Implementation of Interventions and Policies on Opioids and Awareness of Opioid-Related Harms in Canada: A Multistage Mixed Methods Descriptive Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Overview
2.2. Implementation: Scoping Review of Interventions and Policies
2.3. Awareness Outcome: Spontaneous Reports of Opioid-Related Harms
2.4. Awareness Outcome: Social Media Posts
2.5. Health Outcome: Opioid-Related Death (Province of Quebec Only)
3. Results
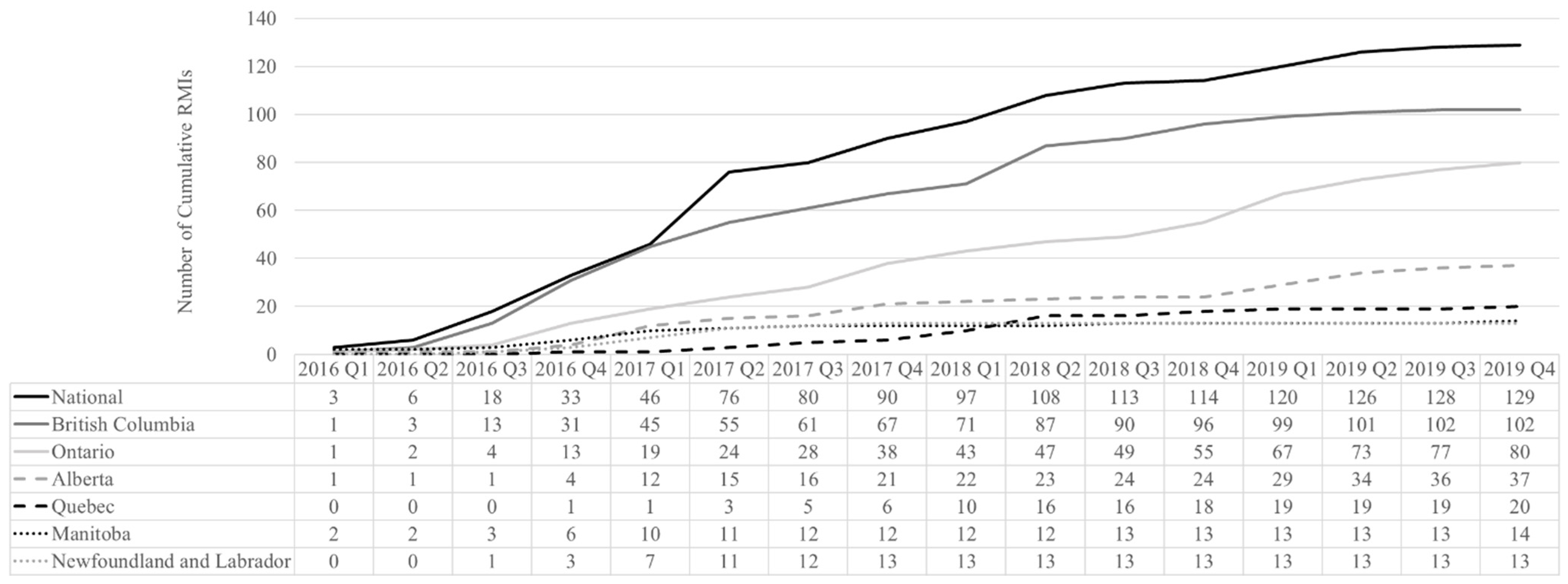
3.1. Implementation: Scoping Review of Interventions and Policies
