Ł.; Jastrzębska, J.; Kaczmarczyk, M.; Myśliwiec, A.; Dragos, P.; López-Sánchez, G.F.; et al. Vitamin D Supplementation and Physical Activity of Young Soccer Players during High-Intensity Training. Nutrients 2019, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Sarmento, H.; Clemente, F.M.; Harper, L.D.; Da Costa, I.T.; Owen, A.; Figueiredo, A.J. Small sided games in soccer—A systematic review. Int. J. Perform. Anal. Sport 2018, 18, 693–749. [Google Scholar] [CrossRef]
- Stølen, T.; Chamari, K.; Castagna, C.; Wisløff, U. Physiology of Soccer: An update. Sports Med. 2005, 35, 501–536. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, A.; Solarz, K.; Majda, F.; Słowińska-Lisowska, M.; Mędraś, M. An Evaluation of the Levels of Vitamin D and Bone Turnover Markers After the Summer and Winter Periods in Polish Professional Soccer Players. J. Hum. Kinet. 2013, 38, 135–140. [Google Scholar] [CrossRef]
- Constantini, N.W.; Arieli, R.; Chodick, G.; Dubnov-Raz, G. High Prevalence of Vitamin D Insufficiency in Athletes and Dancers. Clin. J. Sport Med. 2010, 20, 368–371. [Google Scholar] [CrossRef]
- Książek, A.; Zagrodna, A.; Słowińska-Lisowska, M. Assessment of the Dietary Intake of High-Rank Professional Male Football Players during a Preseason Training Week. Int. J. Environ. Res. Public Health 2020, 17, 8567. [Google Scholar] [CrossRef]
- Bzikowska-Jura, A.; Sobieraj, P.; Raciborski, F. Low Comparability of Nutrition-Related Mobile Apps against the Polish Reference Method—A Validity Study. Nutrients 2021, 13, 2868. [Google Scholar] [CrossRef]
- Jastrzębska, J.; Skalska, M.; Radzimiński, Ł.; López-Sánchez, G.F.; Weiss, K.; Hill, L.; Knechtle, B. Changes of 25(OH)D Concentration, Bone Resorption Markers and Physical Performance as an Effect of Sun Exposure, Supplementation of Vitamin D and Lockdown among Young Soccer Players during a One-Year Training Season. Nutrients 2022, 14, 521. [Google Scholar] [CrossRef]
- Płudowski, P.; Karczmarewicz, E.; Bayer, M.; Carter, G.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dębski, R.; Decsi, T.; Dobrzańska, A.; Franek, E.; et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe—recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency. Endokrynol. Polska 2013, 64, 319–327. [Google Scholar] [CrossRef]
