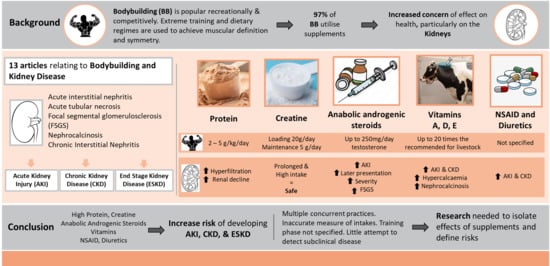
Nutritional and Non-Nutritional Strategies in Bodybuilding: Impact on Kidney Function
Abstract
1. Introduction
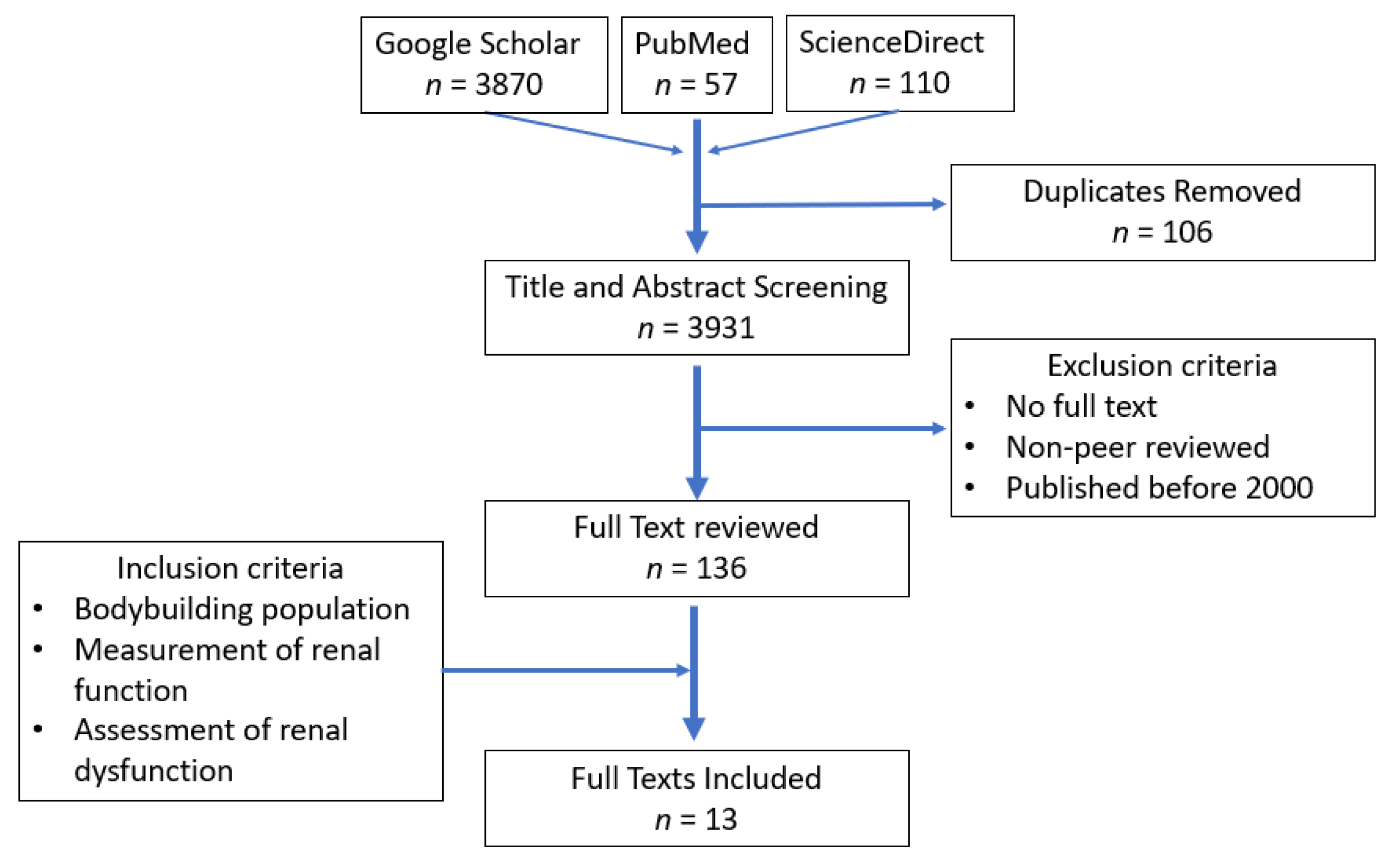
2. Materials and Methods
3. Results
4. Discussion
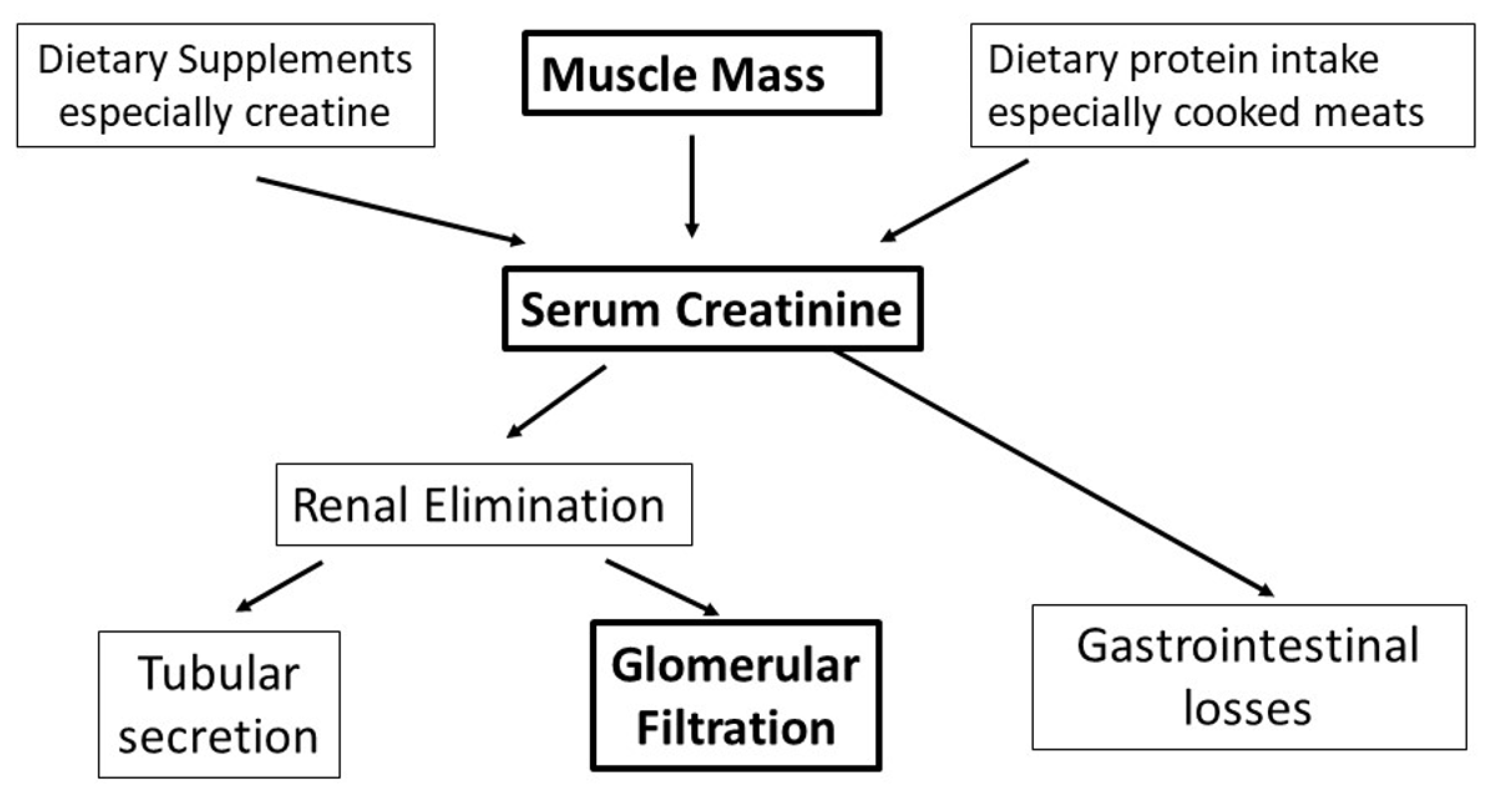
4.1. Measurement of Kidney Function
4.2. Protein Supplementation
4.3. Creatine Supplementation
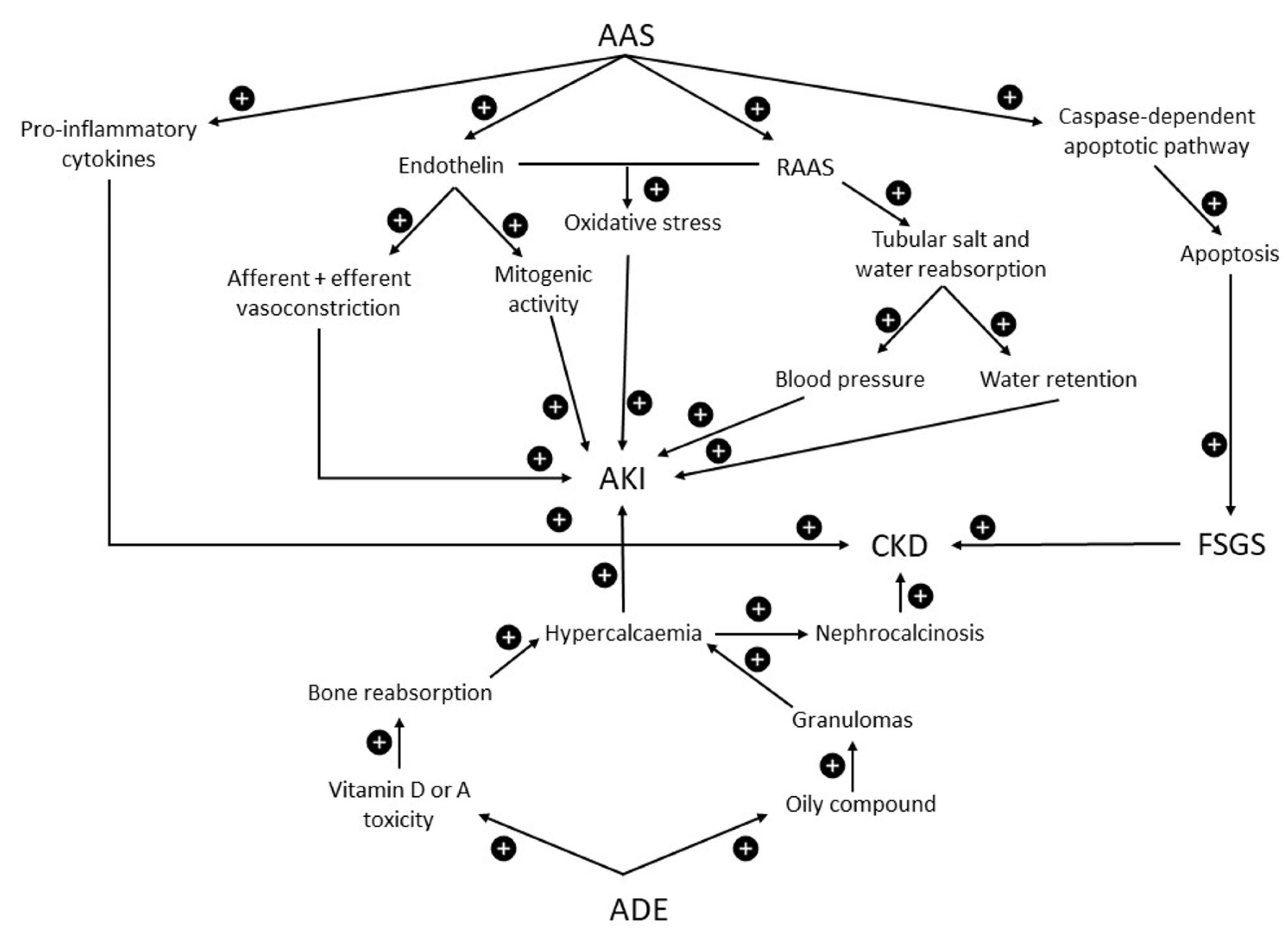
4.4. Anabolic Androgenic Steroids
4.5. Vitamins
4.6. Non-Steroidal Anti-Inflammatory Drugs (NSAID)
4.7. Dehydration and Diuretics
4.8. Other Supplements/Stimulants and Exertional Rhabdomyolysis
4.9. Practical Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lenzi, J.L.; Teixeira, E.L.; de Jesus, G.; Schoenfeld, B.J.; de Salles Painelli, V. Dietary Strategies of Modern Bodybuilders During Different Phases of the Competitive Cycle. J. Strength Cond. Res. 2021, 35, 2546–2551. [Google Scholar] [CrossRef] [PubMed]
- Iraki, J.; Fitschen, P.; Espinar, S.; Helms, E. Nutrition recommendations for bodybuilders in the off-season: A narrative review. Sports 2019, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Edholm, P.; Strandberg, E.; Kadi, F. Lower limb explosive strength capacity in elderly women: Effects of resistance training and healthy diet. J. Appl. Physiol. 2017, 123, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Villareal, D.T.; Aguirre, L.; Gurney, A.B.; Waters, D.L.; Sinacore, D.R.; Colombo, E.; Armamento-Villareal, R.; Qualls, C. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N. Engl. J. Med. 2017, 376, 1943–1955. [Google Scholar] [CrossRef]
- Spendlove, J.; Mitchell, L.; Gifford, J.; Hackett, D.; Slater, G.; Cobley, S.; O’Connor, H. Dietary Intake of Competitive Bodybuilders. Sports Med. 2015, 45, 1041–1063. [Google Scholar] [CrossRef]
- Hackett, D.A. Training, Supplementation, and Pharmacological Practices of Competitive Male Bodybuilders Across Training Phases. J. Strength Cond. Res. 2021, 36, 936–970. [Google Scholar] [CrossRef]
- Ali, A.A.; Almukhtar, S.E.; Sharif, D.A.; Saleem ZS, M.; Muhealdeen, D.N.; Hughson, M.D. Effects of body-building supplements on the kidney: A population-based incidence study of biopsy pathology and clinical characteristics among middle eastern men. BMC Nephrol. 2020, 21, 1–10. [Google Scholar] [CrossRef]
- Gawad, M.A.; Kalawy, H.A. Gym nephropathy ‘bodybuilding versus kidney damaging’. J. Egypt. Soc. Nephrol. Transplant. 2019, 19, 124–128. [Google Scholar] [CrossRef]
- Bhasin, S.; Hatfield, D.L.; Hoffman, J.R.; Kraemer, W.J.; Labotz, M.; Phillips, S.M.; Ratamess, N.A. Anabolic-Androgenic Steroid Use in Sports, Health, and Society. Med. Sci. Sports Exerc. 2021, 53, 1778–1794. [Google Scholar] [CrossRef]
- Ostovar, A.; Haerinejad, M.; Farzaneh, M.R.; Keshavarz, M. Adverse effects of performance-enhancing drugs on the kidney in the male bodybuilders. Sci. Sports 2017, 32, 91–98. [Google Scholar] [CrossRef]
- Filho, S.L.A.P.; Gomes, P.E.A.D.C.; Forte, G.A.; Lima, L.L.L.; Júnior, G.B.D.S.; Meneses, G.; Martins, A.M.C.; Daher, E.D.F. Kidney disease associated with androgenic—Anabolic steroids and vitamin supplements abuse: Be aware! Nefrología 2020, 40, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic re-views and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Andres, S.; Ziegenhagen, R.; Trefflich, I.; Pevny, S.; Schultrich, K.; Braun, H.; Schänzer, W.; Hirsch-Ernst, K.I.; Schäfer, B.; Lampen, A. Creatine and creatine forms intended for sports nutrition. Mol. Nutr. Food Res. 2017, 61, 1600772. [Google Scholar] [CrossRef] [PubMed]
