Evaluation of Celiac Disease by Minimally Invasive Biomarkers in a Spanish Pediatric Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Serological CD Diagnosis
2.3. Genotyping
2.4. Histopathological Evaluation of Intestinal Mucosa
2.5. CD Follow-Up
2.6. Ethical Considerations
2.7. Statistical Analysis
3. Results
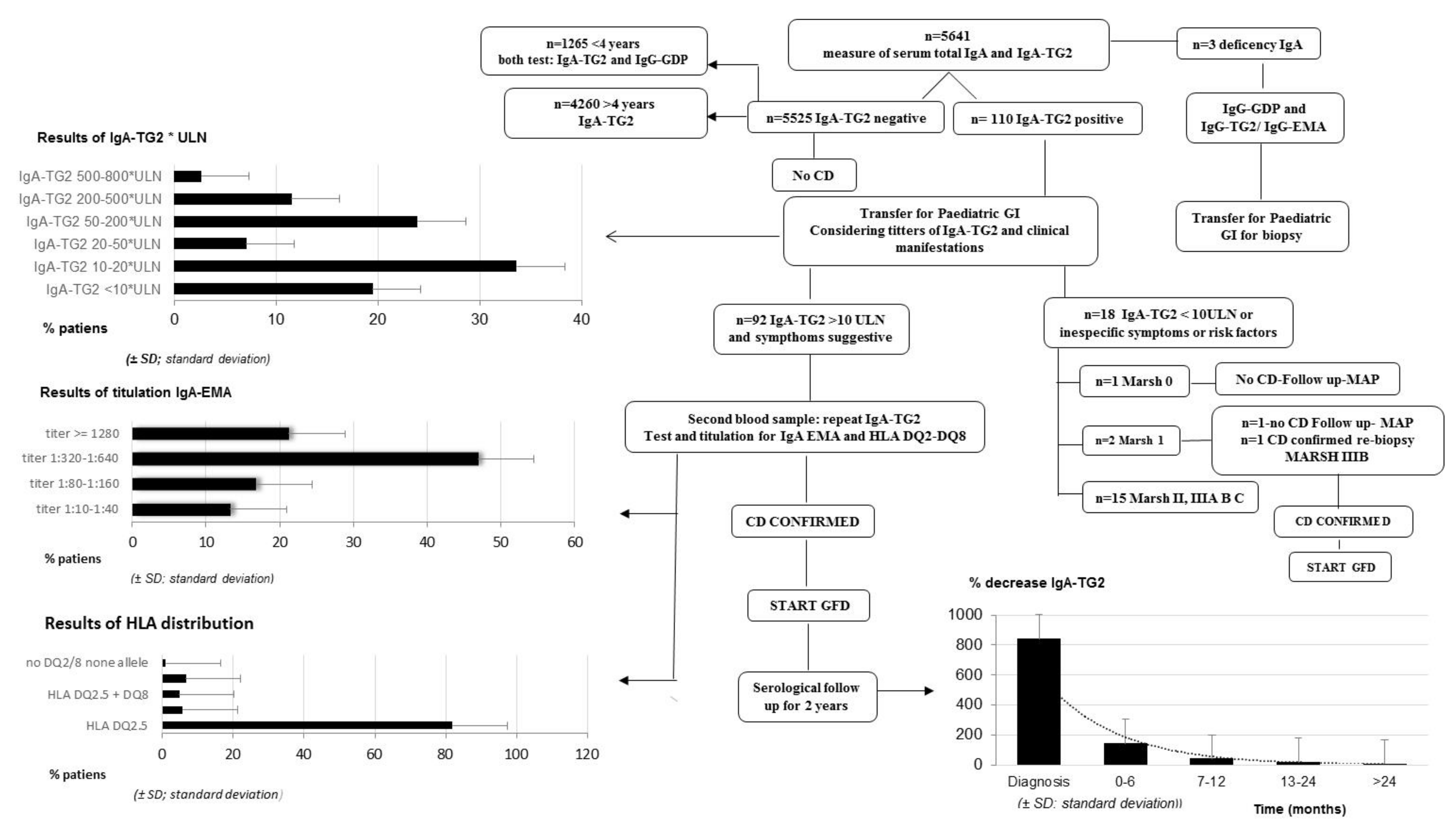
3.1. Serological CD Diagnosis
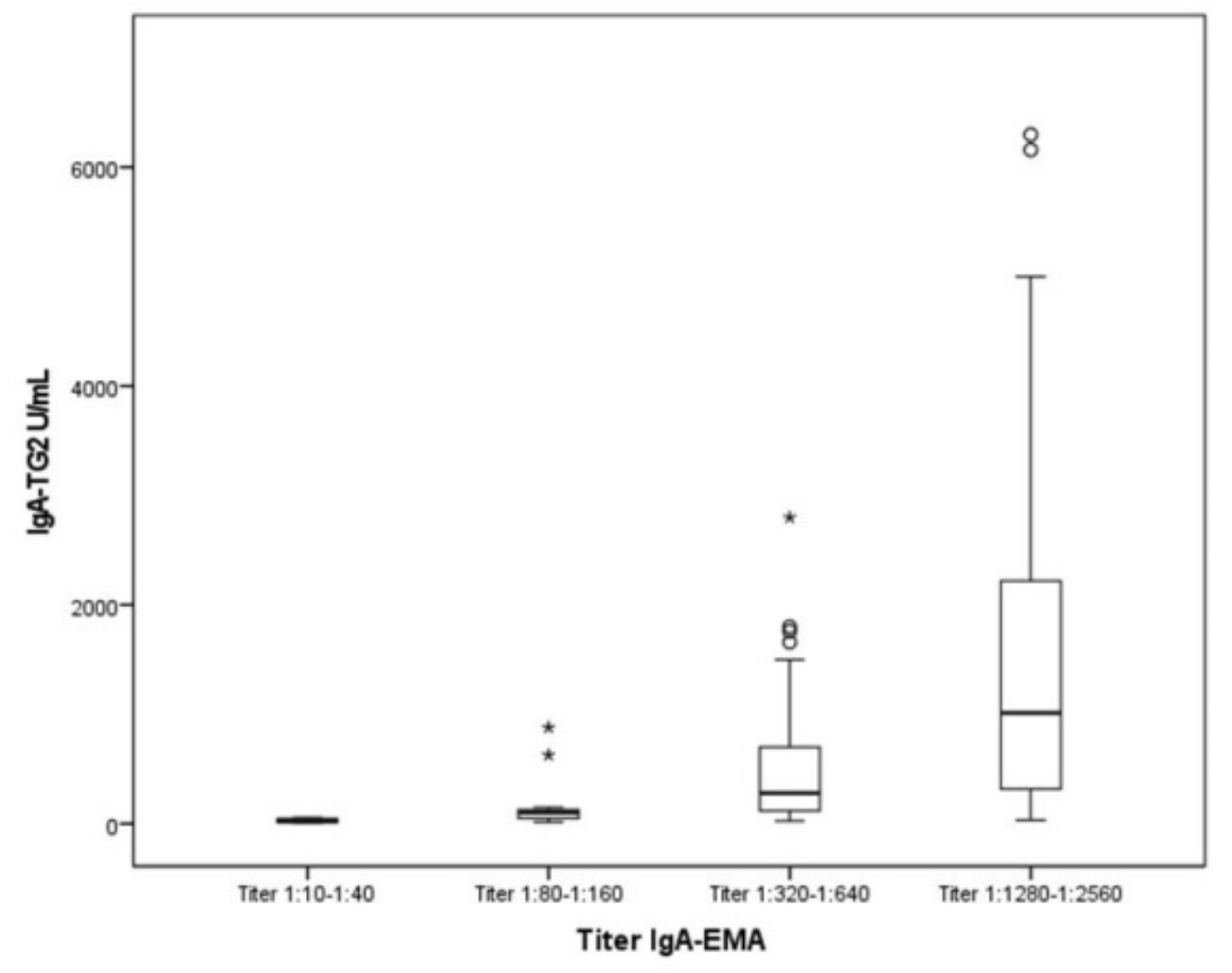
- 100% of pediatric patients for whom IgA-TG2 ≥ 10 ULN (80.53%) were IgA-EMA-positive.