- Léger, L.A.; Lambert, J. A maximal multistage 20-m shuttle run test to predict VO2 max. Eur. J. Appl. Physiol. Occup. Physiol. 1982, 49, 1–12. [Google Scholar] [CrossRef]
- Radzimiński, Ł.; Szwarc, A.; Padrón-Cabo, A.; Jastrzębski, Z. Correlations between body composition, aerobic capacity, speed and distance covered among professional soccer players during official matches. J. Sports Med. Phys. Fit. 2020, 60, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Ramsbottom, R.; Brewer, J.; Williams, C. A progressive shuttle run test to estimate maximal oxygen uptake. Br. J. Sports Med. 1988, 22, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.T.E. Eta squared and partial eta squared as measures of effect size in educational research. Educ. Res. Rev. 2011, 6, 135–147. [Google Scholar] [CrossRef]
- Chan, Y.H. Biostatistics 104: Correlational analysis. Singapore Med. J. 2003, 44, 614–619. [Google Scholar] [PubMed]
- Michalczyk, M.M.; Gołaś, A.; Maszczyk, A.; Kaczka, P.; Zając, A. Influence of Sunlight and Oral D3 Supplementation on Serum 25(OH)D Concentration and Exercise Performance in Elite Soccer Players. Nutrients 2020, 12, 1311. [Google Scholar] [CrossRef] [PubMed]
- Książek, A.; Zagrodna, A.; Dziubek, W.; Pietraszewski, B.; Ochmann, B.; Lisowska, M.S. 25(OH)D3 Levels Relative to Muscle Strength and Maximum Oxygen Uptake in Athletes. J. Hum. Kinet. 2016, 50, 71–77. [Google Scholar] [CrossRef]
- Jastrzębski, Z. Effect of vitamin D supplementation on the level of physical fitness and blood parameters of rowers during the 8-week high intensity training. Facicula Educ Fiz Si Sport 2014, 2, 57–67. [Google Scholar]
- Jastrzębska, M.; Kaczmarczyk, M.; Jastrzębski, Z. Effect of Vitamin D Supplementation on Training Adaptation in Well-Trained Soccer Players. J. Strength Cond. Res. 2016, 30, 2648–2655. [Google Scholar] [CrossRef]
- Misiorowski, W.; Misiorowska, J.; Dębski, R.; Głuszko, P.; Tłustochowicz, W.; Zgliczyński, W. Stanowisko zespołu ekspertów w sprawie stosowania wysokich dawek witaminy D w zapobieganiu i leczeniu jej niedoboru. Medycyna po Dyplomie 2017, 257, 36–45. [Google Scholar]