- Kashani, K.; Rosner, M.H.; Ostermann, M. Creatinine: From physiology to clinical application. Eur. J. Intern. Med. 2020, 72, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Delanaye, P.; Cavalier, E.; Pottel, H. Serum Creatinine: Not So Simple! Nephron 2017, 136, 302–308. [Google Scholar] [CrossRef]
- Levey, A.S.; AInker, L. Assessment of Glomerular Filtration Rate in Health and Disease: A State of the Art Review. Clin. Pharmacol. Ther. 2017, 102, 405–419. [Google Scholar] [CrossRef]
- Ferguson, T.W.; Komenda, P.; Tangri, N. Cystatin C as a biomarker for estimating glomerular filtration rate. Curr. Opin. Nephrol. Hypertens. 2015, 24, 295–300. [Google Scholar] [CrossRef]
- Daher, E.D.F.; Fernandes, P.H.P.D.; Meneses, G.C.; Bezerra, G.F.; Ferreira, L.D.S.L.; Viana, G.D.A.; Martins, A.M.C.; Silva, G.B.D., Jr. Novel Kidney Injury Biomarkers Among Anabolic Androgenic Steroids Users—Evidence of Subclinical Kidney Disease. Asian J. Sports Med. 2018, 9, 1–4. [Google Scholar] [CrossRef]
- Levey, A.S.; Becker, C.; Inker, L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: A systematic review. JAMA 2015, 313, 837–846. [Google Scholar] [CrossRef]
- Zhang, W.R.; Parikh, C.R. Biomarkers of Acute and Chronic Kidney Disease. Annu. Rev. Physiol. 2019, 81, 309–333. [Google Scholar] [CrossRef]
- Bennett, E.; Peters, S.A.E.; Woodward, M. Sex differences in macronutrient intake and adherence to dietary recommendations: Findings from the UK Biobank. BMJ Open 2018, 8, e020017. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Vitale, K.; Getzin, A. Nutrition and Supplement Update for the Endurance Athlete: Review and Recommendations. Nutrients 2019, 11, 1289. [Google Scholar] [CrossRef] [PubMed]
- Akl, A.; Aldabbagh, Y. Does gymnasium harbor an endemic cause of end stage kidney disease? a case report & review of literature. Urol. Nephrol. Open Access J. 2019, 7, 55–57. [Google Scholar] [CrossRef]
- Almukhtar, S.E.; Abbas, A.A.; Muhealdeen, D.N.; Hughson, M.D. Acute kidney injury associated with androgenic steroids and nutritional supplements in bodybuilders. Clin. Kidney J. 2015, 8, 415–419. [Google Scholar] [CrossRef] [PubMed]
- El-Reshaid, W.; El-Reshaid, K.; Al-Bader, S.; Ramadan, A.; Madda, J. Complementary bodybuilding: A potential risk for permanent kidney disease. Saudi J. Kidney Dis. Transplant. 2018, 29, 326–331. [Google Scholar] [CrossRef]
- Hartung, R.; Gerth, J.; Fünfstück, R.; Gröne, H.J.; Stein, G. End-stage renal disease in a bodybuilder: A multifactorial process or simply doping? Nephrol. Dial. Transplant. 2001, 16, 163–165. [Google Scholar] [CrossRef]
- Herlitz, L.C.; Markowitz, G.S.; Farris, A.B.; Schwimmer, J.A.; Stokes, M.B.; Kunis, C.; Colvin, R.B.; D’Agati, V.D. Development of Focal Segmental Glomerulosclerosis after Anabolic Steroid Abuse. J. Am. Soc. Nephrol. 2010, 21, 163–172. [Google Scholar] [CrossRef]
- Antonio, J.; Ellerbroek, A. Case reports on well-trained bodybuilders: Two years on a high protein diet. J. Exerc. Physiol. Online 2018, 21, 14–24. [Google Scholar]
- Antonio, J.; Ellerbroek, A.; Silver, T.; Vargas, L.; Tamayo, A.; Buehn, R.; Peacock, C.A. A High Protein Diet Has No Harmful Effects: A One-Year Crossover Study in Resistance-Trained Males. J. Nutr. Metab. 2016, 2016, 9104792. [Google Scholar] [CrossRef]