- Furthermore, we observed a positive correlation between IgA-TG2 titers ≥ 10 ULN and strong EMA positivity (p < 0.001), as shown in Figure 2. Above an average value of ≥10 ULN (80 U/mL) of IgA-TG2, the IgA-EMA titer was greater than 1:80, even in asymptomatic patients with extra-intestinal symptoms or belonging to the at-risk group.
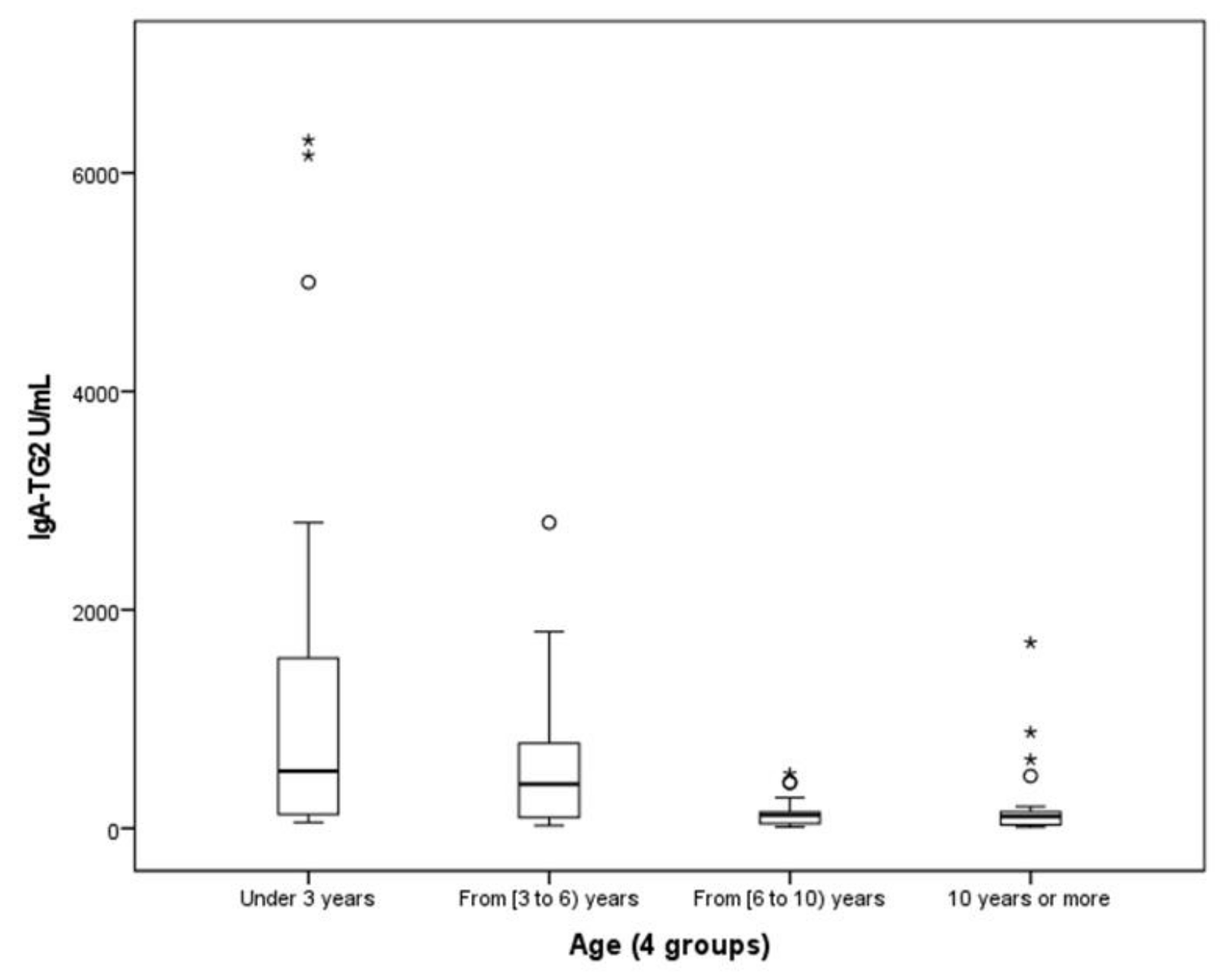
- We found a negative correlation between age and IgA-TG2 (p < 0.001), with higher values in children aged <3 years old (Figure 3).
