Prenatal Exposure to Metals and Neurodevelopment in Infants at Six Months: Rio Birth Cohort Study of Environmental Exposure and Childhood Development (PIPA Project)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
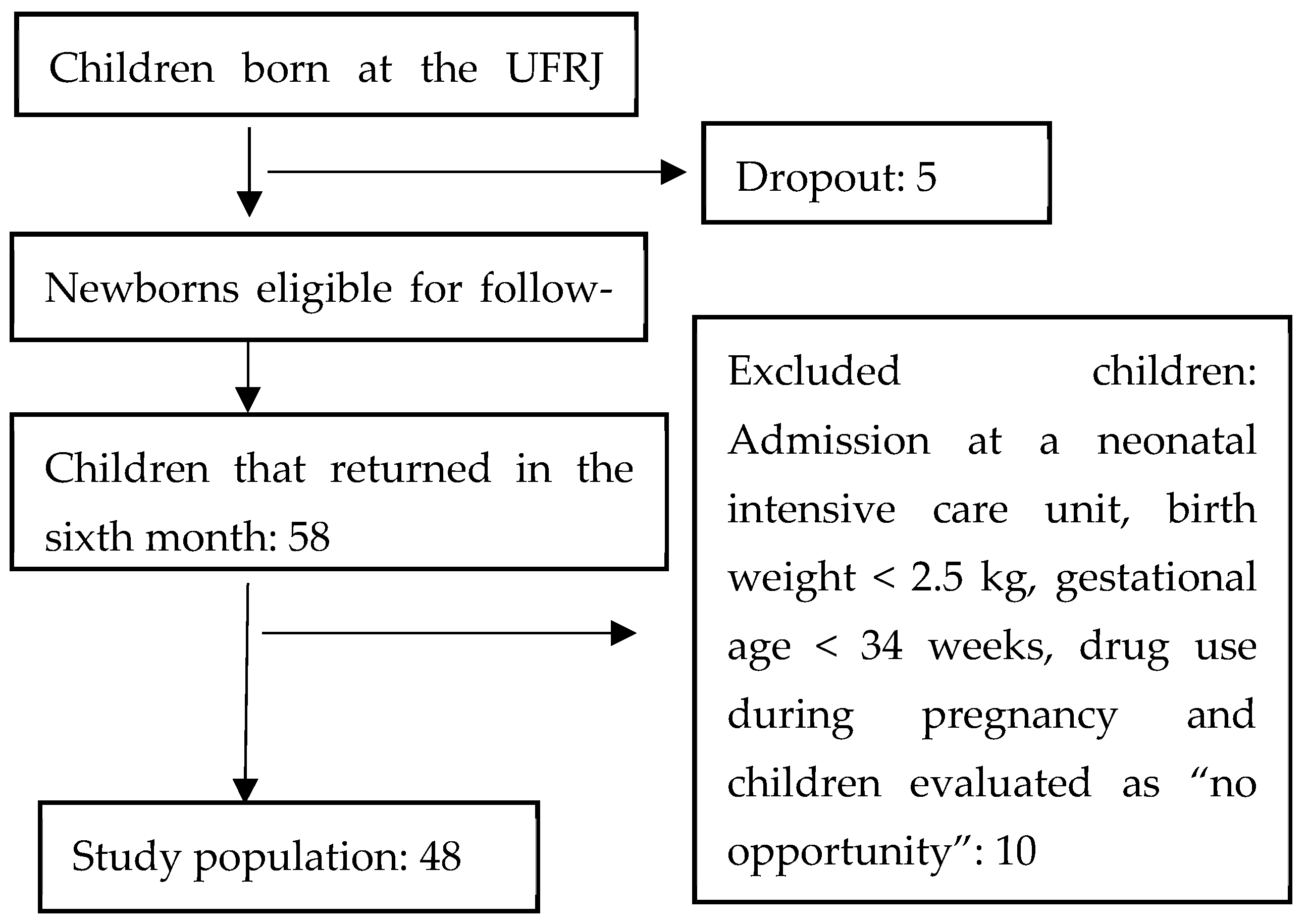
2.2. Study Population
2.3. Arsenic, Cadmium, Lead, and Mercury Determinations
2.4. Denver Development Screening Test II (DDST-II)
2.5. Statistical Analyses
2.6. Covariates
3. Results
4. Discussion
5. Strengths and Limitations of This Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry. ATSDR’s Substance Priority List 2019. Available online: https://www.atsdr.cdc.gov/spl/#2019spl (accessed on 4 May 2021).