- Knight, E.L.; Stampfer, M.J.; Hankinson, S.E.; Spiegelman, D.; Curhan, G.C. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann. Intern. Med. 2003, 138, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.J.; Obi, Y.; Tortorici, A.R.; Kalantar-Zadeh, K. Dietary protein intake and chronic kidney disease. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Frank, H.; Graf, J.; Amann-Gassner, U.; Bratke, R.; Daniel, H.; Heemann, U.; Hauner, H. Effect of short-term high-protein compared with normal-protein diets on renal hemodynamics and associated variables in healthy young men. Am. J. Clin. Nutr. 2009, 90, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Jhee, J.H.; Kee, Y.K.; Park, S.; Kim, H.; Park, J.T.; Han, S.H.; Kang, S.-W.; Yoo, T.-H. High-protein diet with renal hyperfiltration is associated with rapid decline rate of renal function: A community-based prospective cohort study. Nephrol. Dial. Transplant. 2019, 35, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Fouque, D.; Aparicio, M. Eleven reasons to control the protein intake of patients with chronic kidney disease. Nat. Clin. Pract. Nephrol. 2007, 3, 383–392. [Google Scholar] [CrossRef]
- Escalante, G.; Stevenson, S.W.; Barakat, C.; Aragon, A.A.; Schoenfeld, B.J. Peak week recommendations for bodybuilders: An evidence based approach. BMC Sports Sci. Med. Rehabil. 2021, 13, 68. [Google Scholar] [CrossRef]
- Gentil, P.; de Lira, C.A.B.; Paoli, A.; Dos Santos, J.A.B.; da Silva, R.D.T.; Junior, J.R.P.; Magosso, R.F. Nutrition, pharmacological and training strategies adopted by six bodybuilders: Case report and critical review. Eur. J. Transl. Myol. 2017, 27, 51–66. [Google Scholar] [CrossRef]
- Mäestu, J.; Eliakim, A.; Jürimäe, J.; Valter, I.; Jürimäe, T. Anabolic and Catabolic Hormones and Energy Balance of the Male Bodybuilders During the Preparation for the Competition. J. Strength Cond. Res. 2010, 24, 1074–1081. [Google Scholar] [CrossRef]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 1–18. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Karimzadeh, I.; Ezzatzadegan-Jahromi, S.; Sagheb, M.M. Potential Adverse Effects of Creatine Supplement on the Kidney in Athletes and Bodybuilders. Iran. J. Kidney Dis. 2018, 12, 253–260. [Google Scholar]
- Thorsteinsdottir, B.; Grande, J.P.; Garovic, V.D. Acute Renal Failure in a Young Weight Lifter Taking Multiple Food Supplements, Including Creatine Monohydrate. J. Ren. Nutr. 2006, 16, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Taner, B.; Aysim, O.; Abdulkadir, U. The effects of the recommended dose of creatine monohydrate on kidney function. NDT Plus 2010, 4, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Ardalan, M.; Samadifar, Z.; Vahedi, A. Creatine monohydrate supplement induced interstitial nephritis. J. Nephropathol. 2012, 1, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Schilling, B.K.; Stone, M.H.; Utter, A.; Kearney, J.T.; Johnson, M.; Coglianese, R.; Stone, M.E. Creatine supple-mentation and health variables: A retrospective study. Med. Sci. Sports Exerc. 2001, 33, 183–188. [Google Scholar] [CrossRef]
- Lugaresi, R.; Leme, M.; Painelli, V.D.S.; Murai, I.H.; Roschel, H.; Sapienza, M.T.; Gualano, B. Does long-term creatine supplementation impair kidney function in resistance-trained individuals consuming a high-protein diet? J. Int. Soc. Sports Nutr. 2013, 10, 26. [Google Scholar] [CrossRef]
