Optimal Management of Patients with Severe Coronary Artery Disease following Multidisciplinary Heart Team Approach—Insights from Tertiary Cardiovascular Care Center
Abstract
:1. Introduction
2. Methods and Study Design
3. Statistical Analysis
4. Results
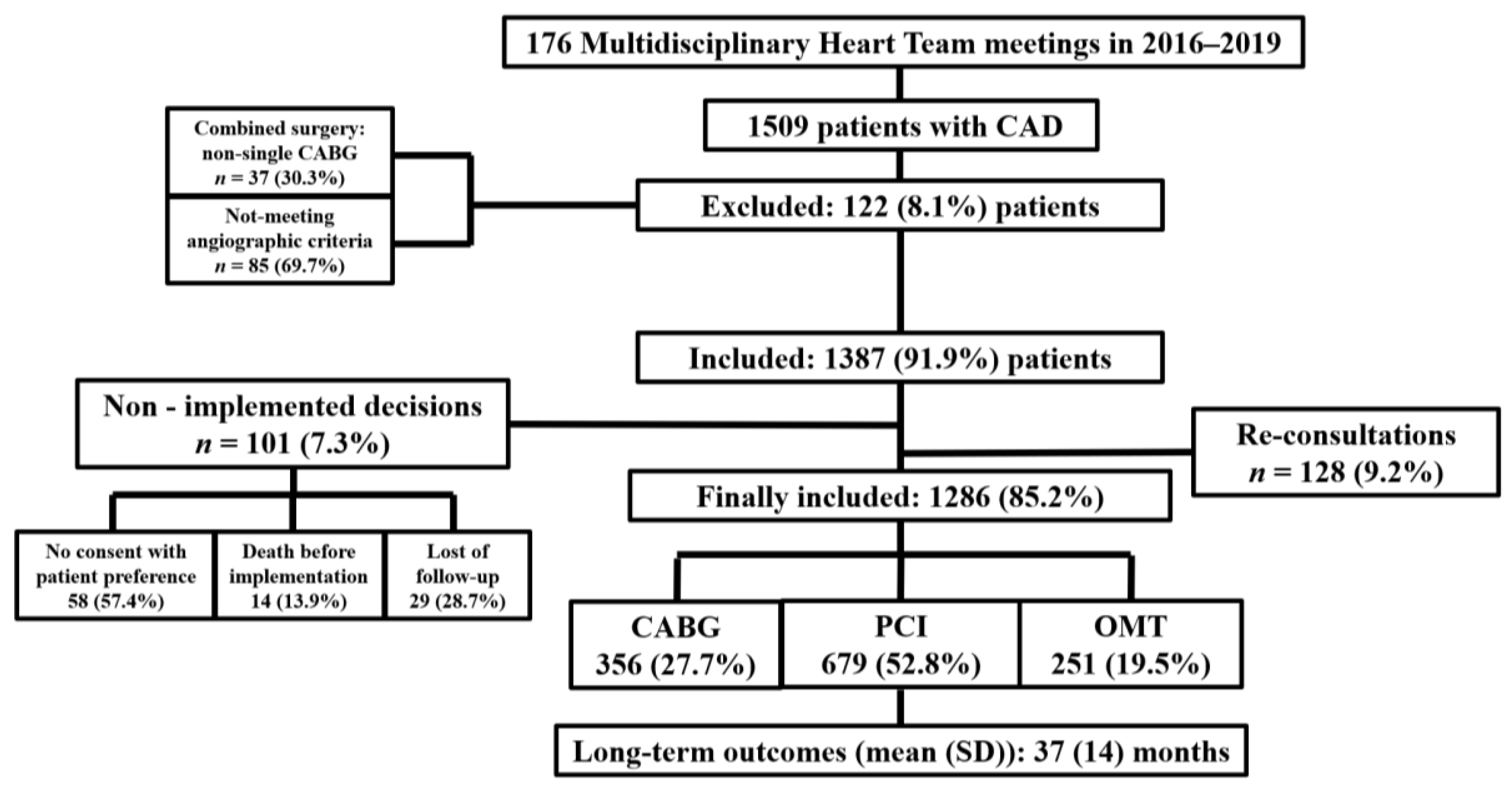
4.1. Study Population and Baseline Characteristics
4.2. Medications at Discharge
4.3. Endpoints
4.4. Quality of Life
4.5. Logistic Regression Analysis
5. Discussion
6. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nichols, M.; Townsend, N.; Scarborough, P.; Rayner, M. Cardiovascular disease in Europe 2014: Epidemiological update. Eur. Heart J. 2014, 35, 2950–2959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, E.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment Elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E.; Ganiats, T.G.; Holmes, D.R.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Non–ST-Elevation Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 64, 139–228. [Google Scholar] [CrossRef] [Green Version]
- Fihn, S.D.; Gardin, J.M.; Abrams, J.; Berra, K.; Blankenship, J.C.; Dallas, A.P.; Douglas, P.S.; Foody, J.M.; Gerber, T.C.; Hinderliter, A.L.; et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012, 126, 3097–3137. [Google Scholar] [CrossRef] [Green Version]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, 362–425. [Google Scholar] [CrossRef] [Green Version]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Serruys, P.W.; Morice, M.C.; Kappetein, A.P.; Colombo, A.; Holmes, D.R.; Mack, M.J.; Ståhle, E.; Feldman, T.E.; van den Brand, M.; Bass, E.J.; et al. Percutaneous Coronary Intervention versus Coronary-Artery Bypass Grafting for Severe Coronary Artery Disease. N. Engl. J. Med. 2009, 360, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Head, S.J.; Kaul, S.; Mack, M.J.; Serruys, P.W.; Taggart, D.P.; Holmes, D.R., Jr.; Leon, M.B.; Marco, J.; Bogers, A.J.J.C.; Kappetein, A.P. The rationale for Heart Team decision-making for patients with stable, complex coronary artery disease. Eur. Heart J. 2013, 34, 2510–2518. [Google Scholar] [CrossRef] [PubMed]
- Head, S.J.; Davierwala, P.M.; Serruys, P.W.; Redwood, S.R.; Colombo, A.; Mack, M.J.; Morice, M.C.; Holmes, D.R., Jr.; Feldman, T.E.; Ståhle, E.; et al. Coronary artery bypass grafting vs. percutaneous coronary intervention for patients with three-vessel disease: Final five-year follow-up of the SYNTAX trial. Eur. Heart J. 2014, 35, 2821–2830. [Google Scholar] [CrossRef]
- Bonzel, T.; Schächinger, V.; Dörge, H. Description of a Heart Team approach to coronary revascularization and its beneficial long-term effect on clinical events after PCI. Clin. Res. Cardiol. 2016, 105, 388–400. [Google Scholar] [CrossRef]