3.2. Genotyping
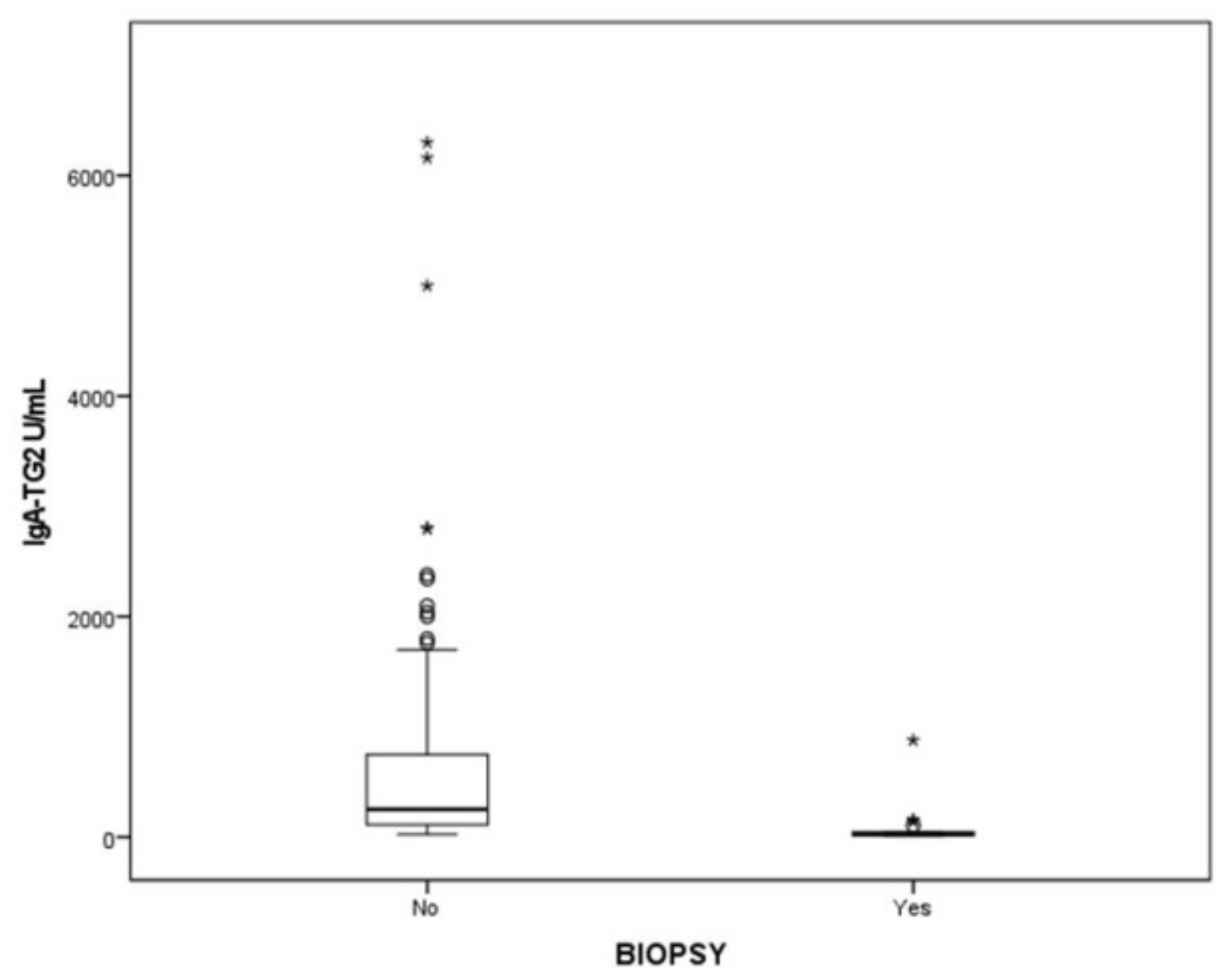
3.3. Histopathological Evaluation of Intestinal Mucosa
3.4. Serologic Response to a GFD
4. Discussion
- This biomarker measured with this precision provides information on CD and gives us greater a guarantee in the follow-up of the response to the GFD, with a minimum cost.