- Sale, C.; Harris, R.C.; Florance, J.; Kumps, A.; Sanvura, R.; Poortmans, J.R. Urinary creatine and methylamine excretion following 4 × 5 g day−1 or 20 × 1 g day−1 of creatine monohydrate for 5 days. J. Sports Sci. 2009, 27, 759–766. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and Creatinine Metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef]
- Poortmans, J.R.; Kumps, A.; Duez, P.; Fofonka, A.; Carpentier, A.; Francaux, M. Effect of Oral Creatine Supplementation on Urinary Methylamine, Formaldehyde, and Formate. Med. Sci. Sports Exerc. 2005, 37, 1717–1720. [Google Scholar] [CrossRef]
- Mitchell, S.C.; Zhang, A.Q. Methylamine in human urine. Clin. Chim. Acta 2001, 312, 107–114. [Google Scholar] [CrossRef]
- Candow, D.G.; Little, J.P.; Chilibeck, P.D.; Abeysekara, S.; Zello, G.A.; Kazachkov, M.; Cornish, S.M.; Yu, P.H. Low-Dose Creatine Combined with Protein during Resistance Training in Older Men. Med. Sci. Sports Exerc. 2008, 40, 1645–1652. [Google Scholar] [CrossRef]
- Post, A.; Tsikas, D.; Bakker, S.J. Creatine is a Conditionally Essential Nutrient in Chronic Kidney Disease: A Hypothesis and Narrative Literature Review. Nutrients 2019, 11, 1044. [Google Scholar] [CrossRef] [PubMed]
- Pope, H.G.; Wood, R.I.; Rogol, A.; Nyberg, F.; Bowers, L.; Bhasin, S. Adverse Health Consequences of Performance-Enhancing Drugs: An Endocrine Society Scientific Statement. Endocr. Rev. 2014, 35, 341–375. [Google Scholar] [CrossRef] [PubMed]
- Sagoe, D.; Molde, H.; Andreassen, C.S.; Torsheim, T.; Pallesen, S. The global epidemiology of anabolic-androgenic steroid use: A meta-analysis and meta-regression analysis. Ann. Epidemiol. 2014, 24, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Daher, E.F.; Júnior, G.B.S.; Queiroz, A.L.; Ramos, L.M.A.; Santos, S.Q.; Barreto, D.M.S.; Guimarães, A.A.C.; Barbosa, C.A.; Franco, L.M.; Patrocínio, R.M.S.V. Acute kidney injury due to anabolic steroid and vitamin supplement abuse: Report of two cases and a literature review. Int. Urol. Nephrol. 2009, 41, 717–723. [Google Scholar] [CrossRef]
- Libório, A.B.; Nasserala, J.C.; Gondim, A.S.; Daher, E. The Case|Renal failure in a bodybuilder athlete. Kidney Int. 2014, 85, 1247–1248. [Google Scholar] [CrossRef][Green Version]
- Rocha, P.N.; Santos, C.S.; Avila, M.O.; Neves, C.L.; Bahiense-Oliveira, M. Hypercalcemia and acute kidney in-jury caused by abuse of a parenteral veterinary compound containing vitamins A, D, and E. Braz. J. Nephrol. 2011, 33, 467–471. [Google Scholar] [CrossRef]
- Dousdampanis, P.; Trigka, K.; Fourtounas, C.; Bargman, J.M. Role of Testosterone in the Pathogenesis, Progression, Prognosis and Comorbidity of Men With Chronic Kidney Disease. Ther. Apher. Dial. 2013, 18, 220–230. [Google Scholar] [CrossRef]
- Quan, A.; Chakravarty, S.; Chen, J.-K.; Loleh, S.; Saini, N.; Harris, R.C.; Capdevila, J.; Quigley, R. Androgens augment proximal tubule transport. Am. J. Physiol. Physiol. 2004, 287, F452–F459. [Google Scholar] [CrossRef]
- Kalk, P.; Thöne-Reineke, C.; Schwarz, A.; Godes, M.; Bauer, C.; Pfab, T.; Hocher, B. Renal phenotype of Et-1 transgenic mice is modulated by androgens. Eur. J. Med. Res. 2009, 14, 55–58. [Google Scholar] [CrossRef]
