Translation, Cross-Cultural Adaptation and Validation of the Myofascial Adhesions for Patients after Breast Cancer (MAP-BC) Evaluation Tool: Spanish Version
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Development of the Spanish Version of the MAP-BC: Translation and Cross-Cultural Adaptation
2.2.1. Preparation
2.2.2. Forward Translation and Reconciliation
2.2.3. Backward Translation
2.2.4. Review and Harmonisation
2.2.5. Pilot Testing
2.3. Assessment of Convergent Validity and Responsiveness
2.3.1. POSAS
2.3.2. MAP-BC
2.4. Statistical Analysis
3. Results
3.1. Development of the Spanish Version of the MAP-BC: Translation and Cross-Cultural Adaptation
3.2. Assessment of Convergent Validity and Responsiveness
4. Discussion
4.1. Development of the Spanish Version of the MAP-BC: Translation and Cross-Cultural Adaptation
4.2. Convergent Validity and Responsiveness
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Final MAP-BC Evaluation Tool: Spanish Version
Appendix A.1. Instrucciones para los Terapeutas:
| Nivel | Instrucciones para la Palpación |
| Piel | Sin presión vertical, deslizar la piel en todas las direcciones relativas a las estructuras anatómicas del nivel miofascial superficial. |
| Superficial | Deslizar la piel y los 6 tejidos subcutáneos del nivel superficial en todas las direcciones relativas al nivel miofascial profundo subyacente. |
| Profundo | Deslizar los tejidos en todas las direcciones relativas a las estructuras óseas subyacentes. |
Appendix A.2. Sistema de puntuación
| Puntuación | Grado de Deslizamiento del Tejido Restringido |
| 0 | No hay restricción en el deslizamiento del tejido. |
| 1 | Restricción limitada que se libera inmediatamente. |
| 2 | Grave restricción en el deslizamiento del tejido. |
| 3 | El deslizamiento del tejido es imposible. |
| 1. CICATRIZ AXILAR (90º Abducción) | 2a. CICATRIZ DEL PECHO (Cirugía Conservadora del Pecho) | 2b. CICATRIZ DE MASTECTOMÍA (Mastectomía) | 3. REGIÓN DEL MÚSCULO PECTORAL (90º Abducción) |
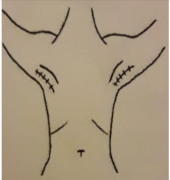 | 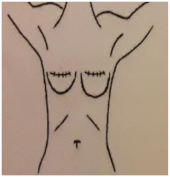 |  | 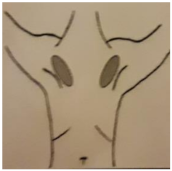 |
| Piel: 0–1–2–3 Superficial: 0–1–2–3 Profundo: 0–1–2–3 PUNTUACIÓN TOTAL: | Piel: 0–1–2–3 Superficial: 0–1–2–3 Profundo: 0–1–2–3 PUNTUACIÓN TOTAL: | Piel: 0–1–2–3 Superficial: 0–1–2–3 Profundo: 0–1–2–3 PUNTUACIÓN TOTAL: | Piel: 0–1–2–3 Superficial: 0–1–2–3 Profundo: 0–1–2–3 PUNTUACIÓN TOTAL: |
| 4. PARED TORÁCICA FRONTAL | 5. PARED TORÁCICA LATERAL | 6. AXILA (90º Abducción) | 7. PLIEGUE INFRAMAMARIO |
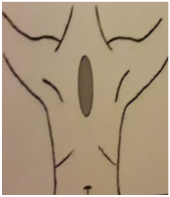 | 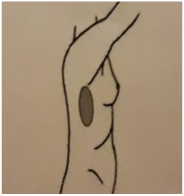 | 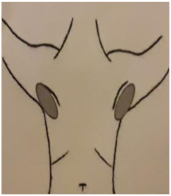 |  |
| Piel: 0–1–2–3 Superficial: 0–1–2–3 Profundo: 0–1–2–3 PUNTUACIÓN TOTAL: | Piel: 0–1–2–3 Superficial: 0–1–2–3 Profundo: 0–1–2–3 PUNTUACIÓN TOTAL: | Piel: 0–1–2–3 Superficial: 0–1–2–3 Profundo: 0–1–2–3 PUNTUACIÓN TOTAL: | Piel: 0–1–2–3 Superficial: 0–1–2–3 Profundo: 0–1–2–3 PUNTUACIÓN TOTAL: |
References
- Soriano-Maldonado, A.; Carrera-Ruiz, Á.; Díez-Fernández, D.M.; Esteban-Simón, A.; Maldonado-Quesada, M.; Moreno-Poza, N.; García-Martínez, M.D.M.; Alcaraz-García, C.; Vázquez-Sousa, R.; Moreno-Martos, H.; et al. Effects of a 12-Week Resistance and Aerobic Exercise Program on Muscular Strength and Quality of Life in Breast Cancer Survivors: Study Protocol for the EFICAN Randomized Controlled Trial. Medicine 2019, 98, e17625. [Google Scholar] [CrossRef] [PubMed]
- SEOM: Sociedad Española de Oncología Médica—SEOM: Sociedad Espa. Available online: https://seom.org/ (accessed on 20 December 2021).