- Morais, S.; Costa, F.G.; Pereira, M.D.L. Heavy Metals and Human Health. In Environmental Health–Emerging Issues and Practice; Oosthuizen, J., Ed.; Intechopen: London, UK, 2012; pp. 227–245. ISBN 978-953-307-854-0. [Google Scholar] [CrossRef]
- Goyer, R.A. Results of lead research: Prenatal exposure and neurological consequences. Environ. Health Perspect. 1996, 104, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Experientia Supplementum, 101; Springer: Basel, Switzerland, 2012. [Google Scholar] [CrossRef]
- Schofield, K. The Metal Neurotoxins: An Important Role in Current Human Neural Epidemics? Int. J. Environ. Res. Public Health 2017, 14, 1511. [Google Scholar] [CrossRef] [PubMed]
- Dórea, J.G. Exposure to environmental neurotoxic substances and neurodevelopment in children from Latin America and the Caribbean. Environ. Res. 2020, 192, 110199. [Google Scholar] [CrossRef] [PubMed]
- Caserta, D.; Grazianom, A.; Lo Monte, G.; Bordi, G.; Moscarini, M. Heavy metals and placental fetal-maternal barrier: A mini-review on the major concerns. Eur. Rev. Med. Pharm. Sci. 2013, 17, 2198–2206. [Google Scholar]
- Lanphear, B.P.; Hornung, R.; Khoury, J.; Yolton, K.; Baghurst, P.; Bellinger, D.C.; Canfield, R.L.; Dietrich, K.N.; Bornschein, R.; Greene, T.; et al. Low-Level Environmental Lead Exposure and Children’s Intellectual Function: An International Pooled Analysis. Environ. Health Perspect. 2005, 113, 894–899. [Google Scholar] [CrossRef]
- Bellinger, D.C. Very low lead exposures and children’s neurodevelopment. Curr. Opin. Pediatr. 2008, 20, 172–177. [Google Scholar] [CrossRef]
- Jurewicz, J.; Polanska, K.; Hanke, W. Chemical exposure early in life and the neurodevelopment of children—An overview of current epidemiological evidence. Ann. Agric. Environ. Med. 2013, 20, 465–486. [Google Scholar]
- Liu, J.; Chen, Y.; Gao, D.; Jing, J.; Hu, Q. Prenatal and postnatal lead exposure and cognitive development of infants followed over the first three years of life: A prospective birth study in the Pearl River Delta region, China. NeuroToxicology 2014, 44, 326–334. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef]
- Ijomone, O.M.; Olung, N.F.; Akingbade, G.T.; Okoh, C.O.; Aschner, M. Environmental influence on neurodevelopmental disorders: Potential association of heavy metal exposure and autism. J. Trace Elements Med. Biol. 2020, 62, 126638. [Google Scholar] [CrossRef] [PubMed]
- Rosalem, A. Association between environmental factors of exposure to lead and lead in spontaneous abortion. Campinas: Universidade Estadual de Campinas. 2004. Available online: https://docs.bvsalud.org/biblioref/ses-sp/2004/ses-16485/ses-16485-1586.pdf (accessed on 26 March 2022).
- Santos, E.O.; De Jesus, I.M.; Câmara, V.D.M.; Brabo, E.D.S.; De Jesus, M.I.; Fayal, K.F.; Asmus, C.I.R.F. Correlation between blood mercury levels in mothers and newborns in Itaituba, Pará State, Brazil. Cadernos Saúde Pública 2007, 23, S622–S629. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.H.; Rezende, V.B.; Quintana, S.M.; Gerlach, R.F.; Barbosa, F.; Tanus-Santos, J.E. The Relationship between Blood and Serum Lead Levels in Peripartum Women and their Respective Umbilica (l Cords. Basic Clin. Pharmacol. Toxicol. 2010, 107, 971–975. [Google Scholar] [CrossRef] [PubMed]
- Rudge, C.V.C.; Calderon, I.M.P.; Rudge, M.V.C.; Volpato, G.; Silva, J.L.P.; Duarte, G.; Neto, C.M.; Sass, N.; Mattar, R.; Röllin, H.B.; et al. Toxic and essential elements in blood from delivering women in selected areas of São Paulo State, Brazil. J. Environ. Monit. 2010, 13, 563–571. [Google Scholar] [CrossRef][Green Version]
- Araujo, M.S.D.A.; Figueiredo, N.D.; Camara, V.M.; Asmus, C.I.F. Maternal-child exposure to metals during pregnancy in Rio de Janeiro city, Brazil: The Rio Birth Cohort Study of Environmental Exposure and Childhood Development (PIPA project). Environ. Res. 2020, 183, 109155. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention. Blood Levels in Children. Available online: https://www.cdc.gov/nceh/lead/prevention/blood-lead-levels.htm (accessed on 8 March 2022).
