A Comparative Multi-System Approach to Characterizing Bioactivity of Commonly Occurring Chemicals
Abstract
:1. Introduction
1.1. Discovery of Common Chemical Exposures
1.2. Exposure to G14 Chemicals
1.3. Importance of Studying Chemical Mixtures
1.4. Use of Two Alternative Models to Assess Chemical Bioactivity
1.5. Bridging the Gap between Exposure Science and Toxicology
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Mixture Calculations and Concentration Selection
2.3. NHBE Bioactivity Screening
2.3.1. Cell Culture Conditions and Exposures
2.3.2. Lactate Dehydrogenase Assay
2.3.3. Cell Titer Glo Assay
2.3.4. 2′,7′-Dichlorofluorescin Diacetate (DCFDA) Assay
2.3.5. NHBE Statistics
2.4. Zebrafish Bioactivity Screening
2.4.1. Zebrafish Husbandry and Exposures
2.4.2. Zebrafish Morphology Screening
2.4.3. Embryonic and Larval Photomotor Response Assays
2.5. Correlation Matrix of Real-World Exposures
2.6. Benchmark Concentration Modeling and Mixture Interaction Assessment
3. Results
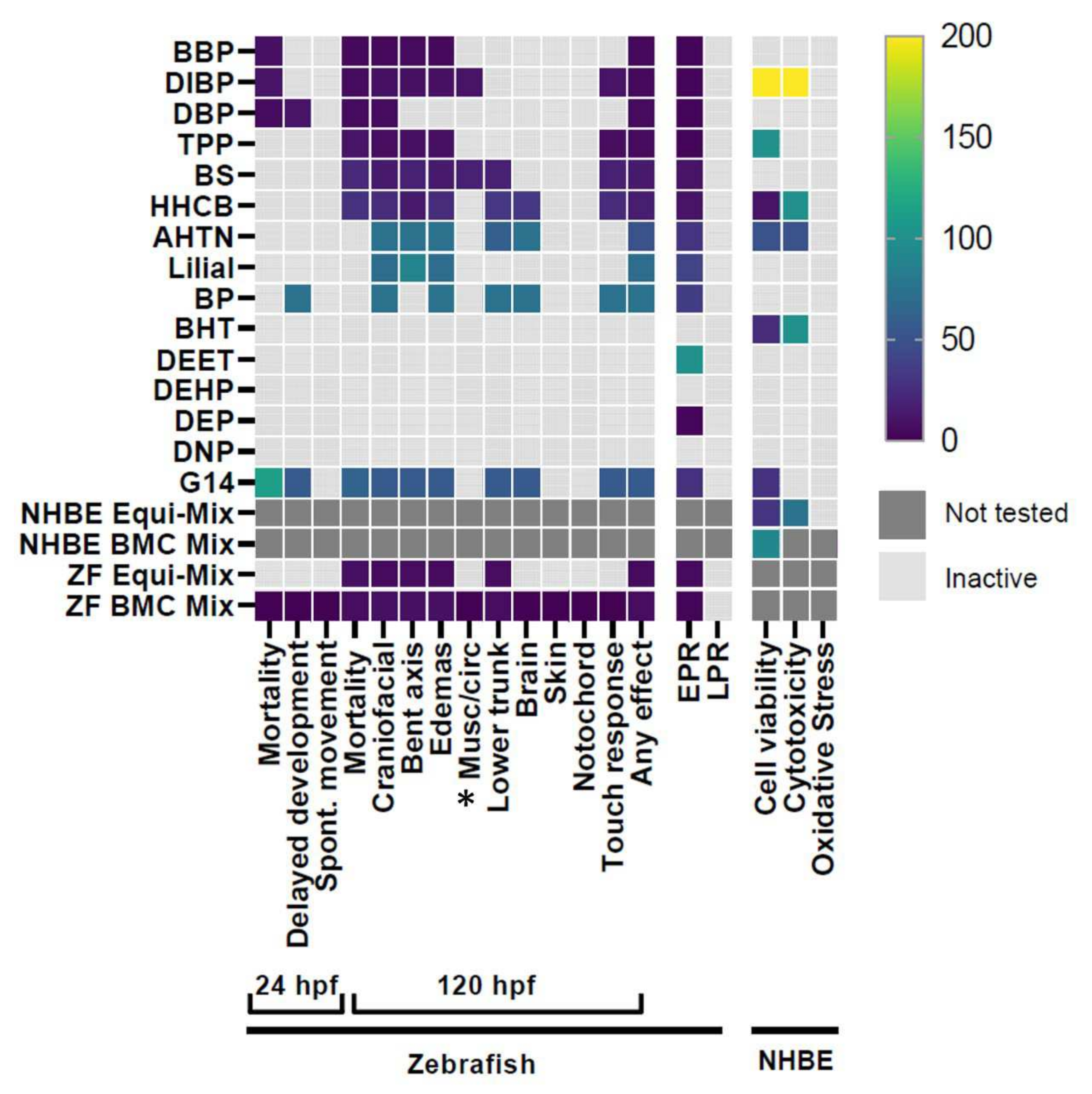
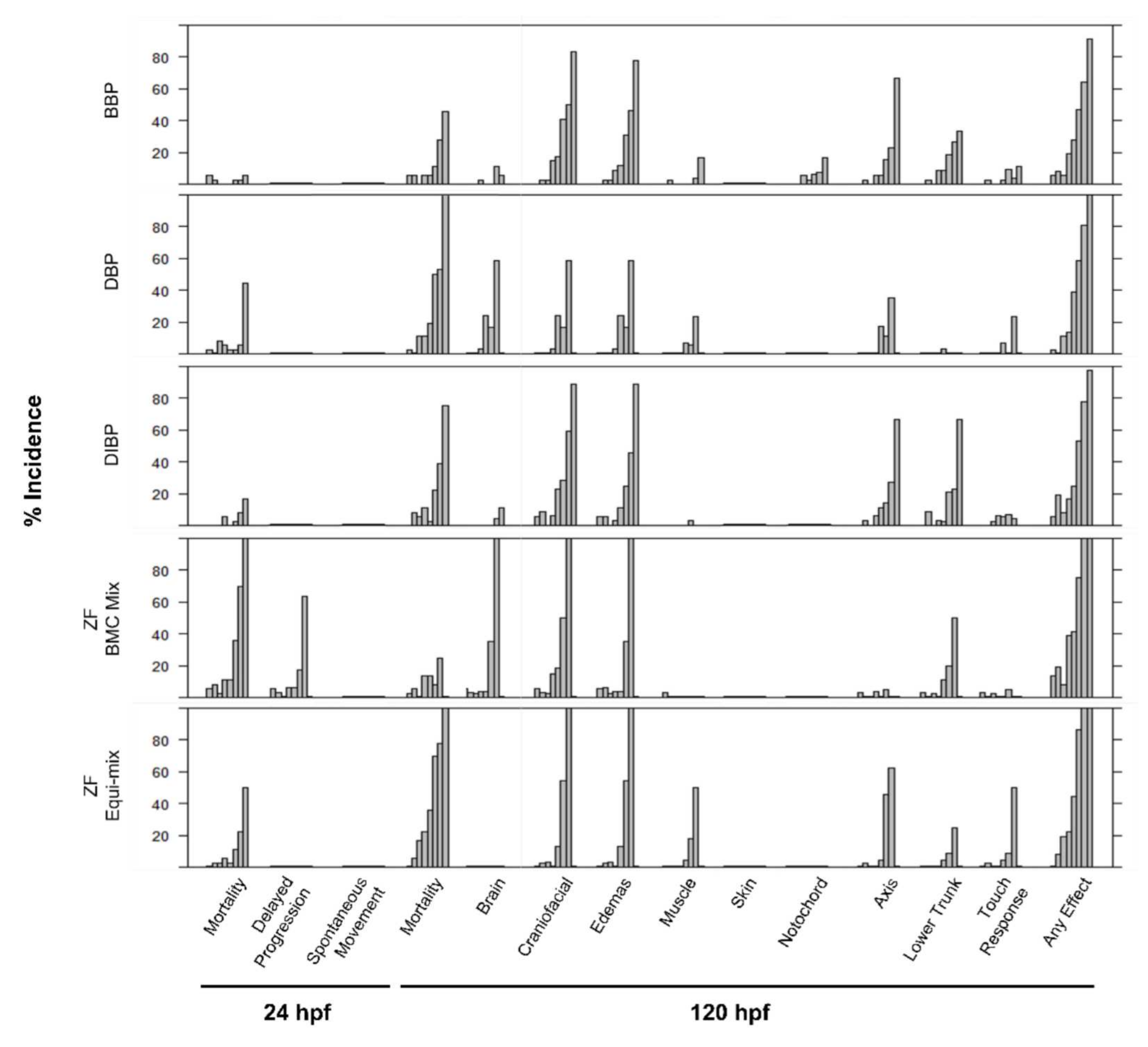
3.1. G14 Mixture and Individual Chemical Screening
3.2. Investigation of Mixture Interactions
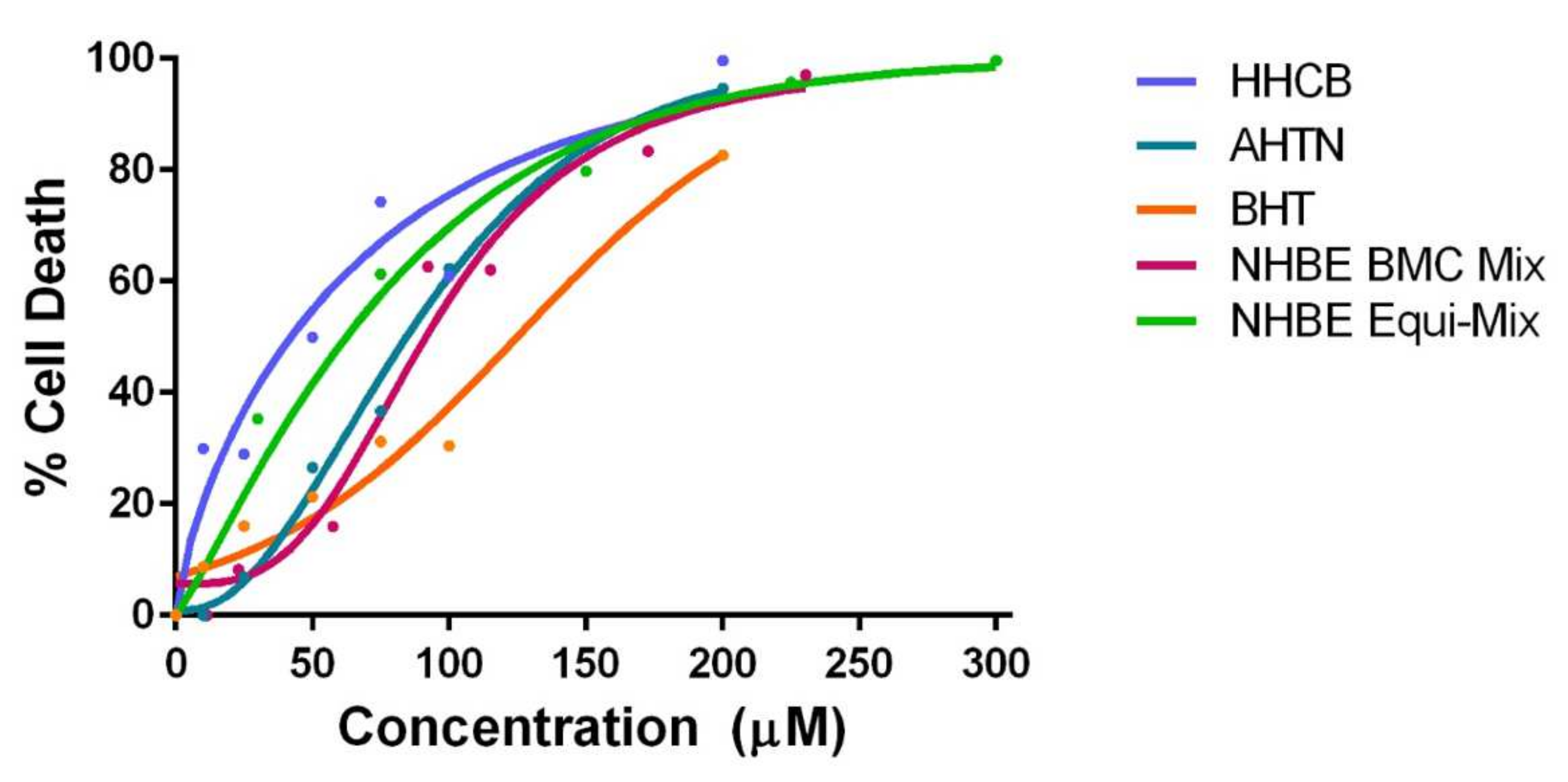
3.2.1. NHBE Mixtures
3.2.2. Zebrafish Mixtures
4. Discussion
4.1. Model-Specific Differences in Bioactive Chemical Detection
4.2. Investigation of Mixture Effects
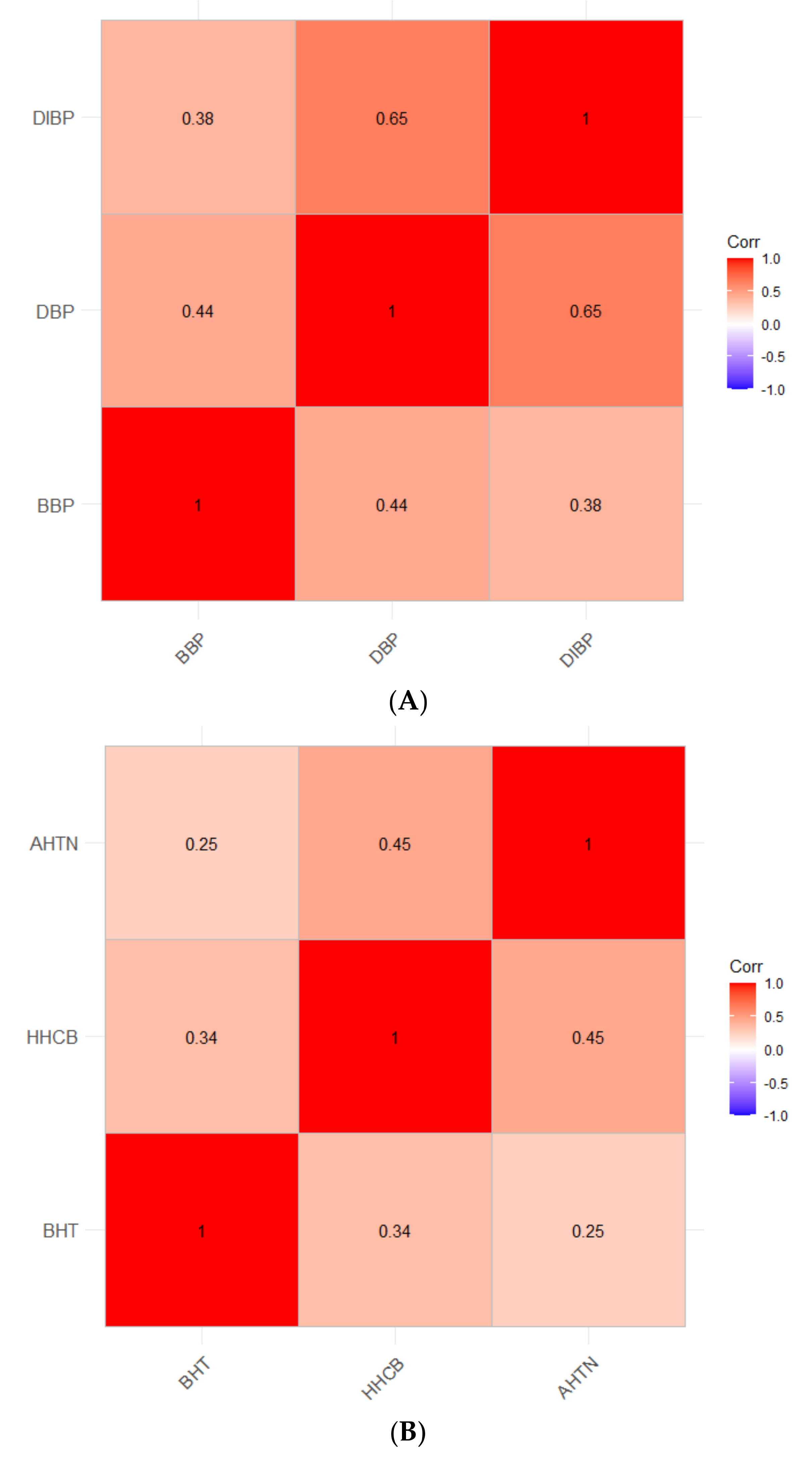
4.3. Correlations of the Most Potent Chemicals Using Real-World Exposure Concentrations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bopp, S.K.; Barouki, R.; Brack, W.; Costa, S.D.; Dorne, J.-L.C.; Drakvik, P.E.; Faust, M.; Karjalainen, T.K.; Kephalopoulos, S.; van Klaveren, J.; et al. Current EU research activities on combined exposure to multiple chemicals. Environ. Int. 2018, 120, 544–562. [Google Scholar] [CrossRef] [PubMed]