- Toth, E.B.; Takacs, I.; Szekeres, L.; Szabo, B.; Bakos, B.; Lakatos, P. Safety and Efficacy of Weekly 30,000 IU Vitamin D Supplementation as a Slower Loading Dose Administration Compared to a Daily Maintenance Schedule in Deficient Patients: A Randomized, Controlled Clinical Trial. J. Pharmacovigil. 2017, 5, 4. [Google Scholar] [CrossRef]
- Sanders, K.M.; Stuart, A.L.; Williamson, E.; Simpson, J.A.; Kotowicz, M.; Young, D.; Nicholson, G. Annual High-Dose Oral Vitamin D and Falls and Fractures in Older Women: A randomized controlled trial. JAMA 2010, 303, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Malihi, Z.; Lawes, C.M.M.; Wu, Z.; Huang, Y.; Waayer, D.; Toop, L.; Khaw, K.-T.; Camargo, A.C.; Scragg, R. Monthly high-dose vitamin D supplementation does not increase kidney stone risk or serum calcium: Results from a randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1578–1587. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P.; Takacs, I.; Boyanov, M.; Belaya, Z.; Diaconu, C.C.; Mokhort, T.; Zherdova, N.; Rasa, I.; Payer, J.; Pilz, S. Clinical Practice in the Prevention, Diagnosis and Treatment of Vitamin D Deficiency: A Central and Eastern European Expert Consensus Statement. Nutrients 2022, 14, 1483. [Google Scholar] [CrossRef] [PubMed]
- Takács, I.; Tóth, B.E.; Szekeres, L.; Szabó, B.; Bakos, B.; Lakatos, P. Randomized clinical trial to comparing efficacy of daily, weekly and monthly administration of vitamin D3. Endocrine 2017, 55, 60–65. [Google Scholar] [CrossRef]
- Mawer, E.B.; Lumb, G.A.; Schaefer, K.; Stanbury, S.W. The Metabolism of Isotopically Labelled Vitamin D3 in Man: The Influence of the State of Vitamin D Nutrition. Clin. Sci. 1971, 40, 39–53. [Google Scholar] [CrossRef]
- Jones, K.S.; Assar, S.; Harnpanich, D.; Bouillon, R.; Lambrechts, D.; Prentice, A.; Schoenmakers, I. 25(OH)D2 Half-Life Is Shorter Than 25(OH)D3 Half-Life and Is Influenced by DBP Concentration and Genotype. J. Clin. Endocrinol. Metab. 2014, 99, 3373–3381. [Google Scholar] [CrossRef]
- Shieh, A.; Chun, R.F.; Ma, C.; Witzel, S.; Meyer, B.; Rafison, B.; Swinkels, L.; Huijs, T.; Pepkowitz, S.; Holmquist, B.; et al. Effects of High-Dose Vitamin D2 Versus D3 on Total and Free 25-Hydroxyvitamin D and Markers of Calcium Balance. J. Clin. Endocrinol. Metab. 2016, 101, 3070–3078. [Google Scholar] [CrossRef]