- Verzola, D.; Villaggio, B.; Procopio, V.; Gandolfo, M.T.; Gianiorio, F.; Famà, A.; Tosetti, F.; Traverso, P.; Deferrari, G.; Garibotto, G. Androgen-mediated apoptosis of kidney tubule cells: Role of c-Jun amino terminal kinase. Biochem. Biophys. Res. Commun. 2009, 387, 531–536. [Google Scholar] [CrossRef]
- Doublier, S.; Lupia, E.; Catanuto, P.; Periera-Simon, S.; Xia, X.; Korach, K.; Berho, M.; Elliot, S.J.; Karl, M. Testosterone and 17 b-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int. 2011, 79, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, P.D.; Leslie, J.A.; Campbell, M.T.; Meldrum, D.R.; Hile, K.L.; Meldrum, K.K. Testosterone exacerbates obstructive renal injury by stimulating TNF-α production and increasing proapoptotic and profibrotic signaling. Am. J. Physiol. Metab. 2008, 294, E435–E443. [Google Scholar] [CrossRef] [PubMed]
- Godswill, A.G.; Somtochukwu, I.V.; O Ikechukwu, A.; Kate, E.C. Health Benefits of Micronutrients (Vitamins and Minerals) and their Associated Deficiency Diseases: A Systematic Review. Int. J. Food Sci. 2020, 3, 1–32. [Google Scholar] [CrossRef]
- Daher, E.D.F.; Martiniano, L.V.M.; Lima, L.L.L.; Filho, N.C.V.L.; Souza, L.E.D.O.; Fernandes, P.H.P.D.; da Silva, G.B., Jr. Acute kidney injury due to excessive and prolonged intramuscular injection of veterinary supplements containing vitamins A, D and E: A series of 16 cases. Nefrología 2017, 37, 61–67. [Google Scholar] [CrossRef]
- Ronsoni, M.F.; Santos, H.D.C.D.; Colombo, B.D.S.; Corrêa, C.G.; Moritz, A.P.G.; Coral, M.H.C.; Van De Sande-Lee, S.; Hohl, A. Hypercalcemia and acute renal insufficiency following use of a veterinary supplement. J. Bras. De Nefrol. 2017, 39, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Vitamin D in Health and Disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Vitamin D: Criteria for safety and efficacy. Nutr. Rev. 2008, 66, S178–S181. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, K.; Ennis, D.M.; Ennis, E.D. Hypercalcemia caused by iatrogenic hypervitaminosis A. J. Am. Diet. Assoc. 2005, 105, 119–121. [Google Scholar] [CrossRef]
- Levi, M.; Ellis, M.A.; Berl, T. Control of renal hemodynamics and glomerular filtration rate in chronic hypercalcemia: Role of prostaglandins, renin-angiotensin system, and calcium. J. Clin. Investig. 1983, 71, 1624–1632. [Google Scholar] [CrossRef]
- Tachamo, N.; Donato, A.; Timilsina, B.; Nazir, S.; Lohani, S.; Dhital, R.; Basnet, S. Hypercalcemia associated with cosmetic injections: A systematic review. Eur. J. Endocrinol. 2018, 178, 425–430. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.A.; Lilja, M.; Mandić, M.; Gustafsson, T.; Larsen, F.J.; Lundberg, T.R. Resistance Training with Co-ingestion of Anti-inflammatory Drugs Attenuates Mitochondrial Function. Front. Physiol. 2017, 8, 1074. [Google Scholar] [CrossRef] [PubMed]
- Omeragic, E.; Marjanovic, A.; Djedjibegovic, J.; Turalic, A.; Dedić, M.; Niksic, H.; Lugusic, A.; Sober, M. Prevalence of use of permitted pharmacological substances for recovery among athletes. Pharmacia 2021, 68, 35–42. [Google Scholar] [CrossRef]
- Gorski, T.; Cadore, E.L.; Pinto, S.S.; da Silva, E.M.; Correa, C.S.; Beltrami, F.G.; Kruel, L.F.M. Use of NSAIDs in triathletes: Prevalence, level of awareness and reasons for use. Br. J. Sports Med. 2011, 45, 85–90. [Google Scholar] [CrossRef]
