The Metabolic Profiles of Metabolically Healthy Obese and Metabolically Unhealthy Obese South African Adults over 10 Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Design, Setting and Participants
2.2. Measurements
2.3. Ethical Considerations
2.4. Statistical Methods
3. Results
3.1. Baseline and Follow-Up Characteristics of the Participants
3.2. Comparison of the Metabolic Profiles of the Different Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CRP | C-reactive protein |
| CVD | cardiovascular disease |
| DBP | diastolic blood pressure |
| HbA1c | glycated haemoglobin |
| HDL | high-density lipoprotein |
| HOMA-IR | homeostatic model assessment of insulin resistance |
| MetS | metabolic syndrome |
| MHNW | metabolically healthy normal weight |
| MHO | metabolically healthy overweight/obesity |
| MHOMHO | maintenance of MHO in 2005 and 2015 |
| MHOMUO | MHO in 2005 and MUO in 2015 |
| MUNW | metabolically unhealthy normal weight |
| MUO | metabolically unhealthy overweight/obesity |
| MUOMUO | MUO in 2005 and 2015 |
| PAI-1act | Plasminogen activator inhibitor-1 activity |
| PURE | Prospective Urban and Rural Epidemiological |
| SBP | systolic blood pressure |
| SD | standard deviation |
References
- Di Cesare, M.; Bentham, J.; Stevens, G.A.; Zhou, B.; Danaei, G.; Lu, Y.; Bixby, H.; Cowan, M.J.; Riley, L.M.; Hajifathalian, K.; et al. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Department of Health; Statistics South Africa; South African Medical Research Council. ICF: South Afrian Demographic and Health Survey 2016. Pretoria, South Africa and Rockville; National Department of Health: Washington, DC, USA, 2019. Available online: https://dhsprogram.com/pubs/pdf (accessed on 8 April 2022).
- Walker, A.R.; Walker, B.F.; Vorster, H.H. Low frequency of adverse sequelae of obesity in South African rural black women. Int. J. Vitam. Nutr. Res. 1989, 59, 224–228. [Google Scholar] [PubMed]
- Phillips, C.M. Metabolically healthy obesity across the life course: Epidemiology, determinants, and implications. Ann. N. Y. Acad. Sci. 2016, 1391, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Pataky, Z.; The RISC Investigators; Makoundou, V.; Nilsson, P.; Gabriel, R.S.; Lalic, K.; Muscelli, E.; Casolaro, A.; Golay, A.; Bobbioni-Harsch, E. Metabolic normality in overweight and obese subjects. Which parameters? Which risks? Int. J. Obes. 2011, 35, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Mbanya, V.N.; Echouffo-Tcheugui, J.B.; Akhtar, H.; Mbanya, J.-C.; Kengne, A.P. Obesity phenotypes in urban and rural Cameroonians: A cross-sectional study. Diabetol. Metab. Syndr. 2015, 7, 21. [Google Scholar] [CrossRef][Green Version]
- Kruger, H.S.; Schutte, A.E.; Walsh, C.M.; Kruger, A.; Rennie, K.L. Body mass index cut-points to identify cardiometabolic risk in black South Africans. Eur. J. Nutr. 2015, 56, 193–202. [Google Scholar] [CrossRef]
- Martin, B.-J.; Verma, S.; Charbonneau, F.; Title, L.M.; Lonn, E.M.; Anderson, T.J. The relationship between anthropometric indexes of adiposity and vascular function in the FATE cohort. Obesity 2013, 21, 266–273. [Google Scholar] [CrossRef]