- Abdulrahman, M.; Alsabbagh, A.; Kuntze, T.; Lauer, B.; Ohlow, M.A. Impact of Hierarchy on Multidisciplinary Heart-Team Recommendations in Patients with Isolated Multivessel Coronary Artery Disease. J. Clin. Med. 2019, 8, 1490. [Google Scholar] [CrossRef] [Green Version]
- Collet, C.; Onuma, Y.; Andreini, D.; Sonck, J.; Pompilio, G.; Mushtaq, S.; La Meir, M.; Miyazaki, Y.; de Mey, J.; Gaemperli, O.; et al. Coronary computed tomography angiography for heart team decision-making in multivessel coronary artery disease. Eur. Heart J. 2018, 39, 3689–3698. [Google Scholar] [CrossRef]
- Patterson, T.; McConkey, H.Z.R.; Ahmed-Jushuf, F.; Moschonas, K.; Nguyen, H.; Karamasis, G.V.; Perera, D.; Clapp, B.R.; Roxburgh, J.; Blauth, C.; et al. Long-term outcomes following Heart Team revascularization recommendations in complex coronary artery disease. J. Am. Heart Assoc. 2019, 8, e011279. [Google Scholar] [CrossRef]
- Dominigues, C.T.; Milojevic, M.; Thuijs, D.J.F.M.; Mieghem, N.M.; Daemen, J.; Domburg, R.T.; Kappetein, A.P.; Head, S.J. Heart Team decision making and long-term outcomes for 1000 consecutive cases of coronary artery disease. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 206–213. [Google Scholar] [CrossRef]
- Young, M.N.; Kolte, D.; Cadigan, M.E.; Laikhter, E.; Sinclair, K.; Pomerantsev, E.; Fifer, M.A.; Sundt, T.M.; Yeh, R.W.; Jaffer, F.A. Multidisciplinary Heart Team Approach for Complex Coronary Artery Disease: Single Center Clinical Presentation. J. Am. Heart Assoc. 2020, 9, e014738. [Google Scholar] [CrossRef]
- Banning, A.P.; Serruys, P.; De Maria, G.L.; Ryan, N.; Walsh, S.; Gonzalo, N.; van Geuns, R.J.; Onuma, Y.; Sabate, M.; Davies, J.; et al. Five-year outcomes after state-of-the-art percutaneous coronary revascularization in patients with de novo three-vessel disease: Final results of the SYNTAX II study. Eur. Heart J. 2021, ehab703. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Anders Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]

| Baseline Characteristic | Overall (1286) | CABG (356) | PCI (679) | OMT (251) | p-Value |
|---|---|---|---|---|---|
| Age, years; mean (SD) | 69.0 (10.0) | 66.9 (9.2) | 68.8 (10.1) | 72.5 (9.9) | <0.01 |
| Gender, male (%) | 963 (74.9) | 289 (81.2) | 495 (72.9) | 179 (71.3) | <0.01 |
| BMI, kg/m2; mean (SD) | 27.9 (3.5) | 27.9 (3.3) | 28.2 (3.7) | 27.2 (3.0) | <0.01 |
| Clinical Presentation | |||||
| ACS, n (%) | 526 (40.9) | 152 (42.7) | 285 (42.0) | 89 (35.5) | 0.14 |
| Cardiogenic shock, n (%) | 44 (3.4) | 4 (1.1) | 29 (4.3) | 11 (4.4) | 0.02 |
| Heart Failure, n (%) | 965 (75.0) | 236 (66.3) | 498 (73.3) | 231 (92.0) | <0.01 |
