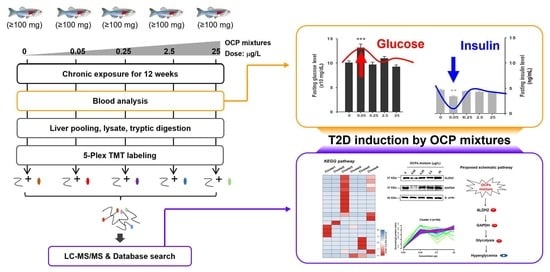
Type 2 Diabetes Induced by Changes in Proteomic Profiling of Zebrafish Chronically Exposed to a Mixture of Organochlorine Pesticides at Low Concentrations
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Zebrafish Husbandry
2.3. Chemical Exposure
2.4. Fasting Glucose and Insulin Measurement
2.5. Protein Extraction from Zebrafish Liver Tissue
2.6. Biological Tryptic Digestion
2.7. TMT Labeling and Peptide Fractionation
2.8. Comparative Analysis of Proteins Using LC-MS/MS
2.9. Immunoblotting
2.10. Peak Alignment and Data Search
2.11. Conservation Analysis
2.12. Bioinformatic Analysis
3. Results and Discussion
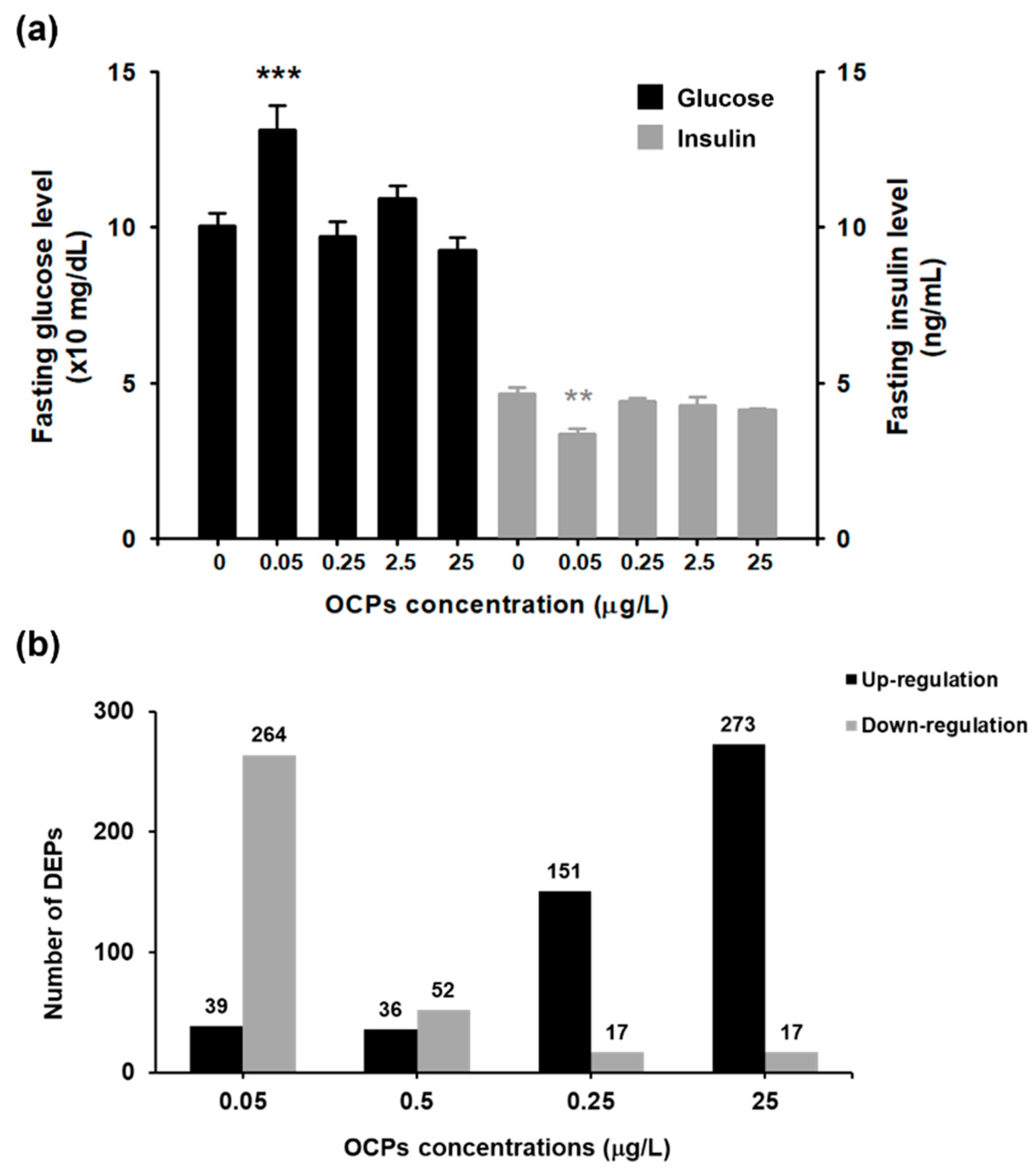
3.1. Fasting Glucose and Insulin Level Analysis
3.2. TMT-Based Proteomic Quantification in Zebrafish Liver
3.3. Evolutionary Conservation Protein between Zebrafish and Human
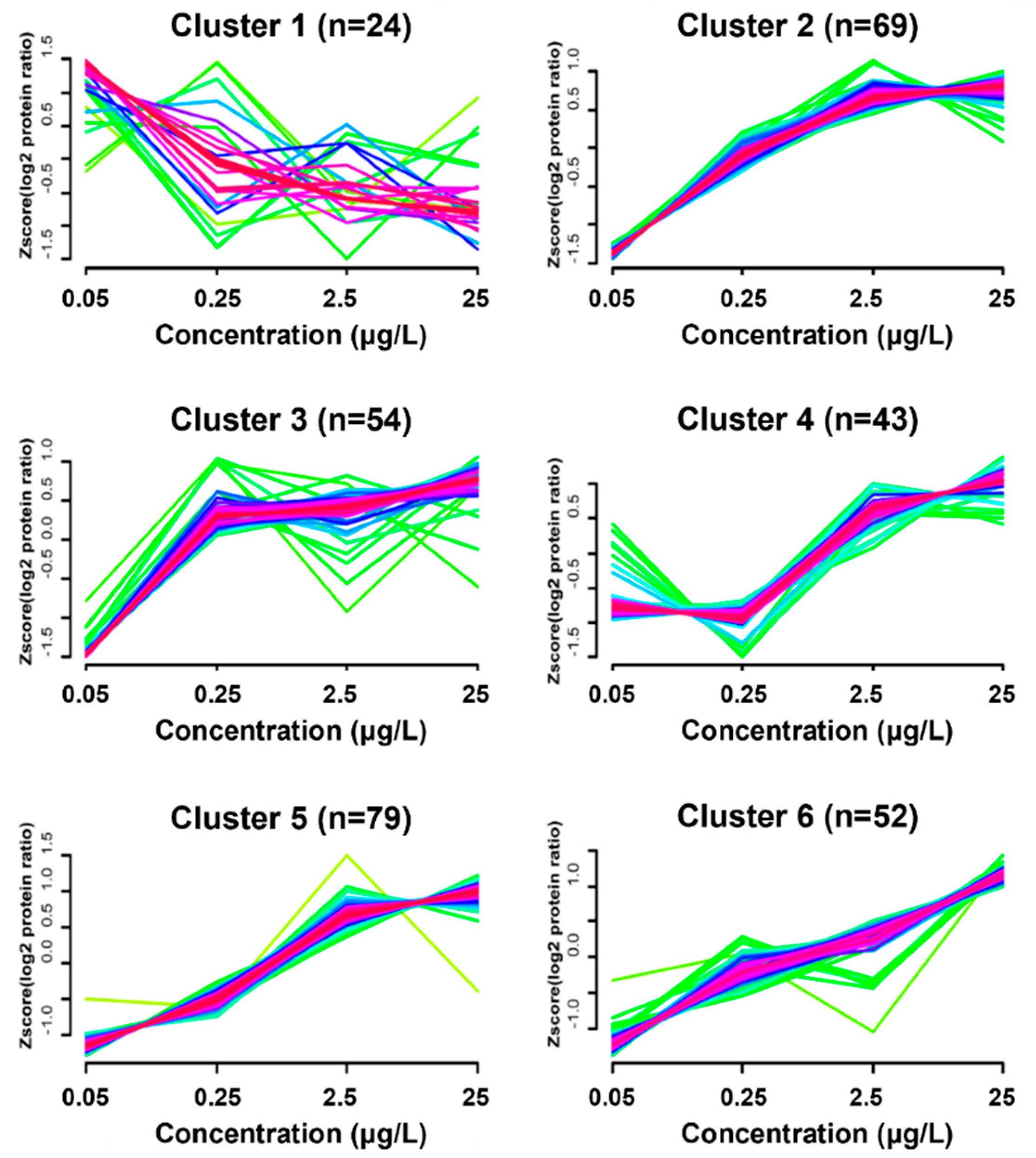
3.4. Dynamic Protein Cluster Analysis
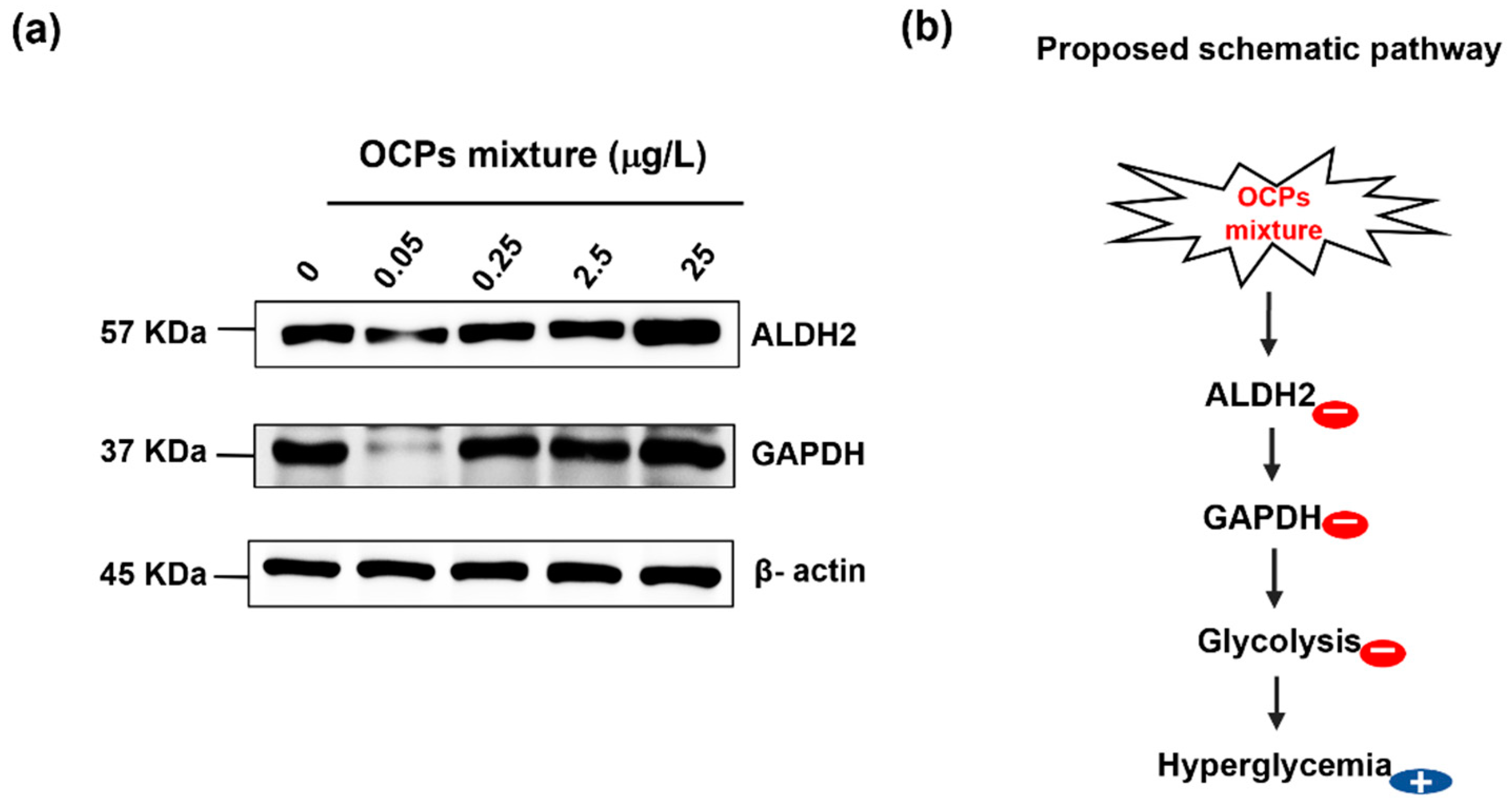
3.5. Biological Functional Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashraf, M.A. Persistent Organic Pollutants (POPs): A Global Issue, a Global Challenge. Environ. Sci. Pollut. Res. Int. 2017, 24, 4223–4227. [Google Scholar] [CrossRef]
- Batt, A.L.; Wathen, J.B.; Lazorchak, J.M.; Olsen, A.R.; Kincaid, T.M. Statistical Survey of Persistent Organic Pollutants: Risk Estimations to Humans and Wildlife through Consumption of Fish from U.S. Rivers. Environ. Sci. Technol. 2017, 51, 3021–3031. [Google Scholar] [CrossRef]
- Foster, K.L.; Braune, B.M.; Gaston, A.J.; Mallory, M.L. Climate Influence on Legacy Organochlorine Pollutants in Arctic Seabirds. Environ. Sci. Technol. 2019, 53, 2518–2528. [Google Scholar] [CrossRef]