- Ruggieri, F.; Majorani, C.; Domanico, F.; Alimonti, A. Mercury in Children: Current State on Exposure through Human Biomonitoring Studies. Int. J. Environ. Res. Public Health 2017, 14, 519. [Google Scholar] [CrossRef]
- Committee on the Toxicological Effects of Methylmercury; Board on Environmental Studies and Toxicology; National Research Council. Toxicological Effects of Methylmercury; National Academy Press: Washington, DC, USA, 2000; Available online: https://www.ncbi.nlm.nih.gov/books/NBK225778/pdf/Bookshelf_NBK225778.pdf (accessed on 8 March 2022).
- Asmus, C.I.F.; Camara, V.M.; Landrigan, P.J.; Claudio, L. A Systematic Review of Children’s Environmental Health in Brazil. Ann. Glob. Health 2016, 82, 132–148. [Google Scholar] [CrossRef]
- Asmus, C.I.R.F.; Barbosa, A.P.; Meyer, A.; Damasceno, N.; Rosa, A.C.S.; Medronho, R.; Da Cunha, A.J.L.A.; Moreira, J.C.; Fernandes, T.V.R.D.B.; Martins, M.; et al. Rio Birth Cohort Study on Environmental Exposure and Childhood Development—PIPA Project. Ann. Glob. Health 2020, 86, 59. [Google Scholar] [CrossRef]
- Frankenburg, W.K.; Dodds, J.; Archer, P.; Shapiro, H.; Bresnick, B. The Denver II: A Major Revision and Restandardization of the Denver Developmental Screening Test. Pediatrics 1992, 89, 91–97. [Google Scholar] [CrossRef]
- Pinto, F.C.D.A.; Isotani, S.M.; Sabatés, A.L.; Perissinoto, J. Denver II: Proposed behaviors compared to those of children from São Paulo. Rev. CEFAC 2015, 17, 1262–1269. [Google Scholar] [CrossRef]
- Frankenburg, W.K. Denver II: Technical manual; Denver Developmental Materials: Denver, CO, USA, 1996. [Google Scholar]
- de Figueiredo, N.D.; Araújo, M.S.; Luiz, R.R.; Câmara, V.D.M.; Jacob, S.D.C.; dos Santos, L.M.G.; Vicentini, S.A.; Asmus, C.I.R.F. Metal mixtures in pregnant women and umbilical cord blood at urban populations—Rio de Janeiro, Brazil. Environ. Sci. Pollut. Res. 2020, 27, 40210–40218. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.L.; Negreiros, M.M. Suspeita de Atraso No Desenvolvimento Neuropsicomotor em Crianças Menores de um Ano Atendidas em Uma Unidade De Saúde Da Família De Rio Branco-Acre. Revista de APS 2013, 16, 60–65. Available online: https://periodicos.ufjf.br/index.php/aps/article/view/14698 (accessed on 15 March 2022).
- Marques, R.C.; Dórea, J.G.; Bastos, W.R.; Rebelo, M.D.F.; Fonseca, M.D.F.; Malm, O. Maternal mercury exposure and neuro-motor development in breastfed infants from Porto Velho (Amazon), Brazil. Int. J. Hyg. Environ. Health 2007, 210, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Halpern, R.; Barros, F.C.; Horta, B.L.; Victora, C.G. Desenvolvimento neuropsicomotor aos 12 meses de idade em uma coorte de base populacional no Sul do Brasil: Diferenciais conforme peso ao nascer e renda familiar. Cadernos Saúde Pública 1996, 12, S73–S78. [Google Scholar] [CrossRef]
- Costa, E.F.; Cavalcante, L.I.C.; Dell’Aglio, D.D. Language development profile of children in Belem, according to Denver developmental screening test. Revista CEFAC 2015, 17, 1090–1102. [Google Scholar] [CrossRef]
- Hirvonen, M.; Ojala, R.; Korhonen, P.; Haataja, P.; Eriksson, K.; Rantanen, K.; Gissler, M.; Luukkaala, T.; Tammela, O. Intellectual disability in children aged less than seven years born moderately and late preterm compared with very preterm and term-born children—A nationwide birth cohort study. J. Intellect. Disabil. Res. 2017, 61, 1034–1054. [Google Scholar] [CrossRef]