- Dixon, H.M.; Armstrong, G.; Barton, M.; Bergmann, A.J.; Bondy, M.; Halbleib, M.L.; Hamilton, W.; Haynes, E.; Herbstman, J.; Hoffman, P.; et al. Discovery of common chemical exposures across three continents using silicone wristbands. R. Soc. Open Sci. 2019, 6, 181836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmann, A.J.; Points, G.L.; Scott, R.P.; Wilson, G.; Anderson, K.A. Development of quantitative screen for 1550 chemicals with GC-MS. Anal. Bioanal. Chem. 2018, 410, 3101–3110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, K.A.; Wambaugh, J.; Grulke, C.M.; Dionisio, K.; Isaacs, K. High-throughput screening of chemicals as functional substitutes using structure-based classification models. Green Chem. 2017, 19, 1063–1074. [Google Scholar] [CrossRef]
- Schettler, T. Human exposure to phthalates via consumer products. Int. J. Androl. 2006, 29, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Berger, K.P.; Kogut, K.R.; Bradman, A.; She, J.; Gavin, Q.; Zahedi, R.; Parra, K.L.; Harley, K.G. Personal care product use as a predictor of urinary concentrations of certain phthalates, parabens, and phenols in the HERMOSA study. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 21–32. [Google Scholar] [CrossRef]
- Homem, V.; Silva, E.; Alves, A.; Santos, L. Scented traces–Dermal exposure of synthetic musk fragrances in personal care products and environmental input assessment. Chemosphere 2015, 139, 276–287. [Google Scholar] [CrossRef] [Green Version]
- DEET. United States Environmental Protection Agency. Available online: https://www.epa.gov/insect-repellents/deet (accessed on 30 November 2021).
- Estill, C.F.; Mayer, A.; Slone, J.; Chen, I.-C.; Zhou, M.; La Guardia, M.J.; Jayatilaka, N.; Ospina, M.; Calafat, A. Assessment of triphenyl phosphate (TPhP) exposure to nail salon workers by air, hand wipe, and urine analysis. Int. J. Hyg. Environ. Health 2021, 231, 113630. [Google Scholar] [CrossRef]
- Estill, C.F.; Slone, J.; Mayer, A.; Chen, I.-C.; La Guardia, M.J. Worker exposure to flame retardants in manufacturing, construction and service industries. Environ. Int. 2020, 135, 105349. [Google Scholar] [CrossRef]
- Schindler, B.K.; Koslitz, S.; Weiss, T.; Broding, H.C.; Brüning, T.; Bünger, J. Exposure of aircraft maintenance technicians to organophosphates from hydraulic fluids and turbine oils: A pilot study. Int. J. Hyg. Environ. Health 2014, 217, 34–37. [Google Scholar] [CrossRef]