- Barker, T.; Henriksen, V.T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Schneider, E.D.; Dixon, B.M.; Weaver, L.K. Higher Serum 25-Hydroxyvitamin D Concentrations Associate with a Faster Recovery of Skeletal Muscle Strength after Muscular Injury. Nutrients 2013, 5, 1253–1275. [Google Scholar] [CrossRef]
- Dahlquist, D.T.; Dieter, B.P.; Koehle, M.S. Plausible ergogenic effects of vitamin D on athletic performance and recovery. J. Int. Soc. Sports Nutr. 2015, 12, 33. [Google Scholar] [CrossRef]
- Koundourakis, N.E.; Androulakis, N.E.; Malliaraki, N.; Margioris, A.N. Vitamin D and Exercise Performance in Professional Soccer Players. PLoS ONE 2014, 9, e101659. [Google Scholar] [CrossRef]
- Paul, D.J.; Nassis, G.P. Testing Strength and Power in Soccer Players: The application of conventional and traditional methods of assessment. J. Strength Cond. Res. 2015, 29, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- Beneke, R.; Böning, D. The limits of human performance. Essays Biochem. 2008, 44, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Ardestani, A.; Parker, B.; Mathur, S.; Clarkson, P.; Pescatello, L.S.; Hoffman, H.J.; Polk, D.M.; Thompson, P.D. Relation of Vitamin D Level to Maximal Oxygen Uptake in Adults. Am. J. Cardiol. 2011, 107, 1246–1249. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Tangpricha, V. Vitamin D and anemia: Insights into an emerging association. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 432–438. [Google Scholar] [CrossRef]
- Gregory, S.M.; Parker, B.A.; Capizzi, J.A.; Grimaldi, A.S.; Clarkson, P.M.; Moeckel-Cole, S.; Thompson, P.D. Changes in Vitamin D are Not Associated with Changes in Cardiorespiratory Fitness. Clin. Med. Res. 2013, 2, 68. [Google Scholar] [CrossRef]
- Lewis, J.R.; Sim, M.; Daly, R.M. The vitamin D and calcium controversy: An update. Curr. Opin. Rheumatol. 2019, 31, 91–97. [Google Scholar] [CrossRef]

| Parameter | Total Group (TG) |
|---|---|
| Age [years] | 17.5 ± 0.70 |
| Body height [cm] | Height 178 ± 0.70 |
| Body weight [kg] | Weight 68.05 ± 9.18 |
| Training period [weeks] | 8 weeks in the preparation period (winter, from mid-January to mid-March) |