3.2. Awareness Outcome: Spontaneous Reports of Opioid-Related Harms
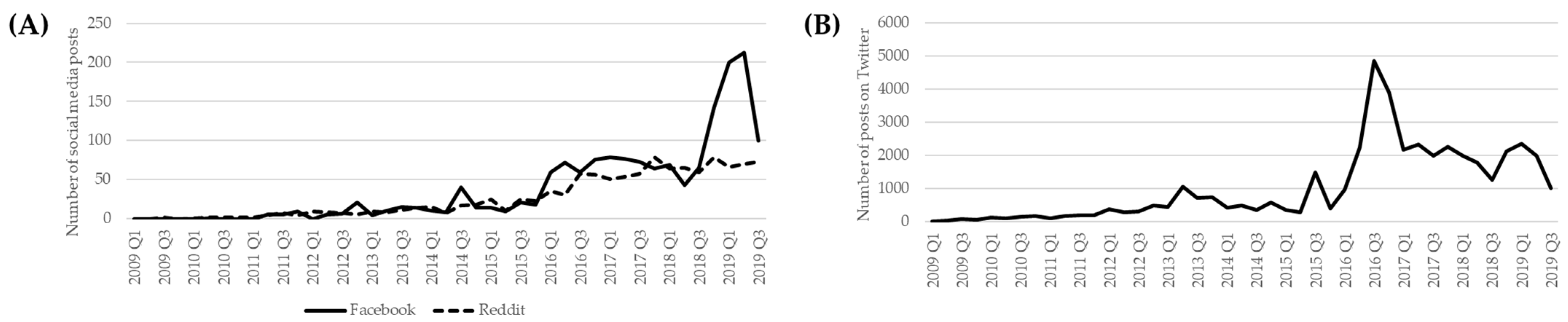
3.3. Awareness Outcome: Social Media Posts
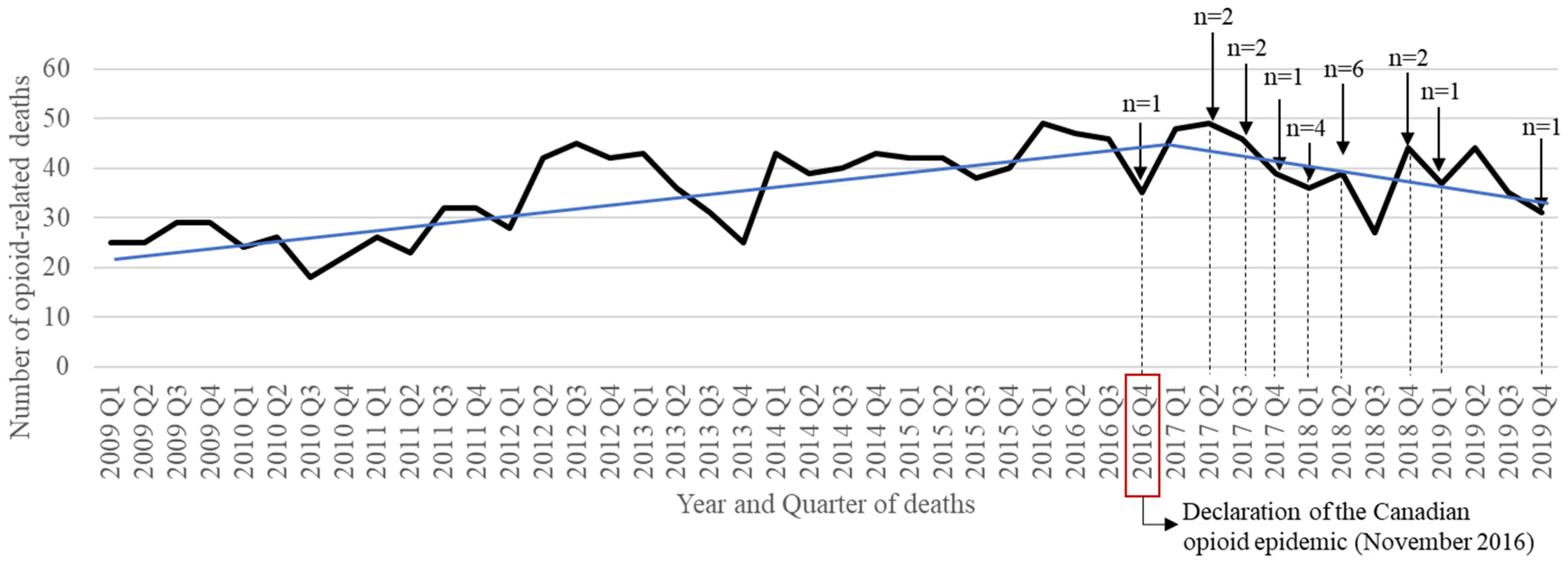
3.4. Health Outcome: Opioid-Related Death (Province of Quebec Only)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Government of Canada. Opioid- and Stimulant-Related Harms in Canada. Government of Canada; 2021. Available online: https://health-infobase.canada.ca/substance-related-harms/opioids-stimulants (accessed on 5 April 2022).