- Steckling, F.M.; Lima, F.D.; Farinha, J.B.; Rosa, P.C.; Royes, L.F.F.; Cuevas, M.J.; Bresciani, G.; Soares, F.A.; González-Gallego, J.; Barcelos, R.P. Diclofenac attenuates inflammation through TLR4 pathway and improves exercise performance after exhaustive swimming. Scand. J. Med. Sci. Sports 2019, 30, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, T.R.; Howatson, G. Analgesic and anti-inflammatory drugs in sports: Implications for exercise performance and training adaptations. Scand. J. Med. Sci. Sports 2018, 28, 2252–2262. [Google Scholar] [CrossRef]
- Lilja, M.; Mandić, M.; Apró, W.; Melin, M.; Olsson, K.; Rosenborg, S.; Gustafsson, T.; Lundberg, T.R. High doses of anti-inflammatory drugs compromise muscle strength and hypertrophic adaptations to resistance training in young adults. Acta Physiol. 2018, 222, e12948. [Google Scholar] [CrossRef]
- Nderitu, P.; Doos, L.; Jones, P.W.; Davies, S.J.; Kadam, U.T. Non-steroidal anti-inflammatory drugs and chronic kidney disease progression: A systematic review. Fam. Pract. 2013, 30, 247–255. [Google Scholar] [CrossRef]
- Chappell, A.J.; Simper, T.N. Nutritional Peak Week and Competition Day Strategies of Competitive Natural Bodybuilders. Sports 2018, 6, 126. [Google Scholar] [CrossRef]
- Mitchell, L.; Hackett, D.; Gifford, J.; Estermann, F.; O’Connor, H. Do Bodybuilders Use Evidence-Based Nutrition Strategies to Manipulate Physique? Sports 2017, 5, 76. [Google Scholar] [CrossRef]
- Mayr, F.B.; Domanovits, H.; Laggner, A.N. Hypokalemic paralysis in a professional bodybuilder. Am. J. Emerg. Med. 2012, 30, 1324–1325. [Google Scholar] [CrossRef] [PubMed]
- Probert, A.; Palmer, F.; Leberman, S. The Fine Line: An insight into ‘risky’ practices of male and female competitive bodybuilders. Ann. Leis. Res. 2007, 10, 272–290. [Google Scholar] [CrossRef]
- Kasper, A.M.; Crighton, B.; Langan-Evans, C.; Riley, P.; Sharma, A.; Close, G.L.; Morton, J.P. Case Study: Extreme Weight Making Causes Relative Energy Deficiency, Dehydration, and Acute Kidney Injury in a Male Mixed Martial Arts Athlete. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, W.; Ren, H.; Chen, X.; Xie, J.; Chen, N. Diuretics associated acute kidney injury: Clinical and pathological analysis. Ren. Fail. 2014, 36, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alvarez, A.; Egan, B.; De Klein, S.; Dima, L.; Maggi, F.M.; Isoniemi, M.; Ribas-Barba, L.; Raats, M.; Meissner, E.M.; Badea, M.; et al. Usage of Plant Food Supplements across Six European Countries: Findings from the PlantLIBRA Consumer Survey. PLoS ONE 2014, 9, e92265. [Google Scholar] [CrossRef] [PubMed]
- García-Cortés, M.; Robles-Díaz, M.; Ortega-Alonso, A.; Medina-Caliz, I.; Andrade, R.J. Hepatotoxicity by Dietary Supplements: A Tabular Listing and Clinical Characteristics. Int. J. Mol. Sci. 2016, 17, 537. [Google Scholar] [CrossRef]
- Helms, E.R.; Aragon, A.A.; Fitschen, P.J. Evidence-based recommendations for natural bodybuilding contest preparation: Nutrition and supplementation. J. Int. Soc. Sport. Nutr. 2014, 11, 20. [Google Scholar] [CrossRef]
- Hackett, D.A.; Johnson, N.A.; Chow, C.-M. Training Practices and Ergogenic Aids Used by Male Bodybuilders. J. Strength Cond. Res. 2013, 27, 1609–1617. [Google Scholar] [CrossRef]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of caffeine on human health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef]