- Barrett, J.R. POPs vs. Fat: Persistent Organic Pollutant Toxicity Targets and Is Modulated by Adipose Tissue. Environ. Health Perspect. 2013, 121, a61. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Fjaere, E.; Lock, E.J.; Naville, D.; Amlund, H.; Meugnier, E.; le Magueresse Battistoni, B.; Froyland, L.; Madsen, L.; Jessen, N.; et al. Chronic Consumption of Farmed Salmon Containing Persistent Organic Pollutants Causes Insulin Resistance and Obesity in Mice. PLoS ONE 2011, 6, e25170. [Google Scholar] [CrossRef]
- Ruzzin, J.; Petersen, R.; Meugnier, E.; Madsen, L.; Lock, E.J.; Lillefosse, H.; Ma, T.; Pesenti, S.; Sonne, S.B.; Marstrand, T.T.; et al. Persistent Organic Pollutant Exposure Leads to Insulin Resistance Syndrome. Environ. Health Perspect. 2010, 118, 465–471. [Google Scholar] [CrossRef]
- Carpenter, D.O. Environmental Contaminants as Risk Factors for Developing Diabetes. Rev. Environ. Health 2008, 23, 59–74. [Google Scholar] [CrossRef]
- Taylor, K.W.; Novak, R.F.; Anderson, H.A.; Birnbaum, L.S.; Blystone, C.; Devito, M.; Jacobs, D.; Kohrle, J.; Lee, D.H.; Rylander, L.; et al. Evaluation of the Association between Persistent Organic Pollutants (POPs) and Diabetes in Epidemiological Studies: A National Toxicology Program Workshop Review. Environ. Health Perspect. 2013, 121, 774–783. [Google Scholar] [CrossRef]
- Wu, H.; Bertrand, K.A.; Choi, A.L.; Hu, F.B.; Laden, F.; Grandjean, P.; Sun, Q. Persistent Organic Pollutants and Type 2 Diabetes: A Prospective Analysis in the Nurses’ Health Study and Meta-Analysis. Environ. Health Perspect. 2013, 121, 153–161. [Google Scholar] [CrossRef]
- La Merrill, M.; Karey, E.; Moshier, E.; Lindtner, C.; la Frano, M.R.; Newman, J.W.; Buettner, C. Perinatal Exposure of Mice to the Pesticide DDT Impairs Energy Expenditure and Metabolism in Adult Female Offspring. PLoS ONE 2014, 9, e103337. [Google Scholar] [CrossRef]
- Howell, G.E., 3rd; Meek, E.; Kilic, J.; Mohns, M.; Mulligan, C.; Chambers, J.E. Exposure to p,p’-Dichlorodiphenyldichloroethylene (DDE) Induces Fasting Hyperglycemia without Insulin Resistance in Male C57BL/6H Mice. Toxicology 2014, 320, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, M.; Melchor, J.J.; Tabares-Villa, F.A.; Taborda, G.; Sepulveda-Arias, J.C. Metabolite Profiling to Monitor Organochlorine Pesticide Exposure in HepG2 Cell Culture. Chromatographia 2016, 79, 1061–1068. [Google Scholar] [CrossRef]