- Allotey, J.; Zamora, J.; Cheong-See, F.; Kalidindi, M.; Arroyo-Manzano, D.; Asztalos, E.; Van Der Post, J.A.M.; Mol, B.W.; Moore, D.; Birtles, D.; et al. Cognitive, motor, behavioural and academic performances of children born preterm: A meta-analysis and systematic review involving 64 061 children. BJOG Int. J. Obstet. Gynaecol. 2017, 125, 16–25. [Google Scholar] [CrossRef]
- Bellinger, D.C. Interpreting epidemiologic studies of developmental neurotoxicity: Conceptual and analytic issues. Neurotoxicology Teratol. 2009, 31, 267–274. [Google Scholar] [CrossRef]
- Selevan, S.G.; Kimmel, C.A.; Mendola, P. Identifying critical windows of exposure for children’s health. Environ. Health Perspect. 2000, 108, 451–455. [Google Scholar] [CrossRef]
- Liang, C.; Wu, X.; Huang, K.; Yan, S.; Li, Z.; Xia, X.; Pan, W.; Sheng, J.; Tao, R.; Tao, Y.; et al. Domain- and sex-specific effects of prenatal exposure to low levels of arsenic on children’s development at 6 months of age: Findings from the Ma’anshan birth cohort study in China. Environ. Int. 2019, 135, 105112. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Liu, B.; Liu, X.; Yu, X. Prenatal exposure to arsenic and neurobehavioral development of newborns in China. Environ. Int. 2018, 121, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Tolins, M.; Ruchirawat, M.; Landrigan, P. The Developmental Neurotoxicity of Arsenic: Cognitive and Behavioral Consequences of Early Life Exposure. Ann. Glob. Health 2014, 80, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Barranco, M.; Lacasaña, M.; Aguilar-Garduño, C.; Alguacil, J.; Gil, F.; Alzaga, B.G.; García, A.R. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: A systematic review and meta-analysis. Sci. Total Environ. 2013, 454–455, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cai, L.; Liu, Y.; Chen, W.; Wang, Q. Association between prenatal cadmium exposure and cognitive development of offspring: A systematic review. Environ. Pollut. 2019, 254, 113081. [Google Scholar] [CrossRef]
- Lewin, A.; Arbuckle, T.E.; Fisher, M.; Liang, C.L.; Marro, L.; Davis, K.; Abdelouahab, N.; Fraser, W.D. Univariate predictors of maternal concentrations of environmental chemicals: The MIREC study. Int. J. Hyg. Environ. Health 2017, 220, 77–85. [Google Scholar] [CrossRef]
- Bulka, C.M.; Bommarito, P.A.; Fry, R.C. Predictors of toxic metal exposures among US women of reproductive age. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 597–612. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for Lead, August 2020. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf (accessed on 21 November 2021).
- Kumar, S.; Sharma, S.; Thaker, R. Occupational, environmental, and lifestyle factors and their contribution to preterm birth—An overview. Indian J. Occup. Environ. Med. 2017, 21, 9–17. [Google Scholar] [CrossRef]
- Ashrap, P.; Watkins, D.J.; Mukherjee, B.; Boss, J.; Richards, M.J.; Rosario, Z.; Vélez-Vega, C.M.; Alshawabkeh, A.; Cordero, J.F.; Meeker, J.D. Maternal blood metal and metalloid concentrations in association with birth outcomes in Northern Puerto Rico. Environ. Int. 2020, 138, 105606. [Google Scholar] [CrossRef]