- Rush, E.C.; Goedecke, J.H.; Jennings, C.; Micklesfield, L.; Dugas, L.; Lambert, E.V.; Plank, L.D. BMI, fat and muscle differences in urban women of five ethnicities from two countries. Int. J. Obes. 2007, 31, 1232–1239. [Google Scholar] [CrossRef]
- Lu, Y.; Hajifathalian, K.; Ezzati, M.; Woodward, M.; Rimm, E.B.; Danaei, G.; D’Este, C. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: A pooled analysis of 97 prospective cohorts with 1·8 million participants. Lancet 2014, 383, 970–983. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Buklijas, T.; Low, F.M.; Beedle, A.S. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat. Rev. Endocrinol. 2009, 5, 401–408. [Google Scholar] [CrossRef]
- Magkos, F. Metabolically healthy obesity: What’s in a name? Am. J. Clin. Nutr. 2019, 110, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Kang, Y.M.; Jang, J.E.; Hwang, J.Y.; Kim, E.H.; Park, J.-Y.; Kim, H.-K.; Lee, W.J. Fatty liver index is a risk determinant of incident type 2 diabetes in a metabolically healthy population with obesity. Obesity 2016, 24, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Rey-López, J.P.; de Rezende, L.F.; Pastor-Valero, M.; Tess, B.H. The prevalence of metabolically healthy obesity: A systematic review and critical evaluation of the definitions used. Obes. Rev. 2014, 15, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.B.; Lavie, C.J.; Blair, S.N. Obesity and Cardiovascular Disease. Circ. Res. 2016, 118, 1752–1770. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Després, J.-P. From syndrome X to cardiometabolic risk: Clinical and public health implications. Proc. Nutr. Soc. 2019, 79, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Gami, A.S.; Witt, B.J.; Howard, D.E.; Erwin, P.J.; Gami, L.A.; Somers, V.K.; Montori, V.M. Metabolic Syndrome and Risk of Incident Cardiovascular Events and Death: A Systematic Review and Meta-Analysis of Longitudinal Studies. J. Am. Coll. Cardiol. 2007, 49, 403–414. [Google Scholar] [CrossRef]
- Reaven, G.M. Insulin Resistance: The Link Between Obesity and Cardiovascular Disease. Med Clin. North Am. 2011, 95, 875–892. [Google Scholar] [CrossRef]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Capó, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic Syndrome Is Associated with Oxidative Stress and Proinflammatory State. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef]
- Teo, K.; Chow, C.K.; Vaz, M.; Rangarajan, S.; Yusufali, A. The Prospective Urban Rural Epidemiology (PURE) study: Examining the impact of societal influences on chronic noncommunicable diseases in low-, middle-, and high-income countries. Am. Heart J. 2009, 158, 1–7. [Google Scholar] [CrossRef]
- ISAK: International Standards for Anthropometric Assessment. Potchefstroom, South Africa: The International Society for the Advancement of Kinanthropometry. 2006. Available online: https://www.worldcat.org/title/international-standards-for-anthropometric-assessment/oclc/224080785 (accessed on 8 April 2022).