| Training unit [min] | Each training session was a continuous 90 min, 5 training units, and a control (friendly) match each week. In addition, the players participated in 2 units of physical education lessons at school (90 min) with a focus on soccer practice. |
| Group | TG | GN | GS | Interactions | p | ES | |||
|---|---|---|---|---|---|---|---|---|---|
| Time-Point | T1 | T2 | T1 | T2 | T1 | T2 | |||
| 25(OH)D [ng/mL] | 26.7 ± 10.01 | 36.9 ± 15.74 † | 25.5 ± 9.52 | 27.2 ± 12.06 | 27.9 ± 10.79 | 47.4 ± 12.21 *† | group time group × time | 0.006 0.0003 0.002 | 0.29 0.44 0.36 |
| Sprint 5 m [s] | 1.01 ± 0.04 | 1.02 ± 0.05 | 1.01 ± 0.06 | 1.01 ± 0.05 | 1.01 ± 0.03 | 1.02 ± 0.05 | group time group × time | 0.80 0.14 0.36 | 0.00 0.09 0.04 |
| Sprint 10 m [s] | 1.77 ± 0.06 | 1.77 ± 0.06 | 1.77 ± 0.07 | 1.75 ± 0.06 | 1.77 ± 0.06 | 1.78 ± 0.06 | group time group × time | 0.45 0.50 0.05 | 0.02 0.02 0.15 |
| Sprint 30 m [s] | 4.24 ± 0.13 | 4.23 ± 0.13 | 4.23 ± 0.18 | 4.21 ± 0.15 | 4.25 ± 0.12 | 4.25 ± 0.10 | group time group × time | 0.54 0.48 0.57 | 0.02 0.02 0.01 |
| MST dist. [m] | 2408 ± 226.0 | 2510 ± 227.1 † | 2395 ± 210.9 | 2492 ± 208.7 | 2422 ± 250.0 | 2530 ± 253.3 † | group time group × time | 0.72 0.000 0.83 | 0.01 0.42 0.00 |
| VO2max [ml/kg/min] | 57.7 ± 3.08 | 59.2 ± 3.05 † | 57.6 ± 2.88 | 58.9 ± 2.82 | 57.9 ± 3.40 | 59.4 ± 3.39 † | group time group × time | 0.73 0.001 0.80 | 0.01 0.41 0.00 |
| 10 jumps [cm] | 37.4 ± 3.55 | 38.5 ± 3.45 | 38.5 ± 3.33 | 39.4 ± 2.66 | 36.1 ± 3.49 | 37.6 ± 4.05 | group time group × time | 0.09 0.07 0.62 | 0.12 0.13 0.01 |
| 10 jumps [W/kg] | 48.3 ± 3.07 | 49.3 ± 2.92 | 49.4 ± 2.97 | 50.0 ± 2.33 | 47.2 ± 2.86 | 48.4 ± 3.36 | group time group × time | 0.07 0.10 0.58 | 0.13 0.11 0.01 |
| SJ [cm] | 36.0 ± 4.09 | 36.6 ± 4.41 | 36.6 ± 3.99 | 37.5 ± 4.46 | 35.3 ± 4.27 | 35.6 ± 4.33 | group time group × time | 0.33 0.28 0.61 | 0.04 0.05 0.01 |
| SJ [W/kg] | 47.1 ± 3.55 | 47.6 ± 3.73 | 47.8 ± 3.50 | 48.4 ± 3.76 | 46.4 ± 3.62 | 46.8 ± 3.67 | group time group × time | 0.30 0.32 0.73 | 0.05 0.04 0.01 |
| CMJ [cm] | 43.9 ± 4.57 | 44.0 ± 3.94 | 45.4 ± 4.8 | 44.7 ± 4.17 | 42.3 ± 3.88 | 43.2 ± 3.67 | group time group × time | 0.14 0.86 0.26 | 0.09 0.00 0.05 |