- Xu, T.; Lim, Y.T.; Chen, L.; Zhao, H.; Low, J.H.; Xia, Y.; Sobota, R.M.; Fang, M. A Novel Mechanism of Monoethylhexyl Phthalate in Lipid Accumulation via Inhibiting Fatty Acid Beta-Oxidation on Hepatic Cells. Environ. Sci. Technol. 2020, 54, 15925–15934. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xue, Q.; Su, X.; Xie, L.; Yan, Y.; Wang, L.; Steinman, A.D. First Identification of the Toxicity of Microcystins on Pancreatic Islet Function in Humans and the Involved Potential Biomarkers. Environ. Sci. Technol. 2016, 50, 3137–3144. [Google Scholar] [CrossRef]
- Cowie, A.M.; Sarty, K.I.; Mercer, A.; Koh, J.; Kidd, K.A.; Martyniuk, C.J. Molecular Networks Related to the Immune System and Mitochondria Are Targets for the Pesticide Dieldrin in the Zebrafish (Danio rerio) Central Nervous System. J. Proteom. 2017, 157, 71–82. [Google Scholar] [CrossRef]
- Patton, E.E.; Tobin, D.M. Spotlight on Zebrafish: The next Wave of Translational Research. Dis. Model. Mech. 2019, 12, dmm039370. [Google Scholar] [CrossRef]
- Bambino, K.; Zhang, C.; Austin, C.; Amarasiriwardena, C.; Arora, M.; Chu, J.; Sadler, K.C. Inorganic Arsenic Causes Fatty Liver and Interacts with Ethanol to Cause Alcoholic Liver Disease in Zebrafish. Dis. Model. Mech. 2018, 11, dmm031575. [Google Scholar] [CrossRef]
- La Merrill, M.; Emond, C.; Kim, M.J.; Antignac, J.-P.; le Bizec, B.; Clément, K.; Birnbaum, L.S.; Barouki, R. Toxicological Function of Adipose Tissue: Focus on Persistent Organic Pollutants. Environ Health Perspect 2013, 121, 162–169. [Google Scholar] [CrossRef]
- Chen, L.; Hu, Y.; He, J.; Chen, J.; Giesy, J.P.; Xie, P. Responses of the Proteome and Metabolome in Livers of Zebrafish Exposed Chronically to Environmentally Relevant Concentrations of Microcystin-LR. Environ. Sci. Technol. 2017, 51, 596–607. [Google Scholar] [CrossRef]