- Gass, J.; Mitchell, S.; Hanna, M. How Do Breast Cancer Surgery Scars Impact Survivorship? Findings from a Nationwide Survey in the United States. BMC Cancer 2019, 19, 342. [Google Scholar] [CrossRef] [PubMed]
- Olsson Möller, U.; Beck, I.; Rydén, L.; Malmström, M. A Comprehensive Approach to Rehabilitation Interventions Following Breast Cancer Treatment—A Systematic Review of Systematic Reviews. BMC Cancer 2019, 19, 472. [Google Scholar] [CrossRef] [PubMed]
- Delay, E.; Gosset, J.; Toussoun, G.; Delaporte, T.; Delbaere, M. Post-Treatment Sequelae after Breast Cancer Conservative Surgery. Ann. Chir. Plast. Esthet. 2008, 53, 135–152. [Google Scholar] [CrossRef]
- Haviland, J.S.; Mannino, M.; Griffin, C.; Porta, N.; Sydenham, M.; Bliss, J.M.; Yarnold, J.R. Late Normal Tissue Effects in the Arm and Shoulder Following Lymphatic Radiotherapy: Results from the UK START (Standardisation of Breast Radiotherapy) Trials. Radiother. Oncol. 2018, 126, 155–162. [Google Scholar] [CrossRef]
- Wasserman, J.B.; Steele-Thornborrow, J.L.; Yuen, J.S.; Halkiotis, M.; Riggins, E.M. Chronic Caesarian Section Scar Pain Treated with Fascial Scar Release Techniques: A Case Series. J. Bodyw. Mov. Ther. 2016, 20, 906–913. [Google Scholar] [CrossRef]
- Sowa, Y.; Morihara, T.; Kushida, R.; Sakaguchi, K.; Taguchi, T.; Numajiri, T. Long-Term Prospective Assessment of Shoulder Function after Breast Reconstruction Involving a Latissimus Dorsi Muscle Flap Transfer and Postoperative Radiotherapy. Breast cancer 2017, 24, 362–368. [Google Scholar] [CrossRef]
- Cheville, A.L.; Tchou, J. Barriers to Rehabilitation Following Surgery for Primary Breast Cancer. J. Surg. Oncol. 2007, 95, 409–418. [Google Scholar] [CrossRef]
- Stubblefield, M.D.; Keole, N. Upper Body Pain and Functional Disorders in Patients with Breast Cancer. PM R 2014, 6, 170–183. [Google Scholar] [CrossRef]
- Serra-Añó, P.; Inglés, M.; Bou-Catalá, C.; Iraola-Lliso, A.; Espí-López, G.V. Effectiveness of Myofascial Release after Breast Cancer Surgery in Women Undergoing Conservative Surgery and Radiotherapy: A Randomized Controlled Trial. Support. Care Cancer 2019, 27, 2633–2641. [Google Scholar] [CrossRef]
- De Groef, A.; Van Kampen, M.; Vervloesem, N.; De Geyter, S.; Dieltjens, E.; Christiaens, M.R.; Neven, P.; Geraerts, I.; Devoogdt, N. An Evaluation Tool for Myofascial Adhesions in Patients after Breast Cancer (MAP-BC Evaluation Tool): Development and Interrater Reliability. PLoS ONE 2017, 12, e0179116. [Google Scholar] [CrossRef] [PubMed]
- De Groef, A.; Van Kampen, M.; Moortgat, P.; Anthonissen, M.; Van den Kerckhove, E.; Christiaens, M.R.; Neven, P.; Geraerts, I.; Devoogdt, N. An Evaluation Tool for Myofascial Adhesions in Patients after Breast Cancer (MAP-BC Evaluation Tool): Concurrent, Face and Content Validity. PLoS ONE 2018, 13, e0193915. [Google Scholar] [CrossRef] [PubMed]