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Samouda, H.; Ruiz-Castell, M.; Karimi, M.; Bocquet, V.; Kuemmerle, A.; Chioti, A.; Dadoun, F.; Stranges, S. Metabolically healthy and unhealthy weight statuses, health issues and related costs: Findings from the 2013–2015 European Health Examination Survey in Luxembourg. Diabetes Metab. 2019, 45, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Kruger, H.S.; Ricci, C.; Pieters, M.; Roux, S.B.-L.; Moss, S.J.; Kruger, I.M.; van Zyl, T.; Schutte, A.E. Lifestyle factors associated with the transition from healthy to unhealthy adiposity among black South African adults over 10 years. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.K.; Zinman, B.; Retnakaran, R. Are Metabolically Healthy Overweight and Obesity Benign Conditions?: A Systematic Review and Meta-Analysis. Ann. Intern. Med. 2013, 159, 758. [Google Scholar] [CrossRef]
- Zheng, R.; Zhou, D.; Zhu, Y. The long-term prognosis of cardiovascular disease and all-cause mortality for metabolically healthy obesity: A systematic review and meta-analysis. J. Epidemiol. Community Health 2016, 70, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Goudge, J.; Gilson, L.; Russell, S.; Gumede, T.; Mills, A. Affordability, availability and acceptability barriers to health care for the chronically ill: Longitudinal case studies from South Africa. BMC Health Serv. Res. 2009, 9, 75. [Google Scholar] [CrossRef]
- Lentz, S.R. Thrombosis in the setting of obesity or inflammatory bowel disease. Blood 2016, 128, 2388–2394. [Google Scholar] [CrossRef]
- Schutte, A.; Schutte, R.; Huisman, H.; van Rooyen, J.M.; Fourie, C.M.; Malan, N.T.; Malan, L.; MC Mels, C.; Smith, W.; Moss, S.J.; et al. Are behavioural risk factors to be blamed for the conversion from optimal blood pressure to hypertensive status in Black South Africans? A 5-year prospective study. Int. J. Epidemiol. 2012, 41, 1114–1123. [Google Scholar] [CrossRef]