- Bocca, B.; Ruggieri, F.; Pino, A.; Rovira, J.; Calamandrei, G.; Mirabella, F.; Martínez, M.; Domingo, J.L.; Alimonti, A.; Schuhmacher, M. Human biomonitoring to evaluate exposure to toxic and essential trace elements during pregnancy. Part B: Predictors of exposure. Environ. Res. 2020, 182, 109108. [Google Scholar] [CrossRef]
- Hadavifar, M.; Rastakhiz, M.; Souvizi, B.; Miri, H.H.; Akrami, R. Biomonitoring of maternal and fetal exposure to mercury in Sabzevar and its affecting risk factors. J. Hazard. Mater. 2019, 388, 121781. [Google Scholar] [CrossRef]
- Bocca, B.; Ruggieri, F.; Pino, A.; Rovira, J.; Calamandrei, G.; Martínez, M.Á.; Domingo, J.L.; Alimonti, A.; Schuhmacher, M. Human biomonitoring to evaluate exposure to toxic and essential trace elements during pregnancy. Part A. concentrations in maternal blood, urine and cord blood. Environ. Res. 2019, 177, 108599. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.C.; Dórea, J.G.; Bernardi, J.V.; Bastos, W.R.; Malm, O. Prenatal and Postnatal Mercury Exposure, Breastfeeding and Neurodevelopment During the First 5 Years. Cogn. Behav. Neurol. 2009, 22, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, P.; Hansen, J.C. Organochlorines and heavy metals in pregnant women from the Disko Bay area in Greenland. Sci. Total Environ. 2000, 245, 195–202. [Google Scholar] [CrossRef]
| Metal | Sample | GM (95% CI) | Min. | P25 | Median | P75 | P90 | P95 | Max | R * (p-Value) |
|---|---|---|---|---|---|---|---|---|---|---|
| Arsenic (µg/L) | mb | 9.46 (7.61–11.18) | 0.33 | 8.27 | 9.89 | 11.79 | 17.47 | 19.21 | 36.48 | 0.87 (<0.001) |
| ucb | 10.07 (9.17–10.98) | 5.06 | 8.29 | 10.27 | 12.06 | 15.37 | 16.95 | 19.94 | ||
| Cadmium (µg/L) | mb | 0.29 (0.18–0.43) | 0.01 | 0.13 | 0.30 | 1.29 | 2.09 | 4.74 | 9.97 | 0.76 (<0.001) |
| ucb | 0.32 (0.21–0.46) | 0.01 | 0.15 | 0.33 | 0.83 | 2.33 | 4.40 | 4.88 | ||
| Lead (µg/dL) | mb | 3.83 (3.32–4.43) | 1.32 | 2.54 | 4.41 | 5.55 | 7.11 | 10.26 | 12.41 | 0.78 (<0.001) |
| ucb | 3.81 (3.81–4.53) | 1.43 | 2.42 | 3.39 | 4.90 | 13.42 | 14.62 | 16.03 | ||
| Mercury (µg/L) | mb | 0.90 (0.74–1.11) | 0.38 | 0.61 | 0.76 | 1.42 | 2.77 | 3.26 | 13.32 | 0.64 (<0.001) |
| ucb | 0.95 (0.82–1.11) | 0.42 | 0.69 | 0.88 | 1.30 | 2.64 | 3.42 | 4.52 |
| Arsenic µg/L | Cadmium µg/L | Lead µg/dL | Mercury µg/L | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal Blood | Cord Blood | Maternal Blood | Cord Blood | Maternal Blood | Cord Blood | Maternal Blood | Cord Blood | |||||||||
| R (p-value) a | ||||||||||||||||
| Mother age (years) | ||||||||||||||||
| 28.54 ± 7.07 * | −0.10 (0.51) | 0.09 (0.54) | −0.05 (0.71) | 0.12 (0.45) | 0.15 (0.29) | 0.29 (0.05) | 0.17 (0.25) | 0.34 (0.02) | ||||||||
| Per capita income (US$) | ||||||||||||||||
| 257 ± 167.38 * | −0.10 (0.54) | 0.06 (0.70) | −0.12 (0.45) | 0.04 (0.78) | −0.21 (0.18) | −0.07 (0.67) | −0.07 (0.64) | 0.18 (0.26) | ||||||||
| Education (years) | ||||||||||||||||
| 14 (5–19) ** | −0.01 (0.93) | 0.01 (0.93) | −0.05 (0.75) | 0.17 (0.27) | −0.14 (0.35) | 0.07 (0.65) | −0.17 (0.23) | −0.06 (0.68) | ||||||||
| GM p-value b (CI 95%) | ||||||||||||||||
| Ethnicity | ||||||||||||||||