- Lee, H.; Gao, Y.; Ko, E.; Lee, J.; Lee, H.-K.; Lee, S.; Choi, M.; Shin, S.; Park, Y.H.; Moon, H.-B.; et al. Nonmonotonic Response of Type 2 Diabetes by Low Concentration Organochlorine Pesticide Mixture: Findings from Multi-Omics in Zebrafish. J. Hazard. Mater. 2021, 416, 125956. [Google Scholar] [CrossRef]
- Hedl, T.J.; San Gil, R.; Cheng, F.; Rayner, S.L.; Davidson, J.M.; de Luca, A.; Villalva, M.D.; Ecroyd, H.; Walker, A.K.; Lee, A. Proteomics Approaches for Biomarker and Drug Target Discovery in ALS and FTD. Front. Neurosci. 2019, 13, 548. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-M.; Bae, S.-G.; Lee, S.-H.; Jacobs, D.R.; Lee, D.-H. Associations between Cigarette Smoking and Total Mortality Differ Depending on Serum Concentrations of Persistent Organic Pollutants among the Elderly. J. Korean Med. Sci. 2013, 28, 1122. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Steffes, M.W.; Sjödin, A.; Jones, R.S.; Needham, L.L.; Jacobs, D.R. Low Dose of Some Persistent Organic Pollutants Predicts Type 2 Diabetes: A Nested Case–Control Study. Environ. Health Perspect. 2010, 118, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Jacobs, D.R.; Porta, M. Association of Serum Concentrations of Persistent Organic Pollutants with the Prevalence of Learning Disability and Attention Deficit Disorder. J. Epidemiol. Community Health 2007, 61, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-M.; Kim, K.-T.; Rhyu, D.-Y. Exposure to a Low Concentration of Mixed Organochlorine Pesticides Impairs Glucose Metabolism and Mitochondrial Function in L6 Myotubes and Zebrafish. J. Hazard. Mater. 2021, 414, 125437. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ko, E.; Shin, S.; Choi, M.; Kim, K.-T. Differential Mitochondrial Dysregulation by Exposure to Individual Organochlorine Pesticides (OCPs) and Their Mixture in Zebrafish Embryos. Environ. Pollut. 2021, 277, 115904. [Google Scholar] [CrossRef]