| LV dysfunction (EF < 50%), n (%) | 1067 (83.0) | 289 (81.2) | 567 (83.5) | 211 (84.1) | 0.56 |
| LV dysfunction (EF < 30%), n (%) | 336 (26.1) | 46 (12.9) | 147 (21.6) | 143 (57.0) | <0.01 |
| LVEDD, cm (SD) | 5.8 (1.0) | 5.5 (0.9) | 5.8 (1.0) | 6.2 (1.0) | <0.01 |
| NYHA class III-IV, n (%) | 441 (34.3) | 95 (26.7) | 217 (32.0) | 129 (51.4) | <0.01 |
| CCS class III-IV, n (%) | 518 (40.3) | 165 (46.3) | 266 (39.2) | 87 (34.7) | 0.01 |
| Diabetes, n (%) | 393 (30.6) | 98 (27.5) | 219 (32.3) | 76 (30.3) | 0.29 |
| Requiring insulin, n (%) | 131 (10.2) | 29 (8.1) | 76 (11.2) | 26 (10.4) | 0.30 |
| Hypertension, n (%) | 1068 (83.0) | 291 (81.7) | 577 (85.0) | 200 (79.7) | 0.12 |
| Previous stroke/TIA, n (%) | 112 (8.7) | 26 (7.3) | 60 (8.8) | 26 (10.4) | 0.42 |
| Atrial fibrillation, n (%) | 351 (27.3) | 67 (18.8) | 189 (27.8) | 95 (37.8) | <0.01 |
| Previous MI, n (%) | 615 (47.8) | 189 (53.1) | 319 (47.0) | 107 (42.6) | 0.03 |
| Previous PCI, n (%) | 382 (29.7) | 91 (25.6) | 217 (32.0) | 74 (29.5) | 0.10 |
| Previous CABG, n (%) | 115 (8.9) | 20 (5.6) | 71 (10.5) | 24 (9.6) | 0.03 |
| PAD, n (%) | 78 (6.1) | 14 (3.9) | 49 (7.2) | 15 (6.0) | 0.11 |
| Carotid AD, n (%) | 133 (10.3) | 34 (9.6) | 77 (11.3) | 22 (8.8) | 0.44 |
| CKD, n (%) | 463 (36.0) | 66 (18.5) | 204 (30.0) | 193 (76.9) | <0.01 |
| Anaemia, n (%) | 428 (33.3) | 89 (25.0) | 182 (26.8) | 157 (62.5) | <0.01 |
| Dyslipidemia, n (%) | 1028 (79.9) | 284 (79.8) | 556 (81.9) | 188 (74.9) | 0.06 |
| COPD, n (%) | 129 (10.0) | 29 (8.1) | 70 (10.3) | 30 (12.0) | 0.29 |
| Severe PH, n (%) | 125 (9.7) | 17 (4.8) | 73 (10.8) | 35 (13.9) | <0.01 |
| Cancer, n (%) | 215 (16.72) | 22 (6.2) | 104 (15.3) | 89 (35.5) | <0.01 |
| Current smoking, n (%) | 234 (18.2) | 65 (18.3) | 132 (19.4) | 37 (14.7) | 0.26 |
| Frailty, n (%) | 261 (20.3) | 9 (2.5) | 89 (13.1) | 163 (64.9) | <0.01 |
| Angiographic Characteristics | |||||
| No. of lesions, mean (SD) | 4.2 (1.5) | 4.2 (1.4) | 4.3 (1.5) | 3.8 (1.4) | <0.01 |
| LM disesase (LM stenosis ≥ 50%), n (%) | 313 (24.3) | 109 (30.6) | 158 (23.3) | 46 (18,3) | <0.01 |
| Bifurcation, n (%) | 927 (72.1) | 261 (73.3) | 491 (72.3) | 175 (69.7) | 0.61 |
| CTO, n (%) | 305 (23.7) | 77 (21.6) | 164 (24.2) | 64 (25.5) | 0.50 |
| SYNTAX score; mean (SD) | 30.2 (6.3) | 31.1 (5.9) | 29.6 (6.5) | 30.6 (6.1) | <0.01 |
| Complete revascularization, n (%) | 630/1035 (60.9) | 233 (65.4) | 397 (58.5) | - | 0.03 |
| EuroSCORE II, %; mean (SD) | 5.5 (1.7) | 3.9 (1.1) | 6.0 (1.4) | 6.5 (1.5) | <0.01 |
| STS score, %; mean (SD) | 3.5 (1.2) | 2.5 (0.8) | 3.9 (1.0) | 4.2 (1.1) | <0.01 |
| Time to procedure, days (SD) | 5.7 (2.3) | 8.4 (1.3) | 4.2 (0.9) | - | <0.01 |