- Carlin, D.J.; Rider, C.; Woychik, R.; Birnbaum, L. Unraveling the Health Effects of Environmental Mixtures: An NIEHS Priority. Environ. Health Perspect. 2013, 121, A6–A8. [Google Scholar] [CrossRef] [PubMed]
- Feron, V.; Groten, J. Toxicological evaluation of chemical mixtures. Food Chem. Toxicol. 2002, 40, 825–839. [Google Scholar] [CrossRef]
- Gennings, C.; Carter, W.H.; Carney, E.W.; Charles, G.D.; Gollapudi, B.B.; Carchman, R.A. A novel flexible approach for evaluating fixed ratio mixtures of full and partial agonists. Toxicol. Sci. 2004, 80, 134–150. [Google Scholar] [CrossRef] [Green Version]
- Drescher, K.; Bödeker, W. Assessment of the Combined Effects of Substances: The Relationship between Concentration Addition and Independent Action. Biometrics 1995, 51, 716. [Google Scholar] [CrossRef]
- Rider, C.; Leblanc, G.A. An integrated addition and interaction model for assessing toxicity of chemical mixtures. Toxicol. Sci. 2005, 87, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Olmstead, A.W.; Leblanc, G.A. Toxicity assessment of environmentally relevant pollutant mixtures using a heuristic model. Integr. Environ. Assess. Manag. 2005, 1, 114–122. [Google Scholar] [CrossRef]
- Kakaley, E.M.; Cardon, M.C.; Gray, L.E.; Hartig, P.C.; Wilson, V.S. Generalized concentration addition model predicts glucocorticoid activity bioassay responses to environmentally detected receptor-ligand mixtures. Toxicol. Sci. 2019, 168, 252–263. [Google Scholar] [CrossRef]
- Gregorio, V.; Chèvre, N.; Junghans, M. Critical issues in using the common mixture toxicity models concentration addition or response addition on species sensitivity distributions: A theoretical approach. Environ. Toxicol. Chem. 2013, 32, 2387–2395. [Google Scholar] [CrossRef]
- Junghans, M.; Backhaus, T.; Faust, M.; Scholze, M.; Grimme, L. Application and validation of approaches for the predictive hazard assessment of realistic pesticide mixtures. Aquat. Toxicol. 2006, 76, 93–110. [Google Scholar] [CrossRef]