- Belzak, L.; Halvesron, J. Evidence synthesis—The opioid crisis in Canada: A national perspective. Health Promot. Chronic Dis. Prev. Can. 2018, 38, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Canadian Public Health Association. The Opioid Crisis in Canada. Canadian Public Health Association; 2016. Available online: https://www.cpha.ca/sites/default/files/uploads/policy/positionstatements/opioid-positionstatement-e.pdf (accessed on 5 April 2022).
- CCSA. The Availability of Take-Home Naloxone in Canada. Canadian Centre on Substance Abuse. 2016. Available online: https://www.ccsa.ca/sites/default/files/2019–05/CCSA-CCENDU-Take-Home-Naloxone-Canada-2016-en.pdf (accessed on 5 April 2022).
- Government of Canada. Federal actions on opioids to date. Government of Canada; 2022. Available online: https://www.canada.ca/en/health-canada/services/opioids/federal-actions/overview.html#a1 (accessed on 5 April 2022).
- Health Canada. Opioid Warning Sticker and Patient Information Handout, and Risk Management Plans. Government of Canada; 2019. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/policies/warning-sticker-opioid-patient-information-handout.html (accessed on 5 April 2022).
- Health Canada. Submission of Targeted Risk Management Plans and Follow-Up Commitments for Prescription Opioid-Containing Products—Guidance for Industry; Government of Canada: Ottawa, ON, Canada, 2018.
- CIOMS Working Group IX. Practical Approaches to Risk Minimisation for Medicinal Products: Report of CIOMS Working Group IX; CIOMS Working Group IX: Geneva, Switzerland, 2014; p. 184. ISBN 978-92-9036-084-4. [Google Scholar]
- Smith, M.Y.; Russell, A.; Bahri, P.; Mol, P.; Frise, S.; Freeman, E.; Morrato, E.H. The RIMES Statement: A Checklist to Assess the Quality of Studies Evaluating Risk Minimization Programs for Medicinal Products. Drug Saf. 2018, 41, 389–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EMA. Guideline on Good Pharmacovigilance Practices (GVP)—Module XVI—Risk Minimisation Measures: Selection of Tools and 5 Effectiveness Indicators (Rev 3); EMA: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Moride, Y.; Lemieux-Uresandi, D.; Castillon, G.; Moura, C.S.; Pilote, L.; Faure, M.; Bernartsky, S. A Systematic Review of Interventions and Programs Targeting Appropriate Prescribing of Opioids. Pain Physician 2019, 22, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Mouchantaf, R.; Auth, D.; Moride, Y.; Raine, J.; Han, S.Y.; Smith, M.Y. Risk Management for the 21st Century: Current Status and Future. Drug Saf. 2021, 44, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Fetters, M.D.; Curry, L.A.; Creswell, J.W. Achieving Integration in Mixed Methods Designs—Principles and Practices. Health Serv. Res. 2013, 48, 2134–2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Health Canada. Actions on Opioids 2016 and 2017; Government of Canada: Ottawa, ON, Canada, 2017; ISBN 978-0-660-23819-7.
- Canada Health Act, RSC 1985, c C-6. 2022. Available online: https://canlii.ca/t/532qv (accessed on 5 April 2022).
- Health Canada. Canada Vigilance Program. Government of Canada. 2018. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/canada-vigilance-program.html (accessed on 5 April 2022).
- Van Hunsel, F.; van Puijenbroek, E.; de Jong-van den Berg, L.; van Grootheest, K. Media attention and the influence on the reporting odds ratio in disproportionality analysis: An example of patient reporting of statins. Pharmacoepidemiol. Drug Saf. 2010, 19, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, D.R. Statistics: Detecting a Rare Adverse Drug Reaction Using Spontaneous Reports. Reg. Anesth. Pain Med. 1998, 23 (Suppl. S2), 183–189. [Google Scholar] [CrossRef] [PubMed]
- Health Canada. Good Pharmacovigilance Practices (GVP) Guidelines (GUI-0102); Health Canada: Ottawa, ON, Canada, 2013.