- Not all countries have the necessary resources and facilities to analyze IgA-EMA. One study performed in 13 Mediterranean countries (western and eastern WHO regions, including Spain) [29] enrolled 1974 pediatric patients and confirmed CD in 25.9% of them. Nevertheless, only 14 patients were diagnosed of CD by the 2012 ESPGHAN guidelines. In that study, 40 Spanish pediatric patients were screened for CD and only three patients could be diagnosed by the ESPGHAN criteria.
- The IgA-EMA test is relatively expensive and requires experienced observers for microscopic evaluation, with potential inter-observer variation.
- IgA-EMA is detected on monkey esophagus by immunofluorescence, which raises ethical concerns related to the use of endangered species as a substrate.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caio, G.; Volta, U.; Sapone, A.; Leffler, D.A.; De Giorgio, R.; Catassi, C.; Fasano, A. Celiac disease: A comprehensive current review. BMC Med. 2019, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Lindfors, K.; Ciacci, C.; Kurppa, K.; Lundin, K.E.A.; Makharia, G.K.; Mearin, M.L.; Murray, J.A.; Verdu, E.F.; Kaukinen, K. Coeliac disease. Nat. Rev. Dis. Primers 2019, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Green, P.; Lebwohl, B.; Greywoode, R. Celiac disease. J. Allergy Clin. Immunol. 2015, 135, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Werkstetter, K.J.; Korponay-Szabó, I.R.; Popp, A.; Villanacci, V.; Salemme, M.; Heilig, G.; Lillevang, S.T.; Mearin, M.L.; Ribes-Koninckx, C.; Thomas, A.; et al. Accuracy in diagnosis of celiac disease without biopsies in clinical practice. Gastroenterology 2017, 153, 924–935. [Google Scholar] [CrossRef]
- Saginur, M.; Alrefaee, F.A.; Spady, D.W.; Girgis, S.A.; Huynh, H.Q.; Prosser, C.I.; Persad, R.; Turner, J.M. Antitissue transglutaminase antibody determination versus upper endoscopic bi-opsy diagnosis of paediatric celiac disease. Paediatr. Child Health 2013, 18, 246–250. [Google Scholar] [CrossRef]
- Bonamico, M.; Thanasi, E.; Mariani, P.; Nenna, R.; Luparia, R.P.L.; Barbera, C.; Morra, I.; Lerro, P.; Guariso, G.; De Giacomo, C.; et al. Duodenal bulb biopsies in celiac disease: A multicenter study. J. Pediatr. Gas-troenterol. Nutr. 2008, 47, 618–622. [Google Scholar] [CrossRef]
- Dahlbom, I.; Korponay-Szabó, I.R.; Kovács, J.B.; Szalai, Z.; Mäki, M.; Hansson, T. Prediction of clinical and mucosal severity of coeliac disease and dermatitis herpetiformis by quantification of IgA/IgG serum antibodies to tissue transglutaminase. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 140–146. [Google Scholar] [CrossRef]
- Mubarak, A.; Wolters, V.M.; Gerritsen, S.M.; Gmelig-Meyling, F.H.J.; Ten Kate, F.J.W.; Houwen, R.H.J. A biopsy is not always necessary to diagnose celiac disease. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 554–557. [Google Scholar] [CrossRef]