- US EPA. Supplementary Guidance for Conducting Health Risk Assessment of Chemical; Risk Assessment Forum Technical Panel; US EPA: Washington, DC, USA, 2000. [Google Scholar]
- Richard, A.M.; Judson, R.S.; Houck, K.; Grulke, C.; Volarath, P.; Thillainadarajah, I.; Yang, C.; Rathman, J.F.; Martin, M.T.; Wambaugh, J.; et al. ToxCast chemical landscape: Paving the road to 21st century toxicology. Chem. Res. Toxicol. 2016, 29, 1225–1251. [Google Scholar] [CrossRef] [Green Version]
- Hasspieler, B.M.; Haffner, G.D.; Adeli, K. In vitro toxicological methods for environmental health testing. Rev. Environ. Health 1996, 11, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.J.; Huang, R.; Austin, C.P.; Xia, M. The future of toxicity testing: A focus on in vitro methods using a quantitative high-throughput screening platform. Drug Discov. Today 2010, 15, 997–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anadón, A.; Martínez, M.A.; Castellano, V.; Martínez-Larrañaga, M.R. The role of in vitro methods as alternatives to animals in toxicity testing. Expert Opin. Drug Metab. Toxicol. 2014, 10, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.J.; Grulke, C.M.; Edwards, J.; McEachran, A.D.; Mansouri, K.; Baker, N.C.; Patlewicz, G.; Shah, I.; Wambaugh, J.F.; Judson, R.S.; et al. The CompTox chemistry dashboard: A community data resource for environmental chemistry. J. Cheminform. 2017, 9, 61. [Google Scholar] [CrossRef]
- Weschler, C.J.; Nazaroff, W. Semivolatile organic compounds in indoor environments. Atmos. Environ. 2008, 42, 9018–9040. [Google Scholar] [CrossRef]
- Bugel, S.M.; Tanguay, R.L.; Planchart, A. Zebrafish: A marvel of high-throughput biology for 21st century toxicology. Curr. Environ. Health Rep. 2014, 1, 341–352. [Google Scholar] [CrossRef] [Green Version]
- Gehrig, J.; Pandey, G.; Westhoff, J.H. Zebrafish as a model for drug screening in genetic kidney diseases. Front. Pediatr. 2018, 6, 183. [Google Scholar] [CrossRef]
- Kaufman, C.K.; White, R.M.; Zon, L. Chemical genetic screening in the zebrafish embryo. Nat. Protoc. 2009, 4, 1422–1432. [Google Scholar] [CrossRef] [Green Version]