| Non-white— 71.4% (35) *** | 9.41 (7.07–11.15) | 0.84 | 10.39 (9.22–11.48) | 0.55 | 0.37 (0.21–0.66) | 0.04 | 0.39 (0.23–0.63) | 0.08 | 3.73 (3.15–4.41) | 0.67 | 3.87 (3.19–4.81) | 0.96 | 0.94 (0.74–1.24) | 0.78 | 0.98 (0.83–1.19) | 0.40 |
| White— 28.6% (14) *** | 9.96 (7.89–12.54) | 9.80 (8.13–11.92) | 0.15 (0.09–0.25) | 0.19 (0.10–0.37) | 3.97 (2.98–5.05) | 3.69 (2.63–5.17) | 0.82 (0.61–1.13) | 0.91 (0.66–1.28) | ||||||||
| Tobacco exposure | ||||||||||||||||
| No—63.3% (31) *** | 10.62 (8.92–12.72) | 0.58 | 10.66 (9.30–12.15) | 0.12 | 0.30 (0.16–0.56) | 0.72 | 0.37 (0.24–0.57) | 0.69 | 4.09 (3.34–9.99) | 0.24 | 3.96 (3.21–4.79) | 0.30 | 0.96 (0.75–1.27) | 0.44 | 0.90 (0.75–1.09) | 0.78 |
| Yes—36.7% (18) *** | 9.59 (8.33–11.14) | 9.31 (8.28–10.47) | 0.27 (0.14–0.50) | 0.37 (0.24–0.57) | 3.50 (2.89–4.24) | 3.61 (2.65–5.15) | 0.84 (0.64–1.12) | 1.03 (0.80–1.36) | ||||||||
| Alcohol consumption | ||||||||||||||||
| No—50.0% (25) *** | 8.65 (5.78–11.95) | 0.16 | 9.98 (8.68–11.36) | 0.50 | 0.24 (0.11–0.51) | 0.79 | 0.32 (0.17–0.59) | 0.88 | 3.67 (2.93–4.63) | 0.66 | 3.62 (2.90–4.57) | 0.39 | 0.84 (0.62–1.26) | 0.40 | 0.89 (0.76–1.06) | 1.00 |
| Yes—50.0% (25) *** | 10.24 (8.78–11.90) | 10.15 (8.90–11.48) | 0.34 (0.20–0.57) | 0.32 (0.19–0.54) | 3.97 (3.37–4.69) | 3.98 (3.06–5.17) | 0.95 (0.77–1.20) | 1.01 (0.78–1.32) | ||||||||
| Arsenic µg/L | Cadmium µg/L | Lead µg/dL | Mercury µg/L | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal Blood | Cord Blood | Maternal Blood | CORD BLOOD | Maternal Blood | Cord Blood | Maternal Blood | Cord Blood | |||||||||
| R (p-value) a | ||||||||||||||||
| Birth weight (kg) | ||||||||||||||||
| 3.3 ± 0.5 * | 0.08 (0.61) | 0.09 (0.54) | 0.01 (0.96) | 0.06 (0.71) | 0.00 (1.00) | 0.04 (0.79) | 0.16 (0.28) | 0.05 (0.75) | ||||||||
| Gestational age (weeks) | ||||||||||||||||
| 39 (36–41) ** | 0.12 (0.43) | 0.03 (0.84) | −0.25 (0.08) | −0.21 (0.16) | −0.19 (0.19) | −0.18 (0.23) | −0.19 (0.19) | −0.24 (0.10) | ||||||||
| GM p-value b,c (95% CI) | ||||||||||||||||
| Gender | ||||||||||||||||
| Male 62.0% (31) *** | 10.39 (9.10–11.75) | 0.33 | 10.26 (9.21–11.35) | 0.39 | 0.27 (0.16–0.46) | 0.75 | 0.32 (0.19–0.54) | 0.91 | 3.51 (2.93–4.24) | 0.16 | 3.59 (2.86–4.65) | 0.36 | 0.94 (0.73- 1.23) | 0.49 | 0.96 (0.77–1.18) | 0.82 |
| Female 38.0% (19) *** | 8.11 (4.85–11.98) | 9.77 (8.12–11.69) | 0.32 (0.14–0.72) | 0.31 (0.17–0.62) | 4.41 (3.56–5.46) | 4.20 (3.30–5.48) | 0.83 (0.63–1.14) | 0.94 (0.76–1.16) | ||||||||
| Apgar at 5 min | ||||||||||||||||
| ≥8 98.0% (49) *** | 9.46 (7.79- 11.19) | 0.14 | 10.07 (9.17–11.02) | - | 0.29 (0.18–0.44) | 0.29 | 0.32 (0.21–0.47) | - | 3.83 (3.33–4.40) | 0.67 | 3.81 (3.21–4.55) | - | 0.90 (0.75–1.12) | 0.26 | 0.95 (0.82–1.12) | - |
| <8 2.0% (1) ***d | - | - | - | - | - | - | - | |||||||||
| Preterm birth (34–37 weeks) | ||||||||||||||||
| No 94.0% (47) *** | 9.28 (7.57–11.01) | 0.30 | 9.91 (9.01–10.84) | 0.11 | 0.28 (0.18–0.44) | 0.40 | 0.32 (0.22–0.49) | 0.91 | 3.76 (3.30–4.38) | 0.05 | 3.70 (3.11–4.44) | 0.16 | 0.90 (0.74–1.11) | 0.71 | 0.94 (0.81–1.10) | 0.83 |
| Yes 6.0% (3) *** | 14.48 (11.24–18.65) | 14.17 (11.40–17.60) | 0.47 (0.15–1.48) | 0.38 (0.12–1.20) | 5.72 (5.64–5.81) | 7.23 (4.00–13.07) | 0.88 (0.38–2.00) | 1.24 (0.55–2.83) | ||||||||