- Alessio, M.G.; Tonutti, E.; Brusca, I.; Radice, A.; Licini, L.; Sonzogni, A.; Florena, A.; Schiaffino, E.; Marus, W.; Sulfaro, S.; et al. Cor-relation between IgA tissue transglutaminase antibody ratio and histological finding in celiac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 44–49. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutri-tion Guidelines for the Diagnosis of Coeliac Disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–157. [Google Scholar] [CrossRef]
- Arigliani, M.; Morassutti, F.R.; Fabris, M.; Melli, P.; Tonutti, E.; Cogo, P. Coeliac disease in infants: Antibodies to deaminated gliadin peptide come first! Ital. J. Pediatr. 2017, 43, 70. [Google Scholar] [CrossRef]
- Kleinman, K.; McDaniel, L.; Molloy, M. Manual Harriet Lane de pediatría. The Johns Hopkins Hospital, 22nd ed.; Elsevier: Amsterdam, The Netherlands, 2021; 1146p. [Google Scholar]
- García Novo, M.D.; Garfia, C.; Acuña Quirós, M.D.; Asensio, J.; Zancada, G.; Barrio Gutiérrez, S.; Manzanares, J.; Solís-Herruzo, J.A. Prevalencia de la enfermedad celiaca en donantes de sangre de la Comunidad de Madrid. Rev. Esp. Enferm. Dig. 2007, 99, 337–342. [Google Scholar] [CrossRef]
- Oberhuber, G.; Granditsch, G.; Vogelsang, H. The histopathology of coeliac disease: Time for a standardized report scheme for pathologists. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1185–1194. [Google Scholar] [CrossRef]
- Murch, S.; Jenkins, H.; Auth, M.; Bremner, R.; Butt, A.; France, S.; Furman, M.; Gillett, P.; Kiparissi, F.; Lawson, M.; et al. Joint BSPGHAN and Coelic UK guidelines for the diagnosis and management of coeliac disease in children. Arch. Dis. Child. 2013, 98, 806–811. [Google Scholar] [CrossRef]
- Van der Windt, D.A.; Jellema, P.; Mulder, C.J.; Kneepkens, C.M.; van der Horst, H.E. Diagnostic testing for celiac disease among patients with abdominal symp-toms: A systematic review. JAMA 2010, 303, 1738–1746. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Ludvigsson, J.; Brantner, T.; Murray, J.; Everhart, J. The Prevalence of celiac disease in the United States American. Am. J. Gastroenterol. 2012, 107, 1538–1544. [Google Scholar] [CrossRef]
- Gidrewicz, D.; Potter, K.; Trevenen, C.L.; Lyon, M.; Butzner, J.D. Evaluation of the ESPGHAN celiac guidelines in a North American pediatric population. Am. J. Gastroenterol. 2015, 110, 760–767. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Ciacci, C.; Green, P.H.; Kaukinen, K.; Korponay-Szabo, I.R.; Kurppa, K.; Murray, J.A.; Lundin, K.E.A.; Maki, M.J.; Popp, A.; et al. Outcome measures in coeliac disease trials: The Tampere recom-mendations. Gut 2018, 67, 1410–1424. [Google Scholar] [CrossRef]
- Kelly, C.P.; Bai, J.C.; Liu, E.; Leffler, D.A. Advances in diagnosis and management of celiac disease. Gastroenterology 2015, 148, 1175–1186. [Google Scholar] [CrossRef]
- Grupo de Trabajo del Protocolo para el Diagnóstico Precoz de la Enfermedad Celiaca. Protocolo para el Diagnóstico Precoz de la Enfermedad Celiaca; Ministerio de Sanidad, Servicios Sociales e Igualdad: Madrid, Spain; Servicio de Evaluación del Servicio Canario de la Salud (SESCS): Canary Islands, Spain, 2018.
