Effects of Buprenorphine Dose and Therapeutic Engagement on Illicit Opiate Use in Opioid Use Disorder Treatment Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Data, Informed Consent and Ethical Approval
Trial Design and Ascertainment Criteria
2.2. Statistical Analyses
2.2.1. Predictors and Outcome
2.2.2. Trial Variable Harmonization
2.2.3. Treatment Variables
2.2.4. Patient Covariates
2.2.5. Generalized Linear Mixed Model
2.2.6. Assessment of Results
3. Results
3.1. Participant Characteristics
3.1.1. Sociodemographics
3.1.2. Drug Use History
3.2. Treatment Variables and Short-Term Lapse
3.2.1. Dose Records and Urinalysis or Self-Report Records
3.2.2. Treatment and Outcome Characteristics
3.3. Results from the Generalized Linear Mixed Model Analysis
Participant Factors
4. Discussion
4.1. Treatment Factor Findings
4.2. Limitations
4.3. Treatment Implications
- The range of mean time weighted dose in clinical trial participants analyzed covers the range of recommended daily doses [14] and the overall sample mean time weighted dose of 11.94 mg (that incorporates non-adherence) is just under the mean daily buprenorphine dose of 13.4 mg reported in 2015–2018 by N = 1105 physicians treating OUD patients characterized as stable patients [41]. This suggests that, despite some differences in patient characteristics, the clinical trial participant finding that increased buprenorphine dose decreased short-term lapse may apply to current office-based patient practice.
- Time-in-trial may represent effects of both patient characteristics and the influence of successful treatment over time [42]. Future analyses to understand time-in-trial associations with short term lapse at different times during treatment may help distinguish patient and treatment related factors influencing time-in-trial association with response to MOUD. Until then, efforts to support retention should result in better response to MOUD.
- In the same study reporting on office-based treatment practice [41], physicians reported they were likely to increase the frequency of office visits in response to all (N = 16) patient vignettes presented. In the same study, most (12/16) patient vignettes elicited scores suggesting no change of dose and only one vignette elicited a score suggesting an increase in dose. Clinic visits provide opportunities to observe and ensure that patients are provided comprehensive individualized treatment, and perhaps clinic visits in a clinical trial have less influence on short-term lapse than clinic visits have on treatment response in an office-based physician MOUD practice. That clinic visit was ranked last among the four treatment variables examined in our analysis of clinical trial participants suggests that the other components of individualized treatment in office-based treatment may have greater effectiveness than currently considered.
4.4. Research Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2019 National Survey on Drug Use and Health; Technical Report HHS Publication No. PEP20-07-01-001; SAMHSA, DHHS: Rockville, ML, USA, 2020.
- Volkow, N.D.; Frieden, T.R.; Hyde, P.S.; Cha, S.S. Medication-assisted therapies–Tackling the opioid-overdose epidemic. N. Engl. J. Med. 2014, 370, 2063–2066. [Google Scholar] [CrossRef]
- Campbell, N.D.; Lovell, A.M. The history of the development of buprenorphine as an addiction therapeutic. Ann. N. Y. Acad. Sci. 2012, 1248, 124–139. [Google Scholar] [CrossRef] [PubMed]