- Chahardehi, A.M.; Arsad, H.; Lim, V. Zebrafish as a successful animal model for screening toxicity of medicinal plants. Plants 2020, 9, 1345. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, B.A.; Wambaugh, J.F.; Allen, B.; Ferguson, S.S.; Sochaski, M.A.; Setzer, R.W.; Houck, K.A.; Strope, C.L.; Cantwell, K.; Judson, R.S.; et al. Incorporating high-throughput exposure predictions with dosimetry-adjusted in vitro bioactivity to inform chemical toxicity testing. Toxicol. Sci. 2015, 148, 121–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wetmore, B.A.; Wambaugh, J.; Ferguson, S.S.; Sochaski, M.A.; Rotroff, D.; Freeman, K.; Clewell, H.J.; Dix, D.J.; Andersen, M.; Houck, K.; et al. Integration of dosimetry, exposure, and high-throughput screening data in chemical toxicity assessment. Toxicol. Sci. 2011, 125, 157–174. [Google Scholar] [CrossRef] [Green Version]
- Nel, A.; Xia, T.; Meng, H.; Wang, X.; Lin, S.; Ji, Z.; Zhang, H. Nanomaterial toxicity testing in the 21st century: Use of a predictive toxicological approach and high-throughput screening. Acc. Chem. Res. 2013, 46, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Schoonen, W.G.E.J.; Westerink, W.M.A.; Horbach, G.J. High-throughput screening for analysis of in vitro toxicity. Exp. Suppl. 2009, 99, 401–452. [Google Scholar] [CrossRef]
- Fernandes, P.B. Technological advances in high-throughput screening. Curr. Opin. Chem. Biol. 1998, 2, 597–603. [Google Scholar] [CrossRef]
- Kent, M.L.; Buchner, C.; Watral, V.G.; Sanders, J.; Ladu, J.; Peterson, T.S.; Tanguay, R.L. Development and maintenance of a specific pathogen-free (SPF) zebrafish research facility for Pseudoloma neurophilia. Dis. Aquat. Org. 2011, 95, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Barton, C.L.; Johnson, E.W.; Tanguay, R.L. Facility Design and health management program at the sinnhuber aquatic research laboratory. Zebrafish 2016, 13, S39–S43. [Google Scholar] [CrossRef] [Green Version]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish. 2000. Available online: http://zfin.org/zf_info/zfbook/zfbk.html (accessed on 30 November 2021).