| Medication at Discharge, n (%) | Overall (1233) | CABG (339) | PCI (654) | OMT (240) | p-Value |
|---|---|---|---|---|---|
| Aspirin | 1150 (93.3) | 302 (89.1) | 631 (96.5) | 217 (90.4) | <0.01 |
| P2Y12 inhibitors | 740 (60.0) | 71 (20.9) | 635 (97.1) | 34 (14.2) | <0.01 |
| Vitamin K antagonist (VKA) | 68 (5.5) | 29 (8.6) | 23 (3.5) | 16 (6.7) | <0.01 |
| Novel oral anticoagulants (NOAC) | 335 (27.2) | 52 (15.3) | 186 (28.4) | 97 (40.4) | <0.01 |
| Statin | 1135 (92.1) | 294 (86.7) | 628 (96.0) | 213 (88.8) | <0.01 |
| ACE inhibitor | 853 (69.2) | 206 (60.8) | 479 (73.2) | 168 (70.0) | <0.01 |
| Angiotensin II-receptor antagonist | 278 (22.5) | 85 (25.1) | 144 (22.0) | 49 (20.4) | 0.37 |
| Beta-blocker | 952 (77.2) | 270 (79.6) | 528 (80.7) | 154 (64.2) | <0.01 |
| Calcium-channel blocker | 294 (23.8) | 71 (20.9) | 157 (24.0) | 66 (27.5) | 0.19 |
| Loop diuretic | 875 (71.0) | 228 (67.3) | 434 (66.4) | 213 (88.8) | <0.01 |
| Aldosterone antagonist | 396 (32.1) | 43 (12.7) | 187 (28.6) | 166 (69.2) | <0.01 |
| Amiodarone | 75 (6.1) | 27 (8.0) | 28 (4.3) | 20 (8.3) | 0.02 |
| Endpoints, n (%) | CABG (356) | PCI (679) | OMT (251) | p Value Overall | CABG vs. PCI HR [95% CI]; p | CABG vs. OMT HR [95% CI]; p | PCI vs. OMT HR [95% CI]; p |
|---|---|---|---|---|---|---|---|
| Primary Endpoint—MACCE | 110 (30.9) | 302 (44.5) | 154 (61.4) | <0.01 | <0.01 | <0.01 | <0.01 |
| Secondary Endpoints | |||||||
| All-cause mortality, stroke, or MI | 69 (19.4) | 136 (20.0) | 139 (55.4) | <0.01 | 0.80 | <0.01 | <0.01 |
| All-cause mortality | 32 (9.0) | 75 (11.0) | 72 (28.7) | <0.01 | 0.30 | <0.01 | <0.01 |
| CV death | 24 (6.7) | 64 (9.4) | 43 (17.1) | <0.01 | 0.14 | <0.01 | <0.01 |
| In-hospital mortality | 17 (4.8) | 25 (3.7) | 11 (4.4) | 0.68 | 0.40 | 0.82 | 0.62 |
| MI | 29 (8.1) | 78 (11.5) | 48 (19.1) | <0.01 | 0.09 | <0.01 | <0.01 |
| Stroke | 21 (5.9) | 14 (2.1) | 24 (9.6) | <0.01 | <0.01 | 0.09 | <0.01 |
| Disabling stroke | 13 (3.7) | 6 (0.9) | 15 (6.0) | <0.01 | <0.01 | 0.18 | <0.01 |
| Repeat/need for revascularization | 38 (10.7) | 165 (24.3) | 19 (7.6) | <0.01 | <0.01 | 0.20 | <0.01 |
| CABG | 9 (2.5) | 17 (2.5) | 0 (0.0) | 0.04 | 0.98 | 0.01 | 0.01 |
| PCI | 29 (8.1) | 148 (21.8) | 19 (7.6) | <0.01 | <0.01 | 0.80 | <0.01 |
| Graft occlusion or stent thrombosis | 20 (5.6) | 40 (5.9) | 0.86 | ||||
| Acute (at ≤1 day) | 2 (0.6) | 5 (0.7) | 0.75 | ||||
| Subacute (within 2–30 days) | 3 (0.8) | 13 (1.9) | 0.18 | ||||
| Late (within 31–365 days) | 9 (2.5) | 8 (1.2) | 0.10 | ||||
| Very late (≥366 days) | 6 (1.7) | 14 (2.1) | 0.68 | ||||
| Postprocedural hospital stay, days; mean (SD) | 9.9 (1.4) | 4.3 (0.7) | <0.01 | ||||