- Alessi, M.C.; Peiretti, F.; Morange, P.; Henry, M.; Nalbone, G.; Juhan-Vague, I. Production of Plasminogen Activator Inhibitor 1 by Human Adipose Tissue: Possible Link Between Visceral Fat Accumulation and Vascular Disease. Diabetes 1997, 46, 860–867. [Google Scholar] [CrossRef]
- Bastelica, D.; Morange, P.; Berthet, B.; Borghi, H.; Lacroix, O.; Grino, M.; Juhan-Vague, I.; Alessi, M.-C. Stromal Cells Are the Main Plasminogen Activator Inhibitor-1-Producing Cells in Human Fat: Evidence of differences between visceral and subcutaneous deposits. Arter. Thromb. Vasc. Biol. 2002, 22, 173–178. [Google Scholar] [CrossRef]
- Mertens, I.; Verrijken, A.; Michiels, J.J.; van der Planken, M.; Ruige, J.B.; Van Gaal, L.F. Among inflammation and coagulation markers, PAI-1 is a true component of the metabolic syndrome. Int. J. Obes. 2006, 30, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, G.K.; Osadnik, K.; Lejawa, M.; Osadnik, T.; Golawski, M.; Lewandowski, P.; Pawlas, N. “Obesity and Insulin Re-sistance” Is the Component of the Metabolic Syndrome Most Strongly Associated with Oxidative Stress. Antioxidants 2022, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, A.O.; Jacobs, D.R., Jr.; Sanchez, O.A.; Goff, D.C., Jr.; Reiner, A.P.; Gross, M.D. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc. Diabetol. 2016, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Häring, H.-U.; Schulze, M.B. Metabolically healthy obesity: The low-hanging fruit in obesity treatment? Lancet Diabetes Endocrinol. 2017, 6, 249–258. [Google Scholar] [CrossRef]
- Eckel, N.; Mühlenbruch, K.; Meidtner, K.; Boeing, H.; Stefan, N.; Schulze, M.B. Characterization of metabolically unhealthy normal-weight individuals: Risk factors and their associations with type 2 diabetes. Metabolism 2015, 64, 862–871. [Google Scholar] [CrossRef]