- Mandrell, D.; Truong, L.; Jephson, C.; Sarker, M.R.; Moore, A.; Lang, C.; Simonich, M.T.; Tanguay, R.L. Automated zebrafish chorion removal and single embryo placement: Optimizing throughput of zebrafish developmental toxicity screens. J. Lab. Autom. 2012, 17, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Truong, L.; Reif, D.M.; Mary, L.S.; Geier, M.C.; Truong, H.D.; Tanguay, R.L. Multidimensional in vivo hazard assessment using zebrafish. Toxicol. Sci. 2014, 137, 212–233. [Google Scholar] [CrossRef] [Green Version]
- Truong, L.; Marvel, S.; Reif, D.M.; Thomas, D.G.; Pande, P.; Dasgupta, S.; Simonich, M.T.; Waters, K.M.; Tanguay, R.L. The multi-dimensional embryonic zebrafish platform predicts flame retardant bioactivity. Reprod. Toxicol. 2020, 96, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Reif, D.M.; Truong, L.; Mandrell, D.; Marvel, S.; Zhang, G.; Tanguay, R.L. High-throughput characterization of chemical-associated embryonic behavioral changes predicts teratogenic outcomes. Arch. Toxicol. 2016, 90, 1459–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokel, D.; Dunn, T.W.; Ahrens, M.B.; Alshut, R.; Cheung, C.Y.J.; Saint-Amant, L.; Bruni, G.; Mateus, R.; van Ham, T.; Shiraki, T.; et al. Identification of nonvisual photomotor response cells in the vertebrate hindbrain. J. Neurosci. 2013, 33, 3834–3843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Truong, L.; Tanguay, R.L.; Reif, D.M. A new statistical approach to characterize chemical-elicited behavioral effects in high-throughput studies using zebrafish. PLoS ONE 2017, 12, e0169408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truong, L.; Bugel, S.M.; Chlebowski, A.; Usenko, C.Y.; Simonich, M.T.; Simonich, S.L.M.; Tanguay, R.L. Optimizing multi-dimensional high throughput screening using zebrafish. Reprod. Toxicol. 2016, 65, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A. Ggcorrplot. 2019. Available online: https://CRAN.R-project.org/package=ggcorrplot (accessed on 11 November 2019).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis. Available online: https://ggplot2.tidyverse.org (accessed on 11 November 2019).
- Gosline, S.; Kim, D.; Pande, P.; Thomas, D.G.; Truong, L.; Hoffman, P.; Barton, M.; Loftus, J.; Moran, A.; Hampton, S.; et al. The Superfund Research Program Analytics Portal: Linking environmental chemical exposure to biological phenotypes. Sci. Data 2022. in review. [Google Scholar]
- US EPA. Benchmark Dose Software (BMDS) 3.2; US EPA: Washington, DC, USA, 2020. [Google Scholar]
- Ayuk-Takem, L.; Amissah, F.; Aguilar, B.J.; Lamango, N.S. Inhibition of polyisoprenylated methylated protein methyl esterase by synthetic musks induces cell degeneration. Environ. Toxicol. 2012, 29, 466–477. [Google Scholar] [CrossRef] [Green Version]
- Ham, J.; Lim, W.; Whang, K.-Y.; Song, G. Butylated hydroxytoluene induces dysregulation of calcium homeostasis and endoplasmic reticulum stress resulting in mouse Leydig cell death. Environ. Pollut. 2019, 256, 113421. [Google Scholar] [CrossRef]
- OSPAR. The Ospar List of Chemicals for Priority Action Suggestions for Future Actions; OSPAR: London, UK, 2019. [Google Scholar]
- Ehiguese, F.O.; Rodgers, M.L.; Araújo, C.V.; Griffitt, R.J.; Martin-Diaz, M.L. Galaxolide and tonalide modulate neuroendocrine activity in marine species from two taxonomic groups. Environ. Res. 2021, 196, 110960. [Google Scholar] [CrossRef]
- Liang, X.; Zhao, Y.; Liu, W.; Li, Z.; Souders, C.L.; Martyniuk, C.J. Butylated hydroxytoluene induces hyperactivity and alters dopamine-related gene expression in larval zebrafish (Danio rerio). Environ. Pollut. 2020, 257, 113624. [Google Scholar] [CrossRef]
- Yang, X.; Sun, Z.; Wang, W.; Zhou, Q.; Shi, G.; Wei, F.; Jiang, G. Developmental toxicity of synthetic phenolic antioxidants to the early life stage of zebrafish. Sci. Total Environ. 2018, 643, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Yamaki, K.; Taneda, S.; Yanagisawa, R.; Inoue, K.-I.; Takano, H.; Yoshino, S. Enhancement of allergic responses in vivo and in vitro by butylated hydroxytoluene. Toxicol. Appl. Pharmacol. 2007, 223, 164–172. [Google Scholar] [CrossRef] [PubMed]
- ECHA. 2,6-di-tert-butyl-p-cresol Substance Infocard. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.004.439 (accessed on 20 November 2021).