- Mokkink, L.B.; Terwee, C.B.; Knol, D.L.; Stratford, P.W.; Alonso, J.; Patrick, D.L.; Bouter, L.M.; De Vet, H.C. The COSMIN Checklist for Evaluating the Methodological Quality of Studies on Measurement Properties: A Clarification of Its Content. BMC Med. Res. Methodol. 2010, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Mokkink, L.B.; Terwee, C.B.; Patrick, D.L.; Alonso, J.; Stratford, P.W.; Knol, D.L.; Bouter, L.M.; De Vet, H.C.W. The COSMIN Checklist for Assessing the Methodological Quality of Studies on Measurement Properties of Health Status Measurement Instruments: An International Delphi Study. Qual. Life Res. 2010, 19, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Wild, D.; Grove, A.; Martin, M.; Eremenco, S.; McElroy, S.; Verjee-Lorenz, A.; Erikson, P. Principles of Good Practice for the Translation and Cultural Adaptation Process for Patient-Reported Outcomes (PRO) Measures: Report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health 2005, 8, 94–104. [Google Scholar] [CrossRef]
- Beaton, D.E.; Bombardier, C.; Guillemin, F.; Ferraz, M.B. Guidelines for the Process of Cross-Cultural Adaptation of Self-Report Measures. Spine 2000, 25, 3186–3191. [Google Scholar] [CrossRef]
- Draaijers, L.J.; Tempelman, F.R.H.; Botman, Y.A.M.; Tuinebreijer, W.E.; Middelkoop, E.; Kreis, R.W.; Van Zuijlen, P.P.M. The Patient and Observer Scar Assessment Scale: A Reliable and Feasible Tool for Scar Evaluation. Plast. Reconstr. Surg. 2004, 113, 1960–1965. [Google Scholar] [CrossRef]
- Truong, P.T.; Lee, J.C.; Soer, B.; Gaul, C.A.; Olivotto, I.A. Reliability and Validity Testing of the Patient and Observer Scar Assessment Scale in Evaluating Linear Scars after Breast Cancer Surgery. Plast. Reconstr. Surg. 2007, 119, 487–494. [Google Scholar] [CrossRef]
- Rodríguez Castillo, T.R.; Sanguineti montalva, A.; Moreno Baeza, N.; Carrillo Gonzalez, K.; Hasbún Nazar, A.; Lopez Nuñez, S. Adaptación Transcultural Del Cuestionario POSAS (Patient and Observer Scar Assessment) Para Valoración de Cicatrices. Rev. Cir. 2019, 71, 385–391. [Google Scholar] [CrossRef][Green Version]
- Portney, L.G.; Watkins, M.P. Fundations of Clinical Research: Applications to Practice (Vol. 892); Prentice Hall: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Stratford, P.W.; Riddle, D.L. Assessing Sensitivity to Change: Choosing the Appropriate Change Coefficient. Health Qual. Life Outcomes 2005, 3, 23. [Google Scholar] [CrossRef]
- de Yébenes Prous, M.J.G.; Rodríguez Salvanés, F.; Carmona Ortells, L. Sensibilidad Al Cambio de Las Medidas de Desenlace. Reumatol. Clin. 2008, 4, 240–247. [Google Scholar] [CrossRef]
- Bae, S.H.; Bae, Y.C. Analysis of Frequency of Use of Different Scar Assessment Scales Based on the Scar Condition and Treatment Method. Arch. Plast. Surg. 2014, 41, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Choo, A.M.H.; Ong, Y.S.; Issa, F. Scar Assessment Tools: How Do They Compare? Front. Surg. 2021, 8, 643098. [Google Scholar] [CrossRef]
- Restrepo, S.; Rojas, S.; Sanabria, A. Cross-Cultural Adaptation and Psychometric Validation of the Patient Scar Assessment Questionnaire to the Spanish Language in Head and Neck Surgery. Int. Wound J. 2020, 17, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.A.L.; Bond, J.S.; Mason, T.; Ludlow, A.; Cridland, P.; O’Kane, S.; Ferguson, M.W.J. Visual Analogue Scale Scoring and Ranking: A Suitable and Sensitive Method for Assessing Scar Quality? Plast. Reconstr. Surg. 2006, 118, 909–918. [Google Scholar] [CrossRef]
- Truong, P.T.; Abnousi, F.; Yong, C.M.; Hayashi, A.; Runkel, J.A.; Phillips, T.; Olivotto, I.A. Standardized Assessment of Breast Cancer Surgical Scars Integrating the Vancouver Scar Scale, Short-Form McGill Pain Questionnaire, and Patients’ Perspectives. Plast. Reconstr. Surg. 2005, 116, 1291–1299. [Google Scholar] [CrossRef]