| CMJ [W/kg] | 53.9 ± 4.26 | 53.9 ± 3.45 | 55.3 ± 4.80 | 54.6 ± 3.75 | 52.4 ± 3.14 | 53.1 ± 3.07 | group time group × time | 0.13 0.96 0.25 | 0.09 0.00 0.06 |
| Variables | 25(OH)D [ng/mL] | |
|---|---|---|
| R | p-Value | |
| Sprint 5 m [s] | 0.0562 | 0.6981 |
| Sprint 10 m [s] | 0.0138 | 0.9242 |
| Sprint 30 m [s] | 0.0920 | 0.5251 |
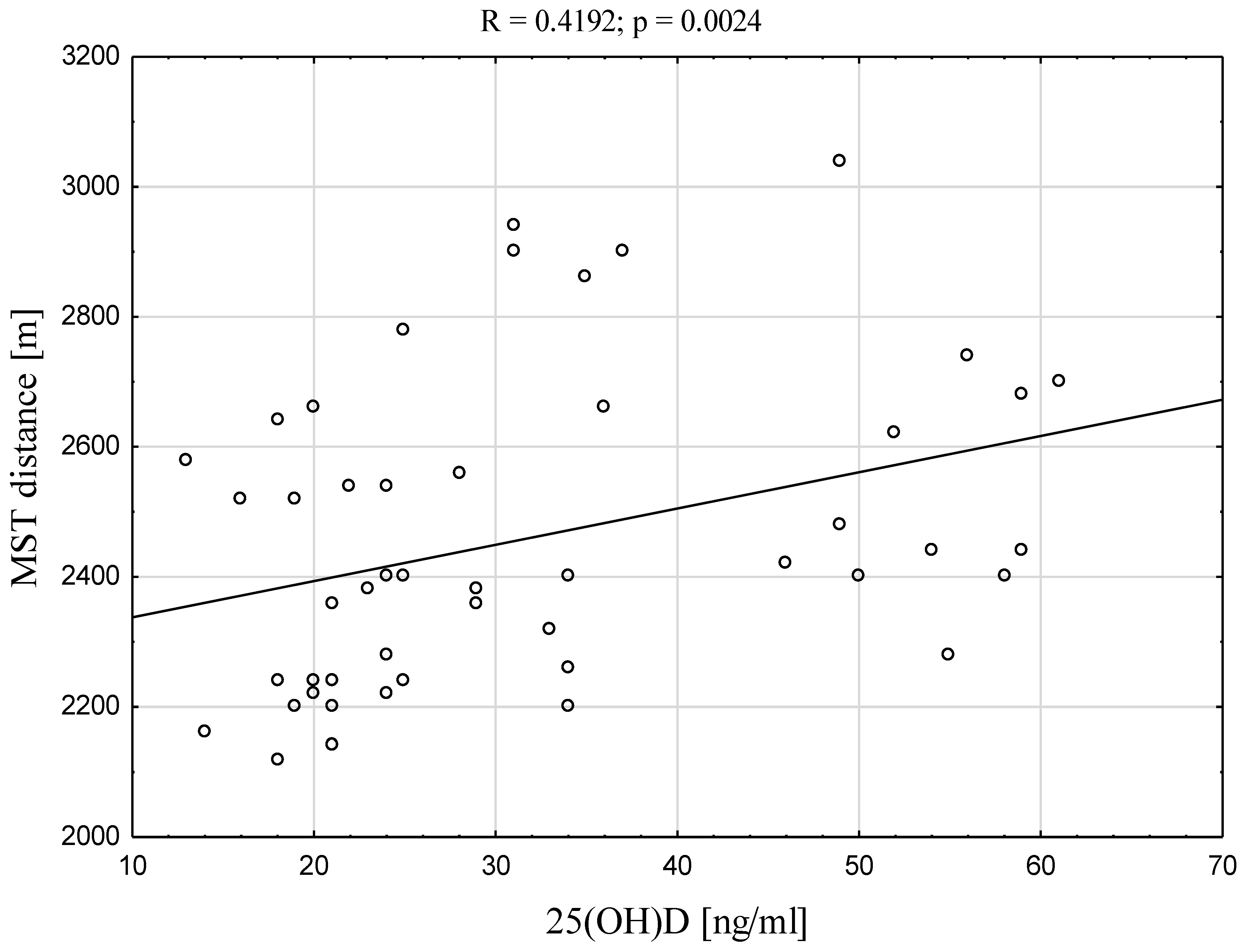
| MST dist. [m] | 0.4192 | 0.0024 * |
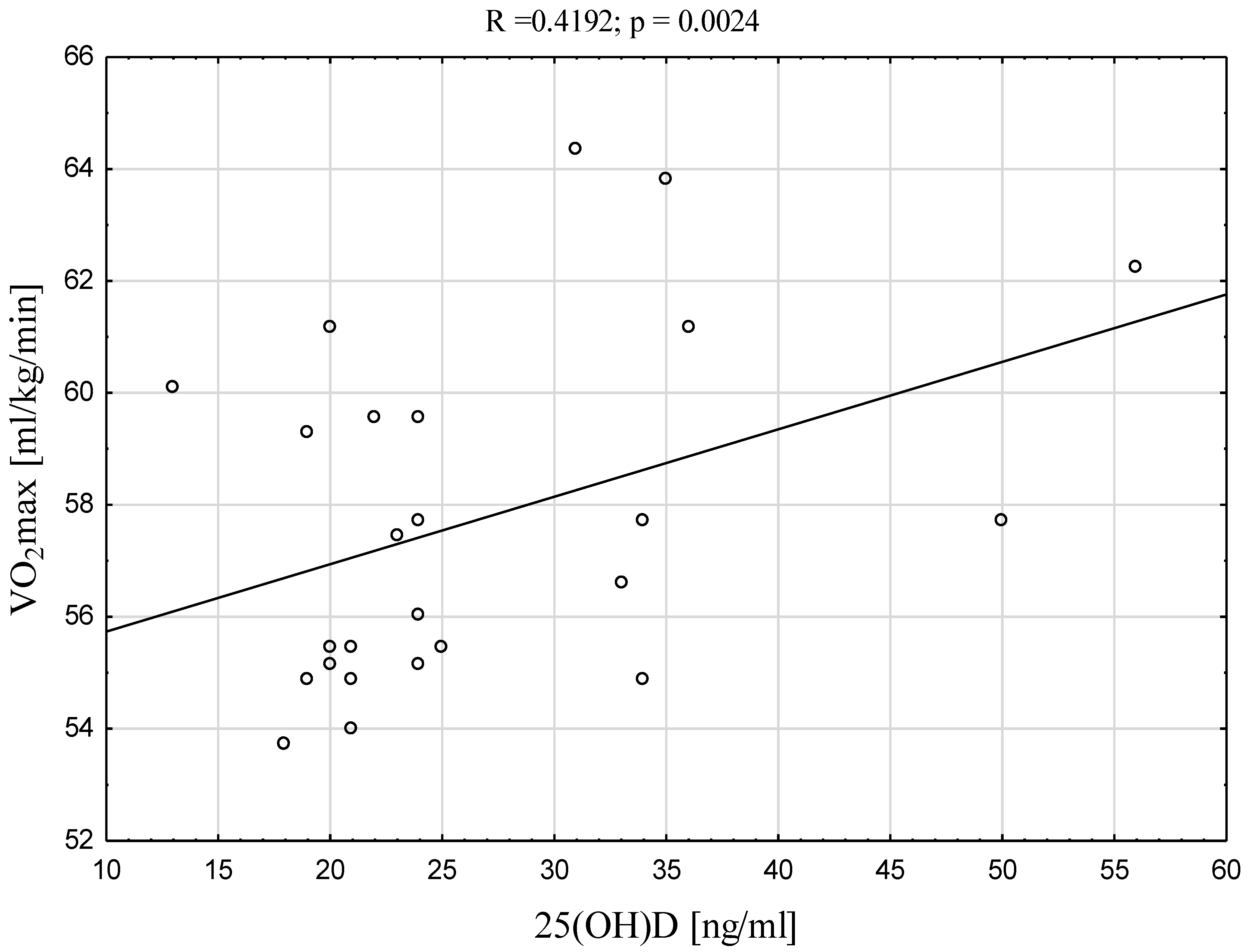
| VO2max [ml/kg/min] | 0.4192 | 0.0024 * |
| 10 jumps [cm] | −0.1878 | 0.1916 |
| 10 jumps [W/kg] | −0.1937 | 0.1777 |
| SJ [cm] | −0.0680 | 0.6390 |
| SJ [W/kg] | −0.0729 | 0.6149 |
| CMJ [cm] | −0.0960 | 0.5074 |
| CMJ [W/kg] | −0.0997 | 0.4910 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brzeziański, M.; Migdalska-Sęk, M.; Czechowska, A.; Radzimiński, Ł.; Jastrzębski, Z.; Brzeziańska-Lasota, E.; Sewerynek, E. Correlation between the Positive Effect of Vitamin D Supplementation and Physical Performance in Young Male Soccer Players. Int. J. Environ. Res. Public Health 2022, 19, 5138. https://doi.org/10.3390/ijerph19095138
Brzeziański M, Migdalska-Sęk M, Czechowska A, Radzimiński Ł, Jastrzębski Z, Brzeziańska-Lasota E, Sewerynek E. Correlation between the Positive Effect of Vitamin D Supplementation and Physical Performance in Young Male Soccer Players. International Journal of Environmental Research and Public Health. 2022; 19(9):5138. https://doi.org/10.3390/ijerph19095138
Chicago/Turabian StyleBrzeziański, Michał, Monika Migdalska-Sęk, Aleksandra Czechowska, Łukasz Radzimiński, Zbigniew Jastrzębski, Ewa Brzeziańska-Lasota, and Ewa Sewerynek. 2022. "Correlation between the Positive Effect of Vitamin D Supplementation and Physical Performance in Young Male Soccer Players" International Journal of Environmental Research and Public Health 19, no. 9: 5138. https://doi.org/10.3390/ijerph19095138
APA StyleBrzeziański, M., Migdalska-Sęk, M., Czechowska, A., Radzimiński, Ł., Jastrzębski, Z., Brzeziańska-Lasota, E., & Sewerynek, E. (2022). Correlation between the Positive Effect of Vitamin D Supplementation and Physical Performance in Young Male Soccer Players. International Journal of Environmental Research and Public Health, 19(9), 5138. https://doi.org/10.3390/ijerph19095138