- Jasinski, D.R.; Pevnick, J.S.; Griffith, J.D. Human pharmacology and abuse potential of the analgesic buprenorphine: A potential agent for treating narcotic addiction. Arch. Gen. Psychiatry 1978, 35, 501–516. [Google Scholar] [CrossRef] [PubMed]
- Bickel, W.K.; Stitzer, M.L.; Bigelow, G.E.; Liebson, I.A.; Jasinski, D.R.; Johnson, R.E. A clinical trial of buprenorphine: Comparison with methadone in the detoxification of heroin addicts. Clin. Pharmacol. Ther. 1988, 43, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.E.; Jaffe, J.H.; Fudala, P.J. A controlled trial of buprenorphine treatment for opioid dependence. JAMA 1992, 267, 2750–2755. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Jacobs, P.; Hillhouse, M.; Hasson, A.; Thomas, C.; Freese, T.; Sparenborg, S.; McCarty, D.; Weiss, R.; Saxon, A.; et al. From research to the real world: Buprenorphine in the decade of the Clinical Trials Network. J. Subst. Abuse Treat. 2010, 38 (Suppl. 1), S53–S60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCance-Katz, E.F. Office-based buprenorphine treatment for opioid-dependent patients. Harv. Rev. Psychiatry 2004, 12, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; McCance-Katz, E.F. Characteristics and prescribing practices of clinicians recently waivered to prescribe buprenorphine for the treatment of opioid use disorder. Addiction 2019, 114, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Lapham, G.; Boudreau, D.M.; Johnson, E.A.; Bobb, J.F.; Matthews, A.G.; McCormack, J.; Liu, D.; Samet, J.H.; Saxon, A.J.; Campbell, C.I.; et al. Prevalence and Treatment of Opioid Use Disorders among Primary Care Patients in Six Health Systems. Drug Alcohol Depend. 2019, 207, 107732. [Google Scholar] [CrossRef]
- Finlay, A.K.; Wong, J.J.; Ellerbe, L.S.; Rubinsky, A.; Gupta, S.; Bowe, T.R.; Schmidt, E.M.; Timko, C.; Burden, J.L.; Harris, A.H.S. Barriers and Facilitators to Implementation of Pharmacotherapy for Opioid Use Disorders in VHA Residential Treatment Programs. J. Stud. Alcohol Drugs 2018, 79, 909–917. [Google Scholar] [CrossRef]
- Gordon, A.J.; Drexler, K.; Hawkins, E.J.; Burden, J.; Codell, N.K.; Mhatre-Owens, A.; Dungan, M.T.; Hagedorn, H. Stepped Care for Opioid Use Disorder Train the Trainer (SCOUTT) initiative: Expanding access to medication treatment for opioid use disorder within Veterans Health Administration facilities. Subst. Abus. 2020, 41, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, E.J.; Malte, C.A.; Gordon, A.J.; Williams, E.C.; Hagedorn, H.J.; Drexler, K.; Blanchard, B.E.; Burden, J.L.; Knoeppel, J.; Danner, A.N.; et al. Accessibility to Medication for Opioid Use Disorder After Interventions to Improve Prescribing Among Nonaddiction Clinics in the US Veterans Health Care System. JAMA Netw. Open 2021, 4, e2137238. [Google Scholar] [CrossRef] [PubMed]
- Expert Panelists, Scientific Reviewers and Field Reviewers. Medications for Opioid Use Disorder. In Treatment Improvement Protocol; Substance Abuse and Mental Health Services Administration: Rockville, ML, USA, 2018; Volume 63. [Google Scholar]
- Consensus and Expert Panelists. Clinical Guideline for the Use of Buprenorphine in the Treatment of Opioid Addiction. In Treatment Improvement Protocol; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2004; Volume 40. [Google Scholar]