- Pestana, D.; Teixeira, D.; Meireles, M.; Marques, C.; Norberto, S.; Sá, C.; Fernandes, V.C.; Correia-Sá, L.; Faria, A.; Guardão, L.; et al. Adipose Tissue Dysfunction as a Central Mechanism Leading to Dysmetabolic Obesity Triggered by Chronic Exposure to p,p’-DDE. Sci. Rep. 2017, 7, 2738. [Google Scholar] [CrossRef]
- Bui Thi, N.H.; Nguyen Thi, N.A.; Audira, G.; Siregar, P.; Liang, S.-T.; Huang, J.-C.; Hsiao, C.-D. Chronic Exposure to Low Concentration Lead Chloride-Induced Anxiety and Loss of Aggression and Memory in Zebrafish. Int. J. Mol. Sci. 2020, 21, 1844. [Google Scholar] [CrossRef]
- Gao, Y.; Lee, H.; Kwon, O.K.; Tan, M.; Kim, K.T.; Lee, S. Global Proteomic Analysis of Lysine Succinylation in Zebrafish (Danio rerio). J. Proteome Res. 2019, 18, 3762–3769. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Khan, A.; Katanic, D.; Thakar, J. Meta-Analysis of Cell- Specific Transcriptomic Data Using Fuzzy c-Means Clustering Discovers Versatile Viral Responsive Genes. BMC Bioinform. 2017, 18, 295. [Google Scholar] [CrossRef]
- Lukowicz, C.; Ellero-Simatos, S.; Régnier, M.; Polizzi, A.; Lasserre, F.; Montagner, A.; Lippi, Y.; Jamin, E.L.; Martin, J.-F.; Naylies, C.; et al. Metabolic Effects of a Chronic Dietary Exposure to a Low-Dose Pesticide Cocktail in Mice: Sexual Dimorphism and Role of the Constitutive Androstane Receptor. Environ. Health Perspect. 2018, 126, 067007. [Google Scholar] [CrossRef]
- Marushka, L.; Hu, X.; Batal, M.; Sadik, T.; Schwartz, H.; Ing, A.; Fediuk, K.; Tikhonov, C.; Chan, H. The Relationship between Persistent Organic Pollutants Exposure and Type 2 Diabetes among First Nations in Ontario and Manitoba, Canada: A Difference in Difference Analysis. Int. J. Environ. Res. Public Health 2018, 15, 539. [Google Scholar] [CrossRef]