| Birth weight adequacy for gestational age | ||||||||||||||||
| AGA 75.6% (34) *** | 9.37 (6.85–11.70) | 0.88 | 10.12 (9.03–11.23) | 0.73 | 0.31 (0.18–0.53) | 0.27 | 0.36 (0.21–0.60) | 0.31 | 3.67 (3.03–4.50) | 0.92 | 3.71 (2.93–4.79) | 0.52 | 0.93 (0.76–1.16) | 0.11 | 1.00 (0.82–1.23) | 0.27 |
| SGA 11.1% (5) *** | 10.01 (6.78–15.09) | 9.60 (6.45–14.26) | 0.13 (0.03–0.64) | 0.14 (0.05–0.35) | 3.62 (2.52–5.14) | 3.30 (2.61–4.02) | 0.52 (0.38–0.77) | 0.68 (0.52–0.98) | ||||||||
| LGA 13.3% (6) *** | 10.40 (6.53–15.80) | 10.52 (7.53–13.68) | 0.19 (0.05–0.62) | 0.29 (0.066–0.88) | 4.15 (3.31–5.20) | 4.63 (3.17–8.24) | 1.18 (0.55–3.99) | 0.93 (0.59–1.46) | ||||||||
| Mother Characteristics | “Not Fail” | “Fail” | p-Value |
|---|---|---|---|
| Mother age (years) a | 29.53 ± 6.03 | 25.88 ± 7.87 | 0.08 d |
| Per capita income (US$) a | 269.77 ± 174.78 | 241.68 ± 157.96 | 0.62 d |
| Education (years) b | 14 (12–14) | 14 (12–16) | 0.75 e |
| Ethnicity c | |||
| Non-white | 22 | 13 | 0.75 f |
| White | 9 | 4 | |
| Tobacco exposure c | |||
| No | 17 | 13 | 0.14 g |
| Yes | 14 | 4 | |
| Alcohol consumption c | |||
| No | 15 | 9 | 0.69 g |
| Yes | 17 | 8 | |
| Birth characteristics | |||
| Birth weight (kg) a | 3.4 ± 0.6 | 3.2 ± 0.4 | 0.22 d |
| Gestational age (weeks) b | 39.0 (38–40) | 39 (38–39) | 1.12 e |
| Gender c | |||
| Male | 22 | 9 | 0.28 g |
| Female | 10 | 8 | |
| Apgar at 5 min c | |||
| ≥8 | 31 | 17 | 1.00 f |
| <8 c | 1 | 0 | |
| Preterm birth (<37 weeks) c | |||
| No | 31 | 15 | 0.27 f |
| Yes | 1 | 2 | |
| Birth weight adequacy for gestational age c | |||
| AGA | 19 | 14 | 0.52 f |
| SGA | 3 | 2 | |
| LGA | 5 | 1 |
| Arsenic µg/L | Cadmium µg/L | Lead µg/dL | Mercury µg/L | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal Blood | Cord Blood | Maternal Blood | Cord Blood | Maternal Blood | Cord Blood | Maternal Blood | Cord Blood | ||||||||||
| GM p-value a (CI 95%) | |||||||||||||||||
| Not fail 64.6% (31) * | 8.47 (6.26–10.55) | 0.03 | 9.51 (8.58–10.59) | 0.14 | 0.28 (0.16–0.50) | 0.99 | 0.33 (0.22–0.52) | 0.64 | 3.73 (3.18–4.33) | 0.84 | 3.57 (2.95–4.39) | 0.73 | 0.93 (0.72–1.25) | 0.69 | 1.02 (0.84–1.26) | 0.33 | |
| Fail 35.4% (17) * | 11.85 (9.39–15.24) | 11.48 (9.53–13.49) | 0.32 (0.13–0.80) | 0.34 (0.14–0.84) | 4.05 (3.10–5.55) | 4.44 (3.18–6.56) | 0.87 (0.66–1.23) | 0.85 (0.66–1.15) | |||||||||
| Arsenic µg/L | Cadmium µg/L | Lead µg/dL | Mercury µg/L | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal Blood | Cord Blood | Maternal Blood | Cord Blood | Maternal Blood | Cord Blood | Maternal Blood | Cord Blood | |||||||||
| GM p-value a (CI 95%) | ||||||||||||||||
| Personal social | ||||||||||||||||
| Not fail 84.0% (40) * | 9.48 (7.62–11.70) | 0.74 | 10.22 (9.17–11.42) | 0.53 | 0.33 (0.20–0.55) | 0.45 | 0.34 (0.21–0.53) | 0.77 | 4.05 (3.48–4.74) | 0.14 | 3.98 (3.26–4.90) | 0.19 | 0.95 (0.76–1.23) | 0.53 | 1.02 (0.86–1.23) | 0.19 |
| Fail 16.0% (8) * | 9.75 (6.78–13.47) | 9.74 (7.33–12.85) | 0.13 (0.04–0.45) | 0.33 (0.15–0.61) | 2.75 (2.13–3.66) | 3.11 (2.23–4.49) | 0.69 (0.55–0.90) | 0.65 (0.49–0.90) | ||||||||
| Fine motor adaptive | ||||||||||||||||
| Not fail 96.0% (48) * | 9.37 (7.51–11.29) | 0.27 | 9.99 (9.07–11.03) | 0.33 | 0.28 (0.18–0.46) | 0.86 | 0.33 (0.23–0.48) | 0.91 | 3.91 (3.40–4.55) | 0.17 | 3.91 (3.30–4.82) | 0.13 | 0.91 (0.75–1.16) | 0.30 | 0.97 (0.83–1.13) | 0.31 |