- Schirru, E.; Danjou, F.; Cicotto, L.; Rossino, R.; Macis, M.D.; Lampis, R.; Jores, R.-D.; Congia, M. Anti-actin IgA antibodies identify celiac disease patients with a Marsh 3 intestinal damage among subjects with moderate anti-TG2 levels. Biomed. Res. Int. 2013, 2013, 630463. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klapp, G.; Masip, E.; Bolonio, M.; Donat, E.; Polo, B.; Ramos, D.; Ribes-Koninckx, C. Celiac dis-ease: The new proposed ESPGHAN diagnostic criteria do work well in a selected population. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Ucci, F.; Astarita, L.; Abkari, A.; Abu-Zekry, M.; Attard, T.; Ben Hariz, M.; Bilbao, J.R.; Boudraa, G.; Boukthir, S.; Costa, S.; et al. Celiac Disease in the Mediterranean Area. BMC Gastroenterol. 2014, 14, 24. [Google Scholar] [CrossRef]
- Donat, E.; Ramos, J.M.; Sánchez-Valverde, F.; Moreno, A.; Martinez, M.-J.; Leis, R.; Peña-Quintana, L.; Castillejo, G.; Fernández, S.F.; Garcia, Z.; et al. ESPGHAN 2012 Guidelines for Coeliac Disease Diagnosis: Validation Through a Retrospective Spanish Multicentric Study. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 284–291. [Google Scholar] [CrossRef]
- Hopper, A.D.; Cross, S.; Hurlstone, D.P.; McAlindon, M.E.; Lobo, A.J.; Hadjivassiliou, M.; Sloan, M.E.; Dixon, S.; Sanders, D.S. Preendoscopy serological testing for coeliac disease: Evaluation of a clinical decision tool. BMJ 2007, 334, 729. [Google Scholar] [CrossRef]
- Sugai, E.; Moreno, M.L.; Hwang, H.J.; Cabanne, A.; Crivelli, A.; Nachman, F.; Vázquez, H.; Niveloni, S.; Argonz, J.; Mazure, R.; et al. Celiac disease serology in patients with different pretest probabilities: Is biopsy avoidable? World J. Gastroenterol. 2010, 16, 3144–3152. [Google Scholar] [CrossRef]
- Smarrazzo, A.; Misak, Z.; Costa, S.; Mičetić-Turk, D.; Abu-Zekry, M.; Kansu, A.; Abkari, A.; Bouziane-Nedjadi, K.; Ben Hariz, M.; Roma, E.; et al. Diagnosis of celiac disease and applicability of ESPGHAN guidelines in Mediterranean countries: A real life prospective study. BMC Gastroenterol. 2017, 17, 17. [Google Scholar] [CrossRef][Green Version]
- Wolf, J.; Petroff, D.; Richter, T.; Auth, M.K.; Uhlig, H.H.; Laass, M.W.; Lauenstein, P.; Krahl, A.; Händel, N.; de Laffolie, J.; et al. Validation of antibody-based strategies for diagnosis of pediatric celiac disease without biopsy. Gastroenterology 2017, 153, 410–419. [Google Scholar] [CrossRef]
- Oyaert, M.; Vermeersch, P.; De Hertogh, G.; Hiele, M.; Vandeputte, N.; Hoffman, I.; Bossuyt, X. Combining antibody tests and taking into account antibody levels improves serologic diagnosis of celiac disease. Clin. Chem. Lab. Med. 2015, 53, 1537–1546. [Google Scholar] [CrossRef]
- Panetta, F.; Torre, G.; Colistro, F.; Ferretti, F.; Daniele, A.; Diamanti, A. Clinical accuracy of anti-tissue transglutaminase as screening test for celiac disease under 2 years. Acta Paediatr. 2011, 100, 728–731. [Google Scholar] [CrossRef]
- Giersiepen, K.; Lelgemann, M.; Stuhldreher, N.; Ronfani, L.; Husby, S.; Koletzko, S.; Korponay-Szabó, I.R. ESPGHAN Working Group on Coeliac Disease Diagnosis. Accuracy of diagnostic antibody tests for coeliac disease in children: Summary of an evidence report. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 229–241. [Google Scholar] [CrossRef]
- Husby, S.; Murray, J.A.; Katzka, D.A. AGA Clinical practice update on diagnosis and monitoring of celiac disease: Changing utility of serology and histologic measures: Expert review. Gastroenterology 2019, 156, 885–889. [Google Scholar] [CrossRef]