- Jorgensen, M.E.; Borch-Johnsen, K.; Bjerregaard, P. A Cross-Sectional Study of the Association between Persistent Organic Pollutants and Glucose Intolerance among Greenland Inuit. Diabetologia 2008, 51, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Lee, D.H. Mitochondrial Toxins and Healthy Lifestyle Meet at the Crossroad of Hormesis. Diabetes Metab. J. 2019, 43, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Lee, Y.M.; Choi, J.Y.; Jacobs, D.R., Jr.; Lee, D.H. Evolutionarily Adapted Hormesis-Inducing Stressors Can Be a Practical Solution to Mitigate Harmful Effects of Chronic Exposure to Low Dose Chemical Mixtures. Environ. Pollut. 2018, 233, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Fronczyk, A.; Moleda, P.; Safranow, K.; Piechota, W.; Majkowska, L. Increased Concentration of C-Reactive Protein in Obese Patients with Type 2 Diabetes Is Associated with Obesity and Presence of Diabetes but Not with Macrovascular and Microvascular Complications or Glycemic Control. Inflammation 2014, 37, 349–357. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thiele, J.R.; Zeller, J.; Bannasch, H.; Stark, G.B.; Peter, K.; Eisenhardt, S.U. Targeting C-Reactive Protein in Inflammatory Disease by Preventing Conformational Changes. Mediat. Inflamm. 2015, 2015, 372432. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-Reactive Protein: A Critical Update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Yudkin, J.S.; Stehouwer, C.D.; Emeis, J.J.; Coppack, S.W. C-Reactive Protein in Healthy Subjects: Associations with Obesity, Insulin Resistance, and Endothelial Dysfunction: A Potential Role for Cytokines Originating from Adipose Tissue? Arterioscler. Thromb. Vasc. Biol. 1999, 19, 972–978. [Google Scholar] [CrossRef]
- Vidali, S.; Aminzadeh, S.; Lambert, B.; Rutherford, T.; Sperl, W.; Kofler, B.; Feichtinger, R.G. Mitochondria: The Ketogenic Diet--A Metabolism-Based Therapy. Int. J. Biochem. Cell Biol. 2015, 63, 55–59. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Liang, X.; Zou, L.; Ong, C.N.; Yuan, J.M.; Koh, W.P.; Pan, A. Serum Amino Acids in Association with Prevalent and Incident Type 2 Diabetes in A Chinese Population. Metabolites 2019, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between Lipids and Branched-Chain Amino Acids in Development of Insulin Resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Babcock, S.A.; Hu, N.; Maris, J.R.; Wang, H.; Ren, J. Mitochondrial Aldehyde Dehydrogenase (ALDH2) Protects against Streptozotocin-Induced Diabetic Cardiomyopathy: Role of GSK3beta and Mitochondrial Function. BMC Med. 2012, 10, 40. [Google Scholar] [CrossRef] [PubMed]
- Murata, C.; Suzuki, Y.; Muramatsu, T.; Taniyama, M.; Atsumi, Y.; Matsuoka, K.; Watanabe, T.; Okazaki, I. Inactive Aldehyde Dehydrogenase 2 Worsens Glycemic Control in Patients with Type 2 Diabetes Mellitus Who Drink Low to Moderate Amounts of Alcohol. Alcohol. Clin. Exp. Res. 2000, 24, 5S–11S. [Google Scholar] [CrossRef] [PubMed]
- Murata, C.; Taniyama, M.; Kuriyama, S.; Muramatsu, T.; Atsumi, Y.; Matsuoka, K.; Suzuki, Y. Meta-Analysis of Three Diabetes Population Studies: Association of Inactive ALDH2 Genotype with Maternal Inheritance of Diabetes. Diabetes Res. Clin. Pract. 2004, 66 (Suppl. 1), S145–S147. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Ohsawa, I.; Kamino, K.; Ando, F.; Shimokata, H. Mitochondrial ALDH2 Deficiency as an Oxidative Stress. Ann. N. Y. Acad. Sci. 2004, 1011, 36–44. [Google Scholar] [CrossRef]
- Hu, J.F.; Zhang, G.J.; Wang, L.; Kang, P.F.; Li, J.; Wang, H.J.; Gao, Q.; Chen, Y.Q. Ethanol at Low Concentration Attenuates Diabetes Induced Lung Injury in Rats Model. J. Diabetes Res. 2014, 2014, 107152. [Google Scholar] [CrossRef]
- Wentzel, P.; Ejdesjo, A.; Eriksson, U.J. Maternal Diabetes in Vivo and High Glucose in Vitro Diminish GAPDH Activity in Rat Embryos. Diabetes 2003, 52, 1222–1228. [Google Scholar] [CrossRef]