| Fail 4.0% (2) * | 11.60 (11.41–11.79) | 11.91 (10.88–13.04) | 0.42 (0.08–2.32) | 0.20 (0.01–3.95) | 2.44 (2.37–2.52) | 2.16 (1.69–2.77) | 0.64 (0.62–0.67) | 0.67 (0.56–0.80) | ||||||||
| Language | ||||||||||||||||
| Not fail 94.0% (47) * | 9.31 (7.58–11.08) | 0.36 | 9.94 (9.11–10.91) | 0.33 | 0.29 (0.17–0.46) | 0.77 | 0.33 (0.21–0.50) | 0.48 | 3.84 (3.30–4.47) | 0.48 | 3.86 (3.28–4.67) | 0.59 | 0.92 (0.75–1.15) | 0.77 | 0.97 (0.83–1.14) | 0.15 |
| Fail 6.0% (3) * | 13.51 (9.79–18.65) | 13.17 (9.86–17.60) | 0.23 (0.15–0.35) | 0.20 (0.12–0.34) | 3.53 (2.21–5.64) | 2.87 (2.07–4.00) | 0.52 (0.39–0.70) | 0.62 (0.55–0.70) | ||||||||
| Gross motor | ||||||||||||||||
| Not fail 80.0% (40) * | 8.69 (6.96–10.51) | 0.07 | 9.56 (8.63–10.51) | 0.07 | 0.26 (0.15–0.41) | 0.77 | 0.29 (0.19–0.45) | 0.41 | 3.59 (3.11–4.11) | 0.31 | 3.48 (2.97–4.16) | 0.23 | 0.88 (0.72–1.14) | 0.80 | 0.93 (0.79–1.09) | 0.85 |
| Fail 20.0% (10) * | 12.75 (10.01–17.84) | 12.06 (9.89–14.96) | 0.41 (0.15–1.18) | 0.43 (0.16–1.15) | 4.77 (3.27–7.28) | 5.21 (3.27–8.81) | 0.95 (0.60–1.55) | 1.04 (0.75–1.49) | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Assis Araujo, M.S.; Froes-Asmus, C.I.R.; de Figueiredo, N.D.; Camara, V.M.; Luiz, R.R.; Prata-Barbosa, A.; Martins, M.M.; Jacob, S.d.C.; Santos, L.M.G.d.; Vicentini Neto, S.A.; et al. Prenatal Exposure to Metals and Neurodevelopment in Infants at Six Months: Rio Birth Cohort Study of Environmental Exposure and Childhood Development (PIPA Project). Int. J. Environ. Res. Public Health 2022, 19, 4295. https://doi.org/10.3390/ijerph19074295
de Assis Araujo MS, Froes-Asmus CIR, de Figueiredo ND, Camara VM, Luiz RR, Prata-Barbosa A, Martins MM, Jacob SdC, Santos LMGd, Vicentini Neto SA, et al. Prenatal Exposure to Metals and Neurodevelopment in Infants at Six Months: Rio Birth Cohort Study of Environmental Exposure and Childhood Development (PIPA Project). International Journal of Environmental Research and Public Health. 2022; 19(7):4295. https://doi.org/10.3390/ijerph19074295
Chicago/Turabian Stylede Assis Araujo, Mônica Seefelder, Carmen Ildes Rodrigues Froes-Asmus, Nataly Damasceno de Figueiredo, Volney Magalhães Camara, Ronir Raggio Luiz, Arnaldo Prata-Barbosa, Marlos Melo Martins, Silvana do Couto Jacob, Lisia Maria Gobbo dos Santos, Santos Alves Vicentini Neto, and et al. 2022. "Prenatal Exposure to Metals and Neurodevelopment in Infants at Six Months: Rio Birth Cohort Study of Environmental Exposure and Childhood Development (PIPA Project)" International Journal of Environmental Research and Public Health 19, no. 7: 4295. https://doi.org/10.3390/ijerph19074295
APA Stylede Assis Araujo, M. S., Froes-Asmus, C. I. R., de Figueiredo, N. D., Camara, V. M., Luiz, R. R., Prata-Barbosa, A., Martins, M. M., Jacob, S. d. C., Santos, L. M. G. d., Vicentini Neto, S. A., Rezende Filho, J. F. d., & Amim Junior, J. (2022). Prenatal Exposure to Metals and Neurodevelopment in Infants at Six Months: Rio Birth Cohort Study of Environmental Exposure and Childhood Development (PIPA Project). International Journal of Environmental Research and Public Health, 19(7), 4295. https://doi.org/10.3390/ijerph19074295