- Houle, B.; Pantazis, A.; Kabudula, C.; Tollman, S.; Clark, S.J. Social patterns and differentials in the fertility transition in the context of HIV/AIDS: Evidence from population surveillance, rural South Africa, 1993–2013. Popul. Health Metr. 2016, 14, 10. [Google Scholar] [CrossRef]
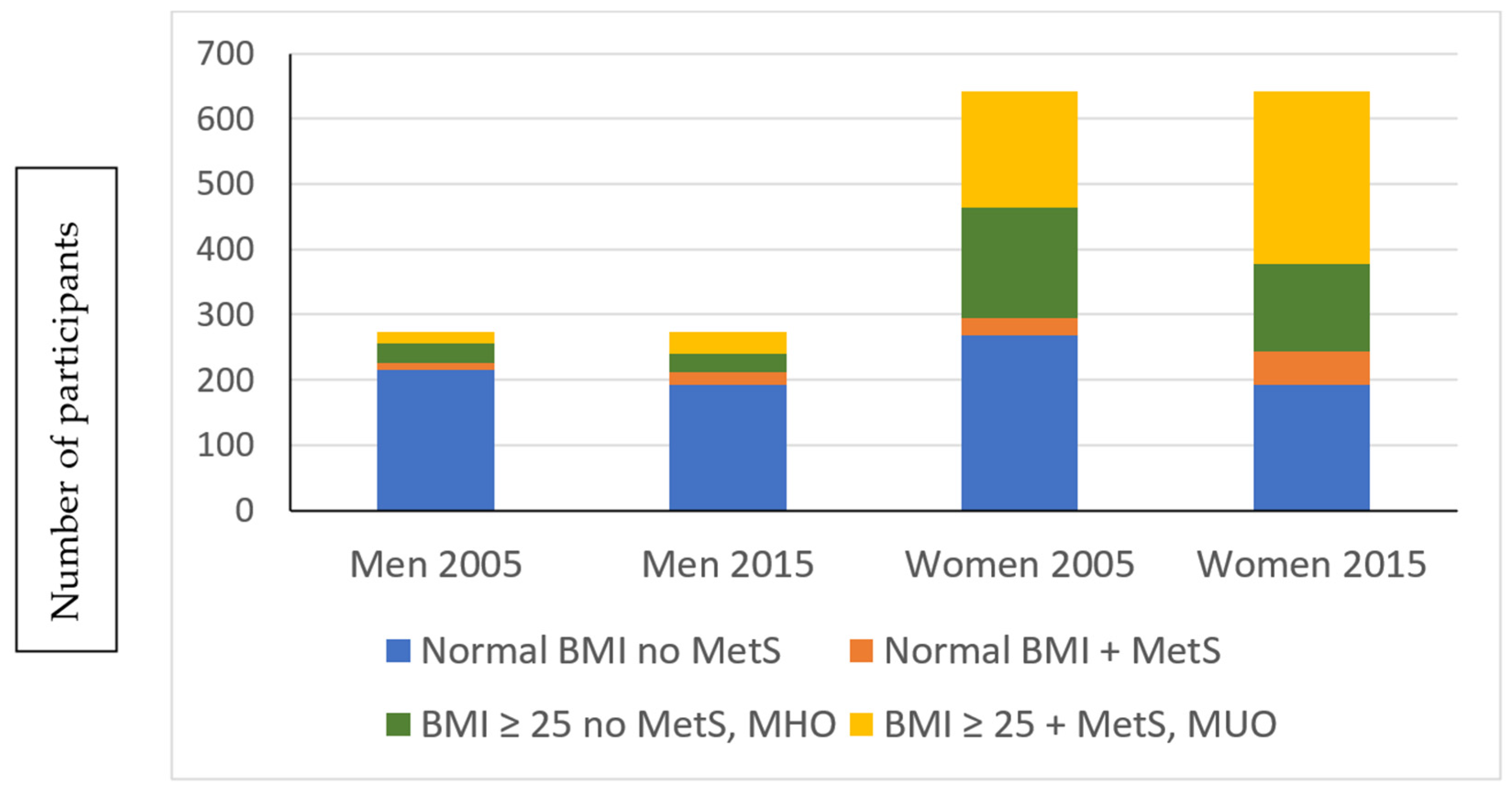
| Variables | Women (n = 642) | Men (n = 275) | ||
|---|---|---|---|---|
| 2005 | 2015 | 2005 | 2015 | |
| Age (years) | 49.3 (43.1–56.4) a | 59.2 (53.0–66.6) b | 49.8 (42.6–57.7) a | 59.7 (52.5–67.9) b |
| Weight (kg) | 63.6 (53.4–78.9) a | 66.0 (53.6–81.1) b | 57.2 (50.5–66.1) a | 57.7 (50.9–67.4) a |
| Height (m) | 1.57 ± 0.06 a | 1.56 ± 0.06 b | 1.67 ± 0.06 a | 1.66 ± 0.07 b |
| Body mass index (kg/m2) | 25.9 (21.8–32.0) a | 27.0 (22.3–33.4) b | 20.1 (18.5–23.4) a | 20.6 (18.3–24.3) b |
| Waist circumference (cm) | 80.8 (71.2–91.5) a | 91.2 (80.1–102.0) b | 75.0 (70.4–82.5) a | 78.9 (72.0–91.0) b |
| Urbanisation: | ||||
| Urban area | 264 (41.1%) | 264 (41.1%) | 120 (43.6%) | 120 (43.6%) |
| Rural area | 378 (58.9%) | 378 (58.9%) | 155 (56.4%) | 155 (56.4%) |
| Educational status: | ||||
| School education | 418 (65.1%) | 418 (65.1%) | 165 (60%) | 165 (60%) |
| No school education | 225 (34.9%) | 225 (34.9%) | 110 (40%) | 110 (40%) |
| Smoking: | ||||
| Smoker | 287 (44.7%) | 210 (32.7%) b | 153 (55.6%) a | 136 (49.4%) b |
| Non-smoking | 355 (55.3%) | 432 (67.3%) | 122 (44.4%) | 139 (50.6%) |
| Baseline Variables | MHNW 2005 and 2015; n = 345 | MHO 2005 and 2015; n = 86 | MUO 2005 And 2015; n = 145 | MHO 2005 to MUO 2015; n = 91 | p |
|---|---|---|---|---|---|
| Male/female ratio | 182/170 (52%/48%) | 14/72 (16%/84%) | 13/132 (9%/91%) | 13/78 (14%/86%) | |
| Age (years) | 47.5 (42.1, 54.6) a | 47.9 (42.4, 54.4) a | 52.9 (45.6, 58.1) b | 47.4 (40.6, 53.7) a | 0.027 |
| Body mass index (kg/m2) | 19.5 (18.0, 21.7) a | 33.0 (26.4, 34.1) b | 32.5 (28.6, 37.1) b | 30.0 (27.0, 32.6) b | <0.0001 |