- Blatnik, M.; Frizzell, N.; Thorpe, S.R.; Baynes, J.W. Inactivation of Glyceraldehyde-3-Phosphate Dehydrogenase by Fumarate in Diabetes: Formation of S-(2-Succinyl)Cysteine, a Novel Chemical Modification of Protein and Possible Biomarker of Mitochondrial Stress. Diabetes 2008, 57, 41–49. [Google Scholar] [CrossRef]
- Nakamura, M.; Tomitori, H.; Suzuki, T.; Sakamoto, A.; Terui, Y.; Saiki, R.; Dohmae, N.; Igarashi, K.; Kashiwagi, K. Inactivation of GAPDH as One Mechanism of Acrolein Toxicity. Biochem. Biophys. Res. Commun. 2013, 430, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Tristan, C.; Shahani, N.; Sedlak, T.W.; Sawa, A. The Diverse Functions of GAPDH: Views from Different Subcellular Compartments. Cell Signal 2011, 23, 317–323. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Lee, H.; Lee, S.; Kim, K.-T. Type 2 Diabetes Induced by Changes in Proteomic Profiling of Zebrafish Chronically Exposed to a Mixture of Organochlorine Pesticides at Low Concentrations. Int. J. Environ. Res. Public Health 2022, 19, 4991. https://doi.org/10.3390/ijerph19094991
Gao Y, Lee H, Lee S, Kim K-T. Type 2 Diabetes Induced by Changes in Proteomic Profiling of Zebrafish Chronically Exposed to a Mixture of Organochlorine Pesticides at Low Concentrations. International Journal of Environmental Research and Public Health. 2022; 19(9):4991. https://doi.org/10.3390/ijerph19094991
Chicago/Turabian StyleGao, Yan, Hyojin Lee, Sangkyu Lee, and Ki-Tae Kim. 2022. "Type 2 Diabetes Induced by Changes in Proteomic Profiling of Zebrafish Chronically Exposed to a Mixture of Organochlorine Pesticides at Low Concentrations" International Journal of Environmental Research and Public Health 19, no. 9: 4991. https://doi.org/10.3390/ijerph19094991
APA StyleGao, Y., Lee, H., Lee, S., & Kim, K.-T. (2022). Type 2 Diabetes Induced by Changes in Proteomic Profiling of Zebrafish Chronically Exposed to a Mixture of Organochlorine Pesticides at Low Concentrations. International Journal of Environmental Research and Public Health, 19(9), 4991. https://doi.org/10.3390/ijerph19094991