- Chappell, A.J.; Simper, T.; Barker, M.E. Nutritional strategies of high level natural bodybuilders during competition preparation. J. Int. Soc. Sports Nutr. 2018, 15, 1–12. [Google Scholar] [CrossRef]
- Dehoney, S.; Wellein, M. Rhabdomyolysis associated with the nutritional supplement Hydroxycut. Am. J. Health Pharm. 2009, 66, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Carol, M.L. Hydroxycut Weight Loss Dietary Supplements: A Contributing Factor in the Development of Exertional Rhabdomyolysis in Three U.S. Army Soldiers. Mil. Med. 2013, 178, e1039–e1042. [Google Scholar] [CrossRef] [PubMed]
- Campana, C.; Griffin, P.L.; Simon, E.L. Caffeine overdose resulting in severe rhabdomyolysis and acute renal failure. Am. J. Emerg. Med. 2014, 32, 111.e3–111.e4. [Google Scholar] [CrossRef] [PubMed]
- Golcuk, Y.; Ozsarac, M.; Golcuk, B.; Gunay, E. Caffeine-induced rhabdomyolysis. Am. J. Emerg. Med. 2014, 32, 100. [Google Scholar] [CrossRef]
- Chiang, W.-F.; Liao, M.-T.; Cheng, C.-J.; Lin, S.-H. Rhabdomyolysis induced by excessive coffee drinking. Hum. Exp. Toxicol. 2013, 33, 878–881. [Google Scholar] [CrossRef]
- Dong Jun, S.U.N.G.; Eun-Ju, C.H.O.I.; Sojung, K.I.M.; Jooyoung, K.I.M. Rhabdomyolysis from resistance exercise and caffeine intake. Iran. J. Public Health 2018, 47, 138. [Google Scholar]
- Gagliano, M.; Corona, D.; Giuffrida, G.; Giaquinta, A.; Tallarita, T.; Zerbo, D.; Sorbello, M.; Paratore, A.; Virgilio, C.; Cappellani, A.; et al. Low-intensity body building exercise induced rhabdomyolysis: A case report. Cases J. 2009, 2, 1–2. [Google Scholar] [CrossRef]
- Patel, D.R.; Gyamfi, R.; Torres, A. Exertional Rhabdomyolysis and Acute Kidney Injury. Physician Sportsmed. 2009, 37, 71–79. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Kim, S.; Ryu, H.Y.; Cha, K.S.; Sung, D.J. Exercise-induced rhabdomyolysis mechanisms and prevention: A literature review. J. Sport Health Sci. 2016, 5, 324–333. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Hubal, M.J. Exercise-Induced Muscle Damage in Humans. Am. J. Phys. Med. Rehabil. 2002, 81, S52–S69. [Google Scholar] [CrossRef]
- Grimmer, N.M.; Gimbar, R.P.; Bursua, A.; Patel, M. Rhabdomyolysis secondary to clenbuterol use and exercise. J. Emerg. Med. 2016, 50, e71–e74. [Google Scholar] [CrossRef] [PubMed]
- Bosch, X.; Poch, E.; Grau, J.M. Rhabdomyolysis and acute kidney injury. N. Engl. J. Med. 2009, 361, 62–72. [Google Scholar] [CrossRef] [PubMed]
| Study | Sex Age | Kidney Function at Presentation | Diagnosis | Protein | Creatine | Anabolic Steroids | Vitamins | Other |
|---|---|---|---|---|---|---|---|---|
| (Thorsteinsdottir, 2006) | Male 24 | Screat 3.8 mg/dL | AIN (biopsy) | NS, Amino acid supplements | 5 g × 3/week for 6 months | Denied | Yes | Diuretics-no NSAID-no |
| (Taner, 2011) | Male 18 | Screat 202 umol/L Uprotein 0.28g/day | ATN (biopsy) | NS | L 20 g/day 5 days M 1 g/day 6 weeks | NS | NS | Diuretics-NS NSAID-NS |
| (Ardalan, 2012) | Male 32 | Screat 4.3 mg/dL, Uprotein 0.85 g/day | AIN (biopsy) | NS | L 20 g/day 3 days M 1 g/day 3 weeks | NS | NS | Diuretics-NS NSAID-NS |
| (Almukhtar, 2015) | 4 males, 20–26 | Screat 230–336 µmol/L | All ATN (biopsy). 3 mild−moderate interstitial fibrosis | 3.2–4.2 g/kg/day | L 15 g/day M 5 g plus 5 g pre- & 5 g post workout | Testosterone +/or nandrolone IM > 400 mg weekly | NS | Diuretics-NS NSAID-NS |