- Fiellin, D.A.; Kleber, H.; Trumble-Hejduk, J.G.; McLellan, A.T.; Kosten, T.R. Consensus statement on office-based treatment of opioid dependence using buprenorphine. J. Subst. Abus. Treat. 2004, 27, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.M.; Lindsay, D.; Williams, J.; Ayers, A.; Schuster, J.; Cilia, A.; Flaherty, M.T.; Mandell, T.; Gordon, A.J.; Stein, B.D. Practice Guidance for Buprenorphine for the Treatment of Opioid Use Disorders: Results of an Expert Panel Process. Subst. Abus. 2015, 36, 209–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwald, M.K.; Comer, S.D.; Fiellin, D.A. Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: Implications for clinical use and policy. Drug Alcohol Depend. 2014, 144, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuckit, M.A. Treatment of Opioid-Use Disorders. N. Engl. J. Med. 2016, 375, 1596–1597. [Google Scholar] [CrossRef] [PubMed]
- Kuhlman, J.J., Jr.; Lalani, S.; Magluilo, J., Jr.; Levine, B.; Darwin, W.D. Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J. Anal. Toxicol. 1996, 20, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Kampman, K.; Jarvis, M. American Society of Addiction Medicine (ASAM) National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. J. Addict. Med. 2015, 9, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Saulle, R.; Vecchi, S.; Gowing, L. Supervised dosing with a long-acting opioid medication in the management of opioid dependence. Cochrane Database Syst. Rev. 2017, 4, CD011983. [Google Scholar] [CrossRef]
- Strong, D.R.; Brown, R.A.; Sims, M.; Herman, D.S.; Anderson, B.J.; Stein, M.D. Persistence on a stress-challenge task before initiating buprenorphine treatment was associated with successful transition from opioid use to early abstinence. J. Addict. Med. 2012, 6, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, W.; Charuvastra, C.; Collins, J.F.; Batki, S.; Brown, L.S., Jr.; Kintaudi, P.; Wesson, D.R.; McNicholas, L.; Tusel, D.J.; Malkerneker, U.; et al. Buprenorphine maintenance treatment of opiate dependence: A multicenter, randomized clinical trial. Addiction 1998, 93, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Fudala, P.J.; Bridge, T.P.; Herbert, S.; Williford, W.O.; Chiang, C.N.; Jones, K.; Collins, J.; Raisch, D.; Casadonte, P.; Goldsmith, R.J.; et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N. Engl. J. Med. 2003, 349, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Fudala, P.; Walsh, R.; Ling, W.; Casadonte, P.; McSherry, F.; Williford, W.; Collins, J.; Raisch, D.; Kilby, S.; Saxon, A.; et al. Office-Based Treatment of Opioid Dependence with Buprenorphine/Naloxone Sublingual Tablets: Results from a Multicenter Study. In Proceedings of the 67th Annual Meeting of College on Problems of Drug Dependence; NIDA Research Monograph; Dewey, W.L., Ed.; NIDA, NIH, HHS, Bethesda: Rockville, ML, USA, 2006. [Google Scholar]
- Saxon, A.J.; Ling, W.; Hillhouse, M.; Thomas, C.; Hasson, A.; Ang, A.; Doraimani, G.; Tasissa, G.; Lokhnygina, Y.; Leimberger, J.; et al. Buprenorphine/Naloxone and methadone effects on laboratory indices of liver health: A randomized trial. Drug Alcohol Depend. 