- Statistics Canada. Table 17-10-0009-01 Population Estimates, Quarterly. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000901 (accessed on 5 April 2022).
- İşgüzar, B. Twitter-Scraper. GitHub. 2019. Available online: https://github.com/bisguzar/twitter-scraper (accessed on 5 April 2022).
- Towards the Heart. Take Home Naloxone. Towards the Heart. 2012. Available online: https://towardtheheart.com/naloxone (accessed on 5 April 2022).
- Tupper, K.W.; McCrae, K.; Garber, I.; Lysyshyn, M.; Wood, E. Initial results of a drug checking pilot program to detect fentanyl adulteration in a Canadian setting. Drug Alcohol Depend. 2018, 190, 242–245. [Google Scholar] [CrossRef]
- College of Physicians and Surgeons of British Columbia. Annual Report 2016/17; College of Physicians and Surgeons of British Columbia: Vancouver, BC, Canada, 2017. [Google Scholar]
- CIHI. Webinar: Opioid Overdose. Canadian Institute for Health Information; 2018. Available online: https://www.cihi.ca/en/bulletin/webinar-opioid-overdose (accessed on 5 April 2022).
- School of Pharmacy. Naloxone and Opioid Resources, University of Waterloo. Available online: https://uwaterloo.ca/pharmacy/resources-services-and-initiatives/health-resources/naloxone-and-opioid-resources (accessed on 5 April 2022).
- Healthy Canadians. End Stigma Campaign. YouTube; 2019. Available online: https://www.youtube.com/watch?v=Lj-lnShfDu0 (accessed on 5 April 2022).
- Health Canada. Opioid Lived Experiences. Health Canada; 2017. Available online: https://www.canada.ca/en/health-canada/services/opioids/awareness-resources.html#t2 (accessed on 5 April 2022).
- Canadian Pain Society. Opioids and Pain Management; Canadian Pain Society: Markham, ON, Canada, 2018. [Google Scholar]
- Health Canada. Drug and Medical Device Highlights 2018: Helping you Maintain and Improve Your Health. Health Canada; 2019. Available online: https://www.canada.ca/en/health-canada/services/publications/drugs-health-products/drug-medical-device-highlights-2018.html (accessed on 5 April 2022).
- Government of Canada. National Report: Apparent Opioid-Related Deaths in Canada, Health Info-Base Canada. 2019. Available online: https://health-infobase.canada.ca/datalab/national-surveillance-opioid-mortality.html (accessed on 5 April 2022).
- Mowatt, G.D.; Goodman, N. Medicinal product regulation and product liability in Canada: Overview, Life Sciences Global Guide 2016/2017, Practical Law. Available online: https://content.next.westlaw.com/2-616-7870?__lrTS=20220201130028830&transitionType=Default&contextData=(sc.Default)&firstPage=true (accessed on 5 April 2022).
- Ahmed, Y.A.; Ahmad, M.N.; Ahmad, N.; Zakaria, N.H. Social media for knowledge-sharing: A systematic literature review. Telemat. Inform. 2019, 37, 72–112. [Google Scholar] [CrossRef]
- Pierce, C.E.; Bouri, K.; Pamer, C.; Proestel, S.; Rodriguez, H.W.; Van Le, H.; Freifeld, C.C.; Brownstein, J.S.; Walderhaug, M.; Edwards, I.R.; et al. Evaluation of Facebook and Twitter Monitoring to Detect Safety Signals for Medical Products: An Analysis of Recent FDA Safety Alerts. Drug Saf. 2017, 40, 317–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pappa, D.; Stergioulas, L.K. Harnessing social media data for pharmacovigilance: A review of current state of the art, challenges and future directions. Int. J. Data Sci. Anal. 2019, 8, 113–135. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-Y.; Lee, Y.-S.; Kim, D.H.; Lee, H.S.; Yang, B.R.; Kim, M.G. The Use of Social Media in Detecting Drug Safety–Related New Black Box Warnings, Labeling Changes, or Withdrawals: Scoping Review. JMIR Public Health Surveill. 2021, 7, e30137. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Rehm, J.; Tyndall, M. Effective Canadian policy to reduce harms from prescription opioids: Learning from past failures. Can. Med. Assoc. J. 2016, 188, 1240–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Drug Administration. Good Pharmacovigilance Practices and Pharmacoepidemiologic Assessment. Food and Drug Administration; 2005. Available online: https://www.fda.gov/files/drugs/published/Good-Pharmacovigilance-Practices-and-Pharmacoepidemiologic-Assessment-March-2005.pdf (accessed on 5 April 2022).