- Elli, L.; Ferrettia, F.; Orlando, S.; Vecchi, M.; Monguzzia, E.; Roncoronia, L.; Schuppan, D. Management of celiac disease in daily clinical. Eur. J. Intern. Med. 2019, 61, 15–24. [Google Scholar] [CrossRef]
- Mubarak, A.; Wolters, V.M.; Gmelig-Meyling, F.H.; Ten Kate, F.J.; Houwen, R.H. Tissue transglutaminase levels above 100U/mL and celiac disease: A prospective study. World J. Gastroenterol. 2012, 18, 4399–4403. [Google Scholar] [CrossRef]
- Clouzeau-Girard, H.; Rebouissoux, L.; Taupin, J.-L.; Le Bail, B.; Kalach, N.; Mi-Chaud, L.; Dabadie, A.; Olives, J.-P.; Blanco, P.; Morali, A.; et al. HLA-DQ genotyping combined with serological markers for the di-agnosis of celiac disease: Is intestinal biopsy still mandatory? J. Pediatr. Gastroenterol. Nutr. 2011, 52, 729–733. [Google Scholar] [CrossRef]
| No Cases | Percentage of Cases | ||
|---|---|---|---|
| 70 | 61.95 | ||
| Chronic or intermitent diarrhea *, weight loss, and/or growth failure * (Classic Triad) | |||
| Gastrointestinal symptoms * | 35 | 30.97 | |
| Clinical symptoms | Recurrent abdominal pain, vomiting, chronic, or recurrent constipation | ||
| Extraintestinal symptoms | |||
| Chronic iron deficency, anaemia * | 29 | 25.66 | |
| Subclinical hypothyroidism | 2 | 1.77 | |
| Arthritis/arthralgia | 1 | 0.88 | |
| Irritability, chronic fatigue | 5 | 4.42 | |
| Neuropaty, TDHA | 2 | 1.77 | |
| Arthritis/arthralgia | 2 | 1.77 | |
| Recurrent aphthous | 1 | 0.88 | |
| Oligo symtomatic or subclinical | 6 | 5.31 | |
| CD first-degree family member | 5 | 4.42 | |
| Type I diabetes mellitus | 1 | 0.88 | |
| Risk factors | Autism | 1 | 0.88 |
| IgA deficiency | 3 | 2.65 | |
| Autoinmunity thyroid disease | 1 | 0.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabo del Riego, J.M.; Núñez Iglesias, M.J.; García-Plata González, C.; Paz Carreira, J.; Álvarez Fernández, T.; Dorado Díaz, A.; Villar Mallo, N.; Penedo Pita, M.; Novío Mallón, S.; Máiz Suárez, L.; et al. Evaluation of Celiac Disease by Minimally Invasive Biomarkers in a Spanish Pediatric Population. Int. J. Environ. Res. Public Health 2022, 19, 5020. https://doi.org/10.3390/ijerph19095020
Cabo del Riego JM, Núñez Iglesias MJ, García-Plata González C, Paz Carreira J, Álvarez Fernández T, Dorado Díaz A, Villar Mallo N, Penedo Pita M, Novío Mallón S, Máiz Suárez L, et al. Evaluation of Celiac Disease by Minimally Invasive Biomarkers in a Spanish Pediatric Population. International Journal of Environmental Research and Public Health. 2022; 19(9):5020. https://doi.org/10.3390/ijerph19095020
Chicago/Turabian StyleCabo del Riego, Julia María, María Jesús Núñez Iglesias, Carmen García-Plata González, José Paz Carreira, Tamara Álvarez Fernández, Ana Dorado Díaz, Noa Villar Mallo, Manuel Penedo Pita, Silvia Novío Mallón, Lola Máiz Suárez, and et al. 2022. "Evaluation of Celiac Disease by Minimally Invasive Biomarkers in a Spanish Pediatric Population" International Journal of Environmental Research and Public Health 19, no. 9: 5020. https://doi.org/10.3390/ijerph19095020
APA StyleCabo del Riego, J. M., Núñez Iglesias, M. J., García-Plata González, C., Paz Carreira, J., Álvarez Fernández, T., Dorado Díaz, A., Villar Mallo, N., Penedo Pita, M., Novío Mallón, S., Máiz Suárez, L., & Freire-Garabal Núñez, M. (2022). Evaluation of Celiac Disease by Minimally Invasive Biomarkers in a Spanish Pediatric Population. International Journal of Environmental Research and Public Health, 19(9), 5020. https://doi.org/10.3390/ijerph19095020