- Sarmah, R.; Bhagabati, S.K.; Dutta, R.; Nath, D.; Pokhrel, H.; Mudoi, L.P.; Sarmah, N.; Sarma, J.; Ahmed, A.M.; Nath, R.J.; et al. Toxicity of a synthetic phenolic antioxidant, butyl hydroxytoluene (BHT), in vertebrate model zebrafish embryo (Danio rerio). Aquac. Res. 2020, 51, 3839–3846. [Google Scholar] [CrossRef]
- Wilson, L.B.; Truong, L.; Simonich, M.T.; Tanguay, R.L. Systematic assessment of exposure variations on observed bioactivity in zebrafish chemical screening. Toxics 2020, 8, 87. [Google Scholar] [CrossRef]
- Panzica-Kelly, J.M.; Zhang, C.X.; Augustine-Rauch, K.A. Optimization and performance assessment of the chorion-off [dechorinated] zebrafish developmental toxicity assay. Toxicol. Sci. 2015, 146, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Tran, C.M.; Lee, H.; Lee, B.; Ra, J.-S.; Kim, K.-T. Effects of the chorion on the developmental toxicity of organophosphate esters in zebrafish embryos. J. Hazard. Mater. 2021, 401, 123389. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.-Y.; Hamid, N.; Ren, Y.-W.; Pei, D.-S. Effects of phthalate acid esters on zebrafish larvae: Development and skeletal morphogenesis. Chemosphere 2020, 246, 125808. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shao, X.; Zhang, Z.; Zou, Y.; Chen, Y.; Han, S.; Wang, S.; Wu, X.; Yang, L.; Chen, Z. Effects of di-n-butyl phthalate and diethyl phthalate on acetylcholinesterase activity and neurotoxicity related gene expression in embryonic zebrafish. Bull. Environ. Contam. Toxicol. 2013, 91, 635–639. [Google Scholar] [CrossRef]
- Blessinger, T.D.; Euling, S.Y.; Wang, L.; Hogan, K.A.; Cai, C.; Klinefelter, G.; Saillenfait, A.-M. Ordinal dose-response modeling approach for the phthalate syndrome. Environ. Int. 2020, 134, 105287. [Google Scholar] [CrossRef]
- Wilson, V.S.; Howdeshell, K.L.; Lambright, C.S.; Furr, J.; Gray, L.E. Differential expression of the phthalate syndrome in male Sprague–Dawley and Wistar rats after in utero DEHP exposure. Toxicol. Lett. 2007, 170, 177–184. [Google Scholar] [CrossRef]
- Welsh, M.; Saunders, P.T.; Fisken, M.; Scott, H.M.; Hutchison, G.R.; Smith, L.B.; Sharpe, R.M. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J. Clin. Investig. 2008, 118, 1479–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, P.M. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int. J. Androl. 2006, 29, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Swan, S.H. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ. Res. 2008, 108, 177–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, M.; Chen, Y. Zebrafish as an emerging model to study gonad development. Comput. Struct. Biotechnol. J. 2020, 18, 2373–2380. [Google Scholar] [CrossRef]
- Kim, S.; Jung, J.; Lee, I.; Jung, D.; Youn, H.; Choi, K. Thyroid disruption by triphenyl phosphate, an organophosphate flame retardant, in zebrafish (Danio rerio) embryos/larvae, and in GH3 and FRTL-5 cell lines. Aquat. Toxicol. 2015, 160, 188–196. [Google Scholar] [CrossRef]
- Cano-Sancho, G.; Smith, A.; La Merrill, M.A. Triphenyl phosphate enhances adipogenic differentiation, glucose uptake and lipolysis via endocrine and noradrenergic mechanisms. Toxicol. Vitr. 2017, 40, 280–288. [Google Scholar] [CrossRef]
- Charles, A.K.; Darbre, P.D. Oestrogenic activity of benzyl salicylate, benzyl benzoate and butylphenylmethylpropional (Lilial) in MCF7 human breast cancer cells in vitro. J. Appl. Toxicol. 2009, 29, 422–434. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Park, Y.J.; Moon, H.-B.; Choi, K. Thyroid Hormone-disrupting potentials of major benzophenones in two cell lines (GH3 and FRTL-5) and embryo-larval zebrafish. Environ. Sci. Technol. 2018, 52, 8858–8865. [Google Scholar] [CrossRef]
- Schreurs, R.H.M.M.; Sonneveld, E.; Jansen, J.H.J.; Seinen, W.; van der Burg, B. Interaction of Polycyclic musks and UV filters with the estrogen receptor (ER), androgen receptor (AR), and progesterone receptor (PR) in reporter gene bioassays. Toxicol. Sci. 2004, 83, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Schreurs, R.H.M.M.; Quaedackers, M.E.; Seinen, W.; van der Burg, B. Transcriptional activation of estrogen receptor ERalpha and ERbeta by polycyclic musks is cell type dependent. Toxicol. Appl. Pharmacol. 2002, 183, 1–9. [Google Scholar] [CrossRef]