| Waist circumference (cm) | 70.8 (66.2, 74.9) a | 85.4 (78.9, 91.9) b | 94.6 (88.6, 101.4) c | 89.4 (83.3, 97.8) b | <0.0001 |
| Alcohol intake (g) | 2.78 (0, 22.9) a | 0 (0, 0.97) b | 0 (0, 6.12) a | 0 (0, 0) b | <0.0001 |
| HIV status | 48 (13.6%) | 6 (7.0%) | 6 (4.1%) | 4 (4.4%) | 0.01 |
| Smoking | 212 (60.2%) | 87 (38.4%) | 61 (42.1%) | 30 (33.0%) | <0.0001 |
| Variables after 10 years | |||||
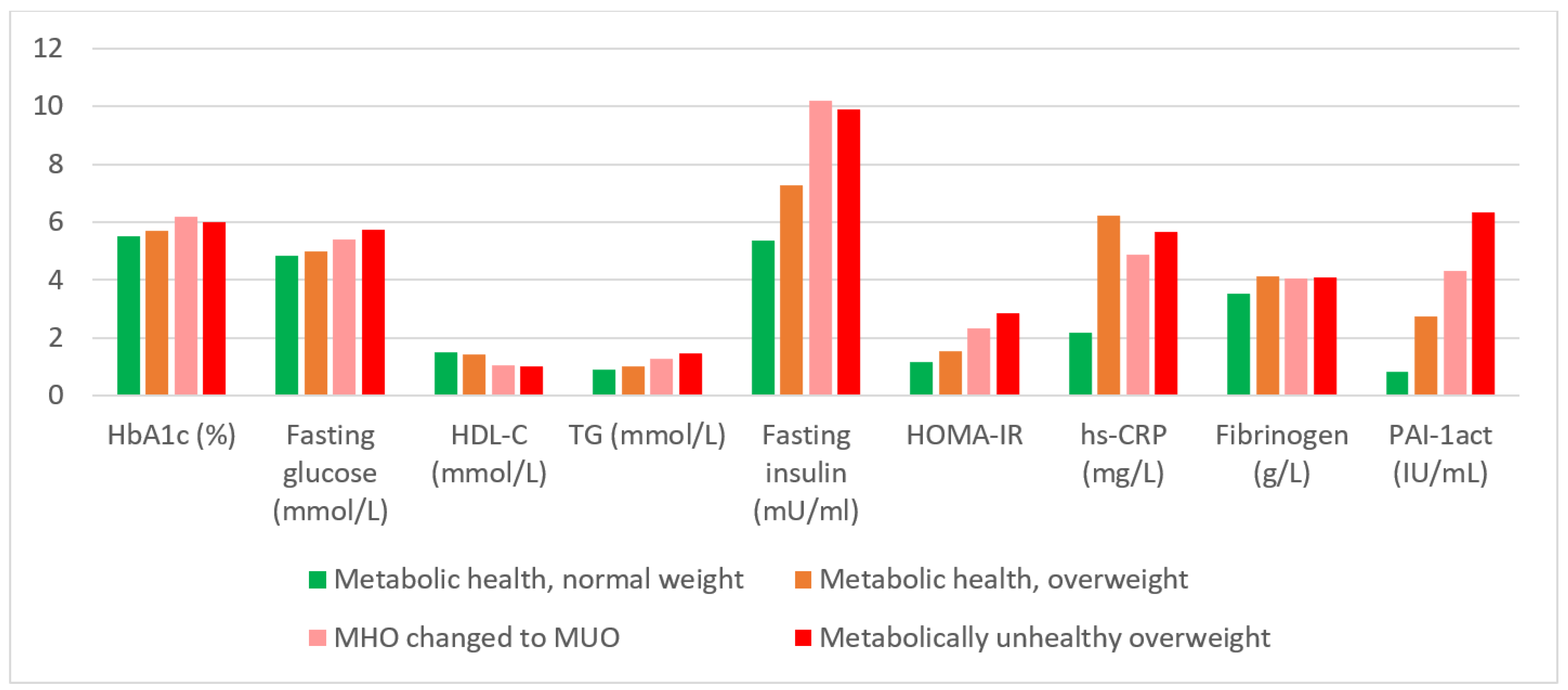
| Variable | MHNW 2005 and 2015; n = 345 | MHO 2005 and 2015; n = 86 | MUO 2005 and 2015; n = 145 | MHO 2005 to MUO 2015; n = 91 | p |
| Body mass index (kg/m2) | 19.8 (18.0, 22.1) a | 32.7 (28.2, 35.6) b | 33.1 (29.1, 37.6) b | 32.0 (29.2, 35.8) b | <0.0001 |
| Waist circumference (cm) | 75.2 (70.0, 80.0) a | 97.7 (91.6, 103.0) b | 102.5 (97.3, 110.6) c | 101.0 (94.4, 110.0) c | <0.0001 |
| Systolic blood pressure (mm Hg) | 127 (112, 147) a | 121 (107, 140) b | 133 (122, 147) c | 133 (122, 150) c | <0.0001 |
| Diastolic blood pressure (mmHg) | 82 (74, 94) a | 80 (72, 91) a | 86 (78, 95) b | 91 (83, 96) c | <0.0001 |
| Glycosylated haemoglobin (%) | 5.50 (5.2, 5.7) a | 5.70 (5.5, 6.0) a | 6.0 (5.7, 6.7) b | 6.20 (5.7, 6.9) c | <0.0001 |
| Total cholesterol (mmol/L) | 4.29 (3.64, 5.04) a | 4.68 (4.05, 5.50) b | 4.93 (4.22, 5.60) c | 4.53 (3.85, 5.48) a,b | <0.0001 |
| HDL cholesterol (mmol/L) | 1.52 (1.20, 1.84) a | 1.42 (1.11, 1.68) b | 1.02 (0.81, 1.18) c | 1.07 (0.93, 1.20) c | <0.0001 |
| Serum triglycerides (mmol/L) | 0.93 (0.71, 1.17) a | 1.04 (0.86, 1.29) a | 1.46 (1.1, 2.14) b | 1.28 (0.95, 2.04) c | <0.0001 |
| Fasting plasma glucose (mmol/L) | 4.85 (4.46, 5.26) a | 4.98 (4.70, 5.28) a | 5.76 (5.08, 6.89) b | 5.39 (4.94, 6.06) c | <0.0001 |
| Fasting plasma insulin (mU/mL) | 5.35 (3.10, 10.3) a | 7.34 (4.8, 10.5) a,b | 9.91 (6.07, 17.2) c | 10.2 (6.06, 15.0) b,c | <0.0001 |
| HOMA-Insulin resistance | 1.16 (0.66, 2.2) a | 1.55 (1.03, 2.19) b | 2.85 (1.46, 4.92) b,c | 2.34 (1.29, 3.74) b | <0.0001 |