| Type of Interventions * | n (%) Total = 413 |
|---|---|
| Harm reduction strategies | 130 (31.5) |
| Education/continuing education | 101 (24.5) |
| For HCPs only | 71 (70.3) |
| For the community | 26 (25.7) |
| For patients only | 4 (4.0) |
| Opioid awareness | 65 (15.7) |
| For the community | 57 (87.7) |
| For patients or family only | 8 (12.3) |
| Policy changes | 51 (12.3) |
| Update/creation of guidelines/standard of practice | 28 (6.8) |
| Knowledge exchange | 16 (3.9) |
| Pharmacovigilance, control, and monitoring | 11 (2.7) |
| Community interventions | 9 (2.2) |
| Prevention measures | 2 (0.5) |
| Patient Characteristics | n (%) Total = 4970 |
|---|---|
| Sex | |
| Male | 2730 (54.9) |
| Female | 1969 (39.6) |
| Unknown | 271 (5.5) |
| Age | |
| Mean ± SD, in years | 38.1 ± 17.0 |
| Neonate (0–<25 days) | 8 (0.2) |
| Infant (>25 days–<1 year) | 16 (0.3) |
| Child (≥1–<13 years) | 44 (0.9) |
| Adolescent (≥13–<18 years) | 76 (1.5) |
| Adult (≥18–<65 years) | 3356 (67.5) |
| Elderly (≥65 years) | 228 (4.6) |
| Unknown | 1242 (25.0) |
| Seriousness of opioid-related harm | |
| Serious | 4881 (98.2) |
| Non-serious | 89 (1.8) |
| Type of reporter | |
| Non-HCP | 3174 (63.9) |
| HCP | 1501 (30.2) |
| Unknown | 295 (6.0) |
| Opioid-related harm a | Total = 6727 |
| Abuse/Misuse/Dependence | 3986 (59.3) |
| Opioid-related death | 1344 (20.0) |
| Overdose | 1159 (17.2) |
| Diversion | 38 (3.5) |
| Patient Characteristics | n (% Total = 1582 |
|---|---|
| Sex | |
| Male | 1074 (67.9) |
| Female | 508 (32.1) |
| Age | |
| Mean ± SD, in years | 44.8 ± 13.3 |
| Pediatric (0–<13 years) | - |
| Adolescent (≥13–<18) | 3 (0.2) |
| Adult (18–<65 years) | 1498 (94.7) |
| Elderly (>65 years) | 80 (5.1) |
| Unknown | 1 (0.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goyer, C.; Castillon, G.; Moride, Y. Implementation of Interventions and Policies on Opioids and Awareness of Opioid-Related Harms in Canada: A Multistage Mixed Methods Descriptive Study. Int. J. Environ. Res. Public Health 2022, 19, 5122. https://doi.org/10.3390/ijerph19095122
Goyer C, Castillon G, Moride Y. Implementation of Interventions and Policies on Opioids and Awareness of Opioid-Related Harms in Canada: A Multistage Mixed Methods Descriptive Study. International Journal of Environmental Research and Public Health. 2022; 19(9):5122. https://doi.org/10.3390/ijerph19095122
Chicago/Turabian StyleGoyer, Camille, Genaro Castillon, and Yola Moride. 2022. "Implementation of Interventions and Policies on Opioids and Awareness of Opioid-Related Harms in Canada: A Multistage Mixed Methods Descriptive Study" International Journal of Environmental Research and Public Health 19, no. 9: 5122. https://doi.org/10.3390/ijerph19095122
APA StyleGoyer, C., Castillon, G., & Moride, Y. (2022). Implementation of Interventions and Policies on Opioids and Awareness of Opioid-Related Harms in Canada: A Multistage Mixed Methods Descriptive Study. International Journal of Environmental Research and Public Health, 19(9), 5122. https://doi.org/10.3390/ijerph19095122