2013, 128, 71–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, R.D.; Potter, J.S.; Provost, S.E.; Huang, Z.; Jacobs, P.; Hasson, A.; Lindblad, R.; Connery, H.S.; Prather, K.; Ling, W. A multi-site, two-phase, Prescription Opioid Addiction Treatment Study (POATS): Rationale, design, and methodology. Contemp. Clin. Trials 2010, 31, 189–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, C.J.; Walsh, S.L.; Bigelow, G.E.; Strain, E.C. Effects associated with double-blind omission of buprenorphine/naloxone over a 98-h period. Psychopharmacology 2006, 189, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Day, E.; Mitcheson, L. Response to commentaries: Neither optimism or nihilism… but reasons for hope. Addiction 2017, 112, 1342–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reese, E.D.; Conway, C.C.; Anand, D.; Bauer, D.J.; Daughters, S.B. Distress tolerance trajectories following substance use treatment. J. Consult. Clin. Psychol. 2019, 87, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Audigier, V.; Husson, F.; Josse, J. A principal component method to impute missing values for mixed data. Adv. Data Anal. Classif. 2016, 10, 5–26. [Google Scholar] [CrossRef]
- Greenwald, M.K.; Johanson, C.E.; Moody, D.E.; Woods, J.H.; Kilbourn, M.R.; Koeppe, R.A.; Schuster, C.R.; Zubieta, J.K. Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology 2003, 28, 2000–2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzicato, L.N.; Hom, J.K.; Sun, M.; Johnson, C.C.; Viner, K.M. Adherence to buprenorphine: An analysis of prescription drug monitoring program data. Drug Alcohol Depend. 2020, 216, 108317. [Google Scholar] [CrossRef] [PubMed]
- Saxon, A.J.; Hser, Y.I.; Woody, G.; Ling, W. Medication-assisted treatment for opioid addiction: Methadone and buprenorphine. J. Food Drug Anal. 2013, 21, S69–S72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, R.E.; Baxter, J.D.; Aweh, G.; O’Connell, E.; Fisher, W.H.; Barton, B.A. Risk Factors for Relapse and Higher Costs Among Medicaid Members with Opioid Dependence or Abuse: Opioid Agonists, Comorbidities, and Treatment History. J. Subst. Abuse Treat. 2015, 57, 75–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Currie, J.M.; Schnell, M.K.; Schwandt, H.; Zhang, J. Prescribing of Opioid Analgesics and Buprenorphine for Opioid Use Disorder During the COVID-19 Pandemic. JAMA Netw. Open 2021, 4, e216147. [Google Scholar] [CrossRef] [PubMed]
- Pytell, J.D.; Rastegar, D.A. Down the drain: Reconsidering routine urine drug testing during the COVID-19 pandemic. J. Subst. Abus. Treat. 2021, 120, 108155. [Google Scholar] [CrossRef] [PubMed]
- Katz, C.; El-Gabalawy, R.; Keyes, K.M.; Martins, S.S.; Sareen, J. Risk factors for incident nonmedical prescription opioid use and abuse and dependence: Results from a longitudinal nationally representative sample. Drug Alcohol Depend. 2013, 132, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lusted, A.; Roerecke, M.; Goldner, E.; Rehm, J.; Fischer, B. Prevalence of pain among nonmedical prescription opioid users in substance use treatment populations: Systematic review and meta-analyses. Pain Physician 2013, 16, E671–E684. [Google Scholar] [PubMed]
- Knudsen, H.K.; Lofwall, M.R.; Lin, L.A.; Walsh, S.L.; Studts, J.L. US physicians’ decision-making during buprenorphine-naloxone treatment: Conjoint analyses of dose and office visit adjustments based on patient progress. Drug Alcohol Depend. 2019, 204, 107490. [Google Scholar] [CrossRef] [PubMed]
- Gossop, M.; Stewart, D.; Marsden, J. Treatment process components and heroin use outcome among methadone patients. Drug Alcohol Depend. 2003, 71, 93–102. [Google Scholar] [CrossRef]