| Component | CABG (356/324) | PCI (679/604) | OMT (251/179) | p-Value |
|---|---|---|---|---|
| Physical Component Summary (PCS) | ||||
| Before CABG, PCI, MHT disscusion; mean (SD) | 71.1 (18.6) | 73.2 (18.4) | 73.8 (15.8) | 0.12 |
| After CABG, PCI, MHT disscusion—at the end of follow up; mean (SD) | 62.0 (17.0) | 65.7 (14.3) | 75.0 (15.9) | <0.01 |
| Mental Component Summary (MCS) | ||||
| Before CABG, PCI, MHT disscusion; mean (SD) | 51.6 (9.4) | 52.0 (9.4) | 52.5 (9.0) | 0.53 |
| After CABG, PCI, MHT disscusion—at the end of follow up; mean (SD) | 43.3 (9.4) | 44.9 (9.7) | 53.3 (8.9) | <0.01 |
| Total | ||||
| Before CABG, PCI, MHT disscusion; mean (SD) | 122.8 (20.2) | 125.2 (20.6) | 126.3 (18.7) | 0.07 |
| After CABG, PCI, MHT disscusio—at the end of follow up; mean (SD) | 105.3 (18.8) | 110.7 (16.8) | 128.3 (19.5) | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonik, S.; Marchel, M.; Pędzich-Placha, E.; Pietrasik, A.; Rdzanek, A.; Huczek, Z.; Kochman, J.; Budnik, M.; Piątkowski, R.; Scisło, P.; et al. Optimal Management of Patients with Severe Coronary Artery Disease following Multidisciplinary Heart Team Approach—Insights from Tertiary Cardiovascular Care Center. Int. J. Environ. Res. Public Health 2022, 19, 3933. https://doi.org/10.3390/ijerph19073933
Jonik S, Marchel M, Pędzich-Placha E, Pietrasik A, Rdzanek A, Huczek Z, Kochman J, Budnik M, Piątkowski R, Scisło P, et al. Optimal Management of Patients with Severe Coronary Artery Disease following Multidisciplinary Heart Team Approach—Insights from Tertiary Cardiovascular Care Center. International Journal of Environmental Research and Public Health. 2022; 19(7):3933. https://doi.org/10.3390/ijerph19073933
Chicago/Turabian StyleJonik, Szymon, Michał Marchel, Ewa Pędzich-Placha, Arkadiusz Pietrasik, Adam Rdzanek, Zenon Huczek, Janusz Kochman, Monika Budnik, Radosław Piątkowski, Piotr Scisło, and et al. 2022. "Optimal Management of Patients with Severe Coronary Artery Disease following Multidisciplinary Heart Team Approach—Insights from Tertiary Cardiovascular Care Center" International Journal of Environmental Research and Public Health 19, no. 7: 3933. https://doi.org/10.3390/ijerph19073933
APA StyleJonik, S., Marchel, M., Pędzich-Placha, E., Pietrasik, A., Rdzanek, A., Huczek, Z., Kochman, J., Budnik, M., Piątkowski, R., Scisło, P., Czub, P., Wilimski, R., Maksym, J., Grabowski, M., Opolski, G., & Mazurek, T. (2022). Optimal Management of Patients with Severe Coronary Artery Disease following Multidisciplinary Heart Team Approach—Insights from Tertiary Cardiovascular Care Center. International Journal of Environmental Research and Public Health, 19(7), 3933. https://doi.org/10.3390/ijerph19073933