| (Hartung, 2001) | Male 27 | Screat 1030 µmol/L, Uprotein 4532 mg/L | Nephrosclerosis, global glomerular sclerosis, CIN (biopsy) | 2 g/kg/day | Creatine-210 g/day | Testosterone-750–1000 mg 6 weekly for 18 months | NS | Diuretics-no NSAID-no |
| (Herlitz., 2010) | 10 males 28–49 | Proteinuria- mean 10.1 g/d (1.3–26.3) Screat 3.0 mg/dL, (1.4–7.8 mg/dL) | FSGS (9) Glomerulomegaly (1) (All biopsy) | 2.8–5.1 g/kg/day | Yes-dose NS | Various combinations of AAS | NS | Diuretics-NS NSAID-NS |
| (El-reshaid, 2018) | 22 males 29 ± 7 | Impaired kidney function or proteinuria and/or haematuria-NS | FSGS (8) Nephrosclerosis (4) AIN (2), CIN (3), Nephrocalcinosis (2), Membranous GN (1), Crescentic GN (1), Sclerosing GN (1) (All biopsy) | high-protein diet (20–30 g/kg/day) | NS | Testosterone 250 mg/day, Growth hormone (up to 100 mg/day) | NS | Diuretics-NS NSAID-NS |
| (Akl, 2019) | Male 26 | Screat 12 mg/dL | FSGS (biopsy) | Yes-NS | Yes-NS | Yes-NS | NS | Diuretics-NS NSAID-NS |
| (Ali, 2020) | 15 males 19–49 | Screat 1.3–8.6 mg/dL | ATN (7), FSGS (2), AIN (1) Membranous GM (2) Nephrocalcinosis (2) Postinfectious GN (1) (All biopsy) | Yes-14/15 NS | Yes-13/15 NS | Yes-12/15 NS | Yes (13) Oral Vit D (11) Injected Vit D (2) | Diuretics-NS NSAID-NS |
| (Rocha, 2011) | Male 19 | Scalcium 13.6 mg/dL Screat 2.64 mg/dL | Nephrocalcinosis | NS | NS | Denied | High dose Vit A, Vit D3, Vit E-over previous year | Diuretics-no NSAID-no |
| (Ronsoni 2017) | Male 24 | Scalcium 13.6 mg/dL Screat 3.1 mg/dL | Nephrocalcinosis (Biopsy not done) | NS | NS | Growth hormone, nandrolone and other testosterone derivatives | High dose Vit A, Vit D3, Vit E-over previous year | Diuretics-NS NSAID-NS |
| (Libório, 2014) | Male 22 | Scalcium 13.8 mg/dL Screat 8.6 mg/dL | Nephrocalcinosis, CIN (biopsy) | NS | NS | NS | High dose Vit D3 over previous 2 years | Diuretics-NS NSAID-NS |
| (Daher, 2017) | 16 males 28 ± 9 | Serum calcium 12 ± 2.2 mg/dL, Serum creatinine 3.9 ± 5.2 mg/dL | AKI (13) Nephrocacinosis (3) Nephrolithiasis (7) (biopsies not done) (Diagnoses not mutually exclusive) | NS | NS | Positive history of AAS (6). NS | High dose Vit A, Vit D3, Vit E-NS | Diuretics-NS NSAID-NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tidmas, V.; Brazier, J.; Hawkins, J.; Forbes, S.C.; Bottoms, L.; Farrington, K. Nutritional and Non-Nutritional Strategies in Bodybuilding: Impact on Kidney Function. Int. J. Environ. Res. Public Health 2022, 19, 4288. https://doi.org/10.3390/ijerph19074288
Tidmas V, Brazier J, Hawkins J, Forbes SC, Bottoms L, Farrington K. Nutritional and Non-Nutritional Strategies in Bodybuilding: Impact on Kidney Function. International Journal of Environmental Research and Public Health. 2022; 19(7):4288. https://doi.org/10.3390/ijerph19074288
Chicago/Turabian StyleTidmas, Victoria, Jon Brazier, Janine Hawkins, Scott C. Forbes, Lindsay Bottoms, and Ken Farrington. 2022. "Nutritional and Non-Nutritional Strategies in Bodybuilding: Impact on Kidney Function" International Journal of Environmental Research and Public Health 19, no. 7: 4288. https://doi.org/10.3390/ijerph19074288
APA StyleTidmas, V., Brazier, J., Hawkins, J., Forbes, S. C., Bottoms, L., & Farrington, K. (2022). Nutritional and Non-Nutritional Strategies in Bodybuilding: Impact on Kidney Function. International Journal of Environmental Research and Public Health, 19(7), 4288. https://doi.org/10.3390/ijerph19074288