- Thomas, C.P.; Doyle, E.; Kreiner, P.W.; Jones, C.M.; Dubenitz, J.; Horan, A.; Stein, B.D. Prescribing patterns of buprenorphine waivered physicians. Drug Alcohol Depend. 2017, 181, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Dela Cruz, A.M.; Walker, R.; Pipes, R.; Wakhlu, S.; Trivedi, M.H. Creation of an algorithm for clinical decision support for treatment of opioid use disorder with buprenorphine in primary care. Addict. Sci. Clin. Pract. 2021, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Shulman, M.; Weiss, R.; Rotrosen, J.; Novo, P.; Costello, E.; Nunes, E.V. Prior National Drug Abuse Treatment Clinical Trials Network (CTN) opioid use disorder trials as background and rationale for NIDA CTN-0100 “optimizing retention, duration and discontinuation strategies for opioid use disorder pharmacotherapy (RDD)”. Addict. Sci. Clin. Pract. 2021, 16, 15. [Google Scholar] [CrossRef] [PubMed]
- McLellan, A.T.; Luborsky, L.; Woody, G.E.; O’Brien, C.P. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J. Nerv. Ment. Dis. 1980, 168, 26–33. [Google Scholar] [CrossRef] [PubMed]
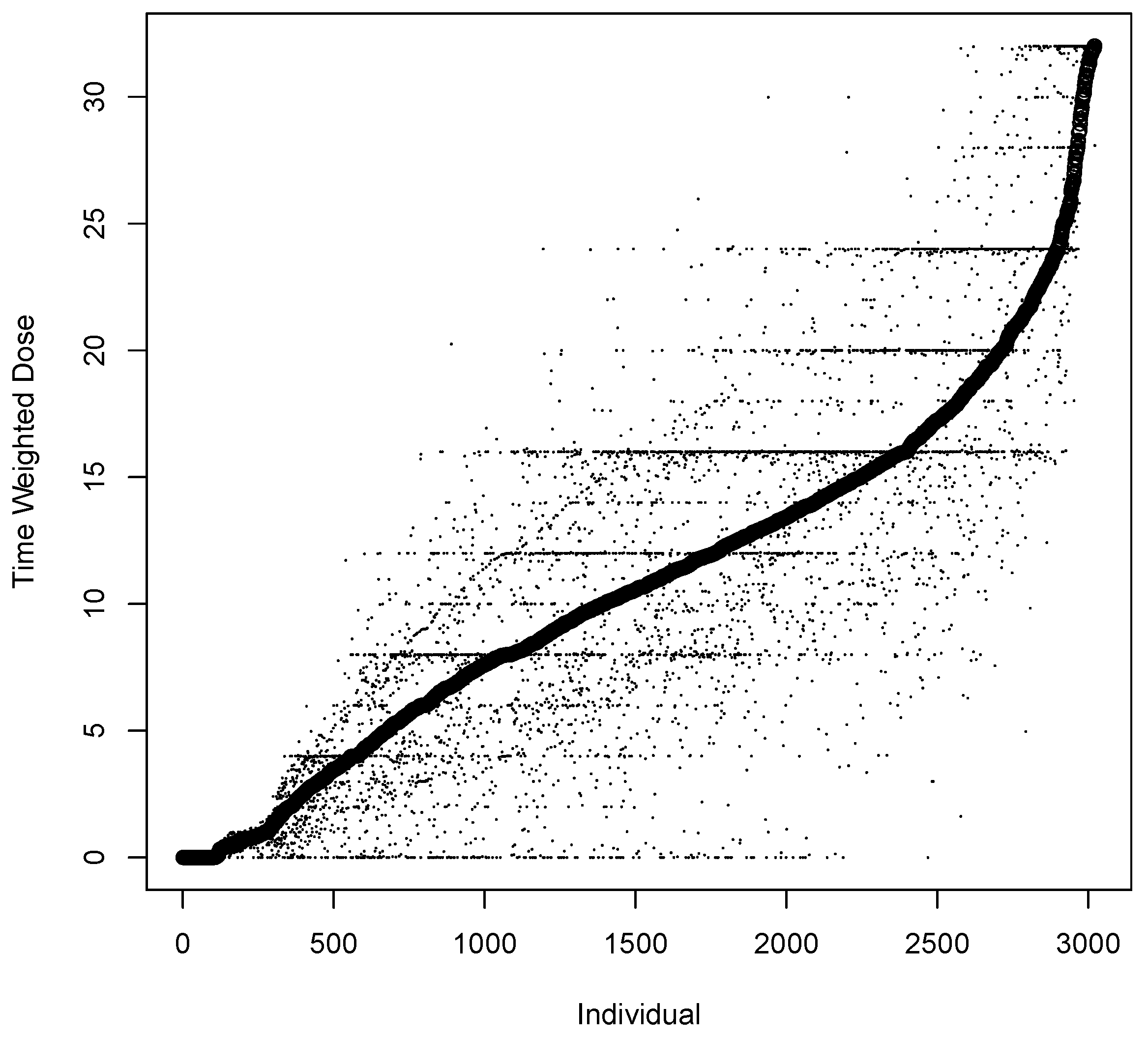
| Variable | Input | Coding | Mean | Median | Range |
|---|---|---|---|---|---|
| Time Weighted Dose (mg) | Dose (mg) | Equation (1) | 11.9 | 12 | 0–32 |
| Time Weighted Adaptive Dose | Fixed or per clinician | Equation (1) | 0.76 | 1.00 | 0–1 |
| Time Weighted Clinic Visit | At home or in clinic | Equation (1) | 0.34 | 0.03 | 0–1 |
| Time-in-Trial (days) | Day of clinic visit | Assigned | 112.7 | 87 | 1–527 |
| Short-term Lapse (Outcome) * | Urinalysis or self-report | Positive = 1 | 0.41 | 0 | 0–1 |
| Variable | Effect * | SE ** | 95% CI | p-Value |
|---|---|---|---|---|
| Intercept | −0.056 | 0.146 | (−0.341, 0.230) | 0.702 |
| Time Weighted Dose | −0.463 | 0.033 | (−0.525, −0.400) | <0.001 |
| Time Weighted Adaptive Dose | −0.354 | 0.039 | (−0.430, −0.279) | <0.001 |
| Time Weighted Clinic Visit | −0.209 | 0.035 | (−0.278, −0.140) | <0.001 |
| Time-in-Trial | −0.283 | 0.032 | (−0.346, −0.221) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergen, A.W.; Baurley, J.W.; Ervin, C.M.; McMahan, C.S.; Bible, J.; Stafford, R.S.; Mudumbai, S.C.; Saxon, A.J. Effects of Buprenorphine Dose and Therapeutic Engagement on Illicit Opiate Use in Opioid Use Disorder Treatment Trials. Int. J. Environ. Res. Public Health 2022, 19, 4106. https://doi.org/10.3390/ijerph19074106
Bergen AW, Baurley JW, Ervin CM, McMahan CS, Bible J, Stafford RS, Mudumbai SC, Saxon AJ. Effects of Buprenorphine Dose and Therapeutic Engagement on Illicit Opiate Use in Opioid Use Disorder Treatment Trials. International Journal of Environmental Research and Public Health. 2022; 19(7):4106. https://doi.org/10.3390/ijerph19074106
Chicago/Turabian StyleBergen, Andrew W., James W. Baurley, Carolyn M. Ervin, Christopher S. McMahan, Joe Bible, Randall S. Stafford, Seshadri C. Mudumbai, and Andrew J. Saxon. 2022. "Effects of Buprenorphine Dose and Therapeutic Engagement on Illicit Opiate Use in Opioid Use Disorder Treatment Trials" International Journal of Environmental Research and Public Health 19, no. 7: 4106. https://doi.org/10.3390/ijerph19074106
APA StyleBergen, A. W., Baurley, J. W., Ervin, C. M., McMahan, C. S., Bible, J., Stafford, R. S., Mudumbai, S. C., & Saxon, A. J. (2022). Effects of Buprenorphine Dose and Therapeutic Engagement on Illicit Opiate Use in Opioid Use Disorder Treatment Trials. International Journal of Environmental Research and Public Health, 19(7), 4106. https://doi.org/10.3390/ijerph19074106