| C-reactive protein (mg/L) | 2.2 (0.92, 5.52) a | 6.21 (2.35, 9.87) b | 5.67 (2.91, 9.74) b | 4.89 (2.23, 9.62) b | <0.0001 |
| Total fibrinogen (g/L) | 3.52 (3.11, 4.12) a | 4.14 (3.65, 4.51) b | 4.09 (3.52, 4.60) b | 4.06 (3.66, 4.57) b | <0.0001 |
| Plasminogen activator inhibitor-1act (IU/mL) | 0.85 (0.00, 4.39) a | 2.76 (0.00, 6.19) b | 6.34 (1.17, 12.3) c | 4.30 (0.74, 12.1) c | <0.0001 |
| HIV status | 89 (23.3%) | 32 (14.0%) | 2 (8.0%) | 4 (4.9%) | 0.001 |
| Smoking | 189 (51.8%) | 57 (25.8%) | 7 (28.0%) | 16 (21.6%) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruger, H.S.; De Lange-Loots, Z.; Kruger, I.M.; Pieters, M. The Metabolic Profiles of Metabolically Healthy Obese and Metabolically Unhealthy Obese South African Adults over 10 Years. Int. J. Environ. Res. Public Health 2022, 19, 5061. https://doi.org/10.3390/ijerph19095061
Kruger HS, De Lange-Loots Z, Kruger IM, Pieters M. The Metabolic Profiles of Metabolically Healthy Obese and Metabolically Unhealthy Obese South African Adults over 10 Years. International Journal of Environmental Research and Public Health. 2022; 19(9):5061. https://doi.org/10.3390/ijerph19095061
Chicago/Turabian StyleKruger, Herculina Salome, Zelda De Lange-Loots, Iolanthé Marike Kruger, and Marlien Pieters. 2022. "The Metabolic Profiles of Metabolically Healthy Obese and Metabolically Unhealthy Obese South African Adults over 10 Years" International Journal of Environmental Research and Public Health 19, no. 9: 5061. https://doi.org/10.3390/ijerph19095061
APA StyleKruger, H. S., De Lange-Loots, Z., Kruger, I. M., & Pieters, M. (2022). The Metabolic Profiles of Metabolically Healthy Obese and Metabolically Unhealthy Obese South African Adults over 10 Years. International Journal of Environmental Research and Public Health, 19(9), 5061. https://doi.org/10.3390/ijerph19095061