| Demographics and Characteristics | Number of Subjects = 77(%) |
|---|---|
| Age at Diagnosis, years | Mean ± SD: 59.51 ± 11.93 Minimum: 31 Maximum: 85 |
| <45 | 8 (10.38) |
| 45–54 | 22 (28.57) |
| 55–64 | 15 (19.48) |
| +65 | 32 (41.55) |
| Body Mass Index, Baseline, kg/m2 | Mean ± SD: 31.5 ± 4.94 Minimum: 17.97 Maximum: 49.33 |
| Normal: 18.5–24.9 | 28 (36.8) |
| Overweight: 25–29.9 | 30 (38.9) |
| Obese: ≥30 | 18 (23.7) |
| Ethnicity | |
| Caucasian | 74 (96.1) |
| African | 1 (1.3) |
| Latin American | 2 (2.6) |
| Type of Breast Cancer | |
| Ductal Carcinoma in situ (DCIS) | 19 (24.6) |
| Invasive Ductal Carcinoma (IDC) | 54 (70.1) |
| Lobular Carcinoma in situ | 3 (3.9) |
| Invasive Lobular Carcinoma | 1 (1.3) |
| Stage of Breast Cancer | |
| 0 | 7 (9.7) |
| IA | 44 (61.1) |
| IIA | 11 (14.3) |
| IIIA | 1 (1.3) |
| IB | 3 (4.1) |
| IIB | 6 (8.3) |
| Type of breast surgery | |
| Simple unilateral mastectomy | 17 (22.0) |
| Breast conserving surgery | 60 (77.9) |
| Numbers of lymph nodes removed | Mean ± SD: 2.01 ± 1.12 Minimum: 1 Maximum:6 |
| Positive Lymph Nodes | |
| Yes | 11 (14) |
| No | 66 (86) |
| Side Involved | |
| Right | 42 (54.5) |
| Left | 34 (44.1) |
| Bilateral | 1 (1.2) |
| Involved side to hand dominance | |
| Ipsilateral side | 43 (55.8) |
| Contralateral side | 34 (44.1) |
| Adjuvant Therapy | |
| Yes | 19 (24.7) |
| No | 58 (75.3) |
| Type of Adjuvant Therapy | |
| Radiation | 2 (2.6) |
| Chemotherapy | 16 (20.8) |
| POSAS (T1) | Mean ± SD: 24.81 ± 10.68 |
| POSAS (T2) | Mean ± SD: 16.87 ± 12.60 |
| MAP-BC (T1) | Mean ± SD: 24.50 ± 11.54 |
| MAP-BC (T2) | Mean ± SD: 17.16 ± 17.03 |
| MAP-BC | Mean (SD) | p-Value | SRM | ES | Correlations between the MAP-BC and POSAS Scales |
|---|---|---|---|---|---|
| T1 evaluation | 24.50 (11.54) | T1 evaluation: r = 0.438 (p < 0.001) | |||
| T2 evaluation | 17.16 (17.03) | 0.000 | 0.45 | 0.63 | T2 evaluation: r = 0.816 (p < 0.001) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casuso-Holgado, M.J.; Ostos-Díaz, B.; Muñoz-Fernández, M.J. Translation, Cross-Cultural Adaptation and Validation of the Myofascial Adhesions for Patients after Breast Cancer (MAP-BC) Evaluation Tool: Spanish Version. Int. J. Environ. Res. Public Health 2022, 19, 4337. https://doi.org/10.3390/ijerph19074337
Casuso-Holgado MJ, Ostos-Díaz B, Muñoz-Fernández MJ. Translation, Cross-Cultural Adaptation and Validation of the Myofascial Adhesions for Patients after Breast Cancer (MAP-BC) Evaluation Tool: Spanish Version. International Journal of Environmental Research and Public Health. 2022; 19(7):4337. https://doi.org/10.3390/ijerph19074337
Chicago/Turabian StyleCasuso-Holgado, María Jesús, Beatriz Ostos-Díaz, and María Jesús Muñoz-Fernández. 2022. "Translation, Cross-Cultural Adaptation and Validation of the Myofascial Adhesions for Patients after Breast Cancer (MAP-BC) Evaluation Tool: Spanish Version" International Journal of Environmental Research and Public Health 19, no. 7: 4337. https://doi.org/10.3390/ijerph19074337
APA StyleCasuso-Holgado, M. J., Ostos-Díaz, B., & Muñoz-Fernández, M. J. (2022). Translation, Cross-Cultural Adaptation and Validation of the Myofascial Adhesions for Patients after Breast Cancer (MAP-BC) Evaluation Tool: Spanish Version. International Journal of Environmental Research and Public Health, 19(7), 4337. https://doi.org/10.3390/ijerph19074337