- Cavanagh, J.-A.E.; Trought, K.; Mitchell, C.; Northcott, G.; Tremblay, L.A. Assessment of endocrine disruption and oxidative potential of bisphenol-A, triclosan, nonylphenol, diethylhexyl phthalate, galaxolide, and carbamazepine, common contaminants of municipal biosolids. Toxicol. Vitr. 2018, 48, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Seralini, G.-E.; Jungers, G. Endocrine disruptors also function as nervous disruptors and can be renamed endocrine and nervous disruptors (ENDs). Toxicol. Rep. 2021, 8, 1538–1557. [Google Scholar] [CrossRef] [PubMed]
- Kajta, M.; Wójtowicz, A.K. Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacol. Rep. 2013, 65, 1632–1639. [Google Scholar] [CrossRef]
- Casals-Casas, C.; Feige, J.N.; Desvergne, B. Interference of pollutants with PPARs: Endocrine disruption meets metabolism. Int. J. Obes. 2008, 32, S53–S61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casals-Casas, C.; Desvergne, B. Endocrine disruptors: From endocrine to metabolic disruption. Annu. Rev. Physiol. 2011, 73, 135–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milla, S.; Depiereux, S.; Kestemont, P. The effects of estrogenic and androgenic endocrine disruptors on the immune system of fish: A review. Ecotoxicology 2011, 20, 305–319. [Google Scholar] [CrossRef]
- Bansal, A.; Henao-Mejia, J.; Simmons, R.A. Immune system: An Emerging player in mediating effects of endocrine dis-ruptors on metabolic health. Endocrinology 2018, 159, 32–45. [Google Scholar] [CrossRef]
- Selderslaghs, I.W.; Hooyberghs, J.; De Coen, W.; Witters, H.E. Locomotor activity in zebrafish embryos: A new method to assess developmental neurotoxicity. Neurotox. Teratol. 2010, 32, 460–471. [Google Scholar] [CrossRef]
- Kokel, D.; Bryan, J.; Laggner, C.; White, R.; Cheung, C.Y.J.; Mateus, R.; Healey, D.; Kim, S.; Werdich, A.A.; Haggarty, S.J.; et al. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat. Chem. Biol. 2010, 6, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Api, A.M.; San, R.H. Genotoxicity tests with 6-acetyl-1,1,2,4,4,7-hexamethyltetralineand 1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethylcyclopenta-g-2-benzopyran. Mutat. Res. 1999, 446, 67–81. [Google Scholar] [CrossRef]
- Reiner, J.L.; Kannan, K. A survey of polycyclic musks in selected household commodities from the United States. Chemosphere 2006, 62, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, W.; Zhou, Q.; Zhou, Q.; Zhang, Y.; Zhu, L. Polycyclic musks in the environment: A review of their concentrations and distribution, ecological effects and behavior, current concerns and future prospects. Crit. Rev. Environ. Sci. Technol. 2021, 51, 323–377. [Google Scholar] [CrossRef]
- Lange, C.; Kuch, B.; Metzger, J.W. Occurrence and fate of synthetic musk fragrances in a small German river. J. Hazard. Mater. 2015, 282, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Schmidtkunz, C.; Küpper, K.; Weber, T.; Leng, G.; Kolossa-Gehring, M. A biomonitoring study assessing the exposure of young German adults to butylated hydroxytoluene (BHT). Int. J. Hyg. Environ. Health 2020, 228, 113541. [Google Scholar] [CrossRef]
- Zhang, R.; Li, J.; Cui, X. Tissue distribution, excretion, and metabolism of 2,6-di-tert-butyl-hydroxytoluene in mice. Sci. Total Environ. 2020, 739, 139862. [Google Scholar] [CrossRef] [PubMed]
- Hutter, H.-P.; Wallner, P.; Hartl, W.; Uhl, M.; Lorbeer, G.; Gminski, R.; Mersch-Sundermann, V.; Kundi, M. Higher blood concentrations of synthetic musks in women above fifty years than in younger women. Int. J. Hyg. Environ. Health 2010, 213, 124–130. [Google Scholar] [CrossRef]
- Liu, R.; Mabury, S.A. Synthetic phenolic antioxidants: A Review of environmental occurrence, fate, human exposure, and toxicity. Environ. Sci. Technol. 2020, 54, 11706–11719. [Google Scholar] [CrossRef]
- Wang, W.; Kannan, K. Quantitative identification of and exposure to synthetic phenolic antioxidants, including butylated hydroxytoluene, in urine. Environ. Int. 2019, 128, 24–29. [Google Scholar] [CrossRef]
- Liu, R.; Mabury, S.A. Synthetic phenolic antioxidants and transformation products in human sera from United States donors. Environ. Sci. Technol. Lett. 2018, 5, 419–423. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, S.; Qiu, T.; Hu, X.; Lu, Y.; Du, P.; Xie, L.; Yang, Y.; Zhao, F.; Zhu, Y.; et al. Investigation of phthalate metabolites in urine and daily phthalate intakes among three age groups in Beijing, China. Environ. Pollut. 2020, 260, 114005. [Google Scholar] [CrossRef]
- Romero-Franco, M.; Hernández-Ramírez, R.U.; Calafat, A.M.; Cebrián, M.E.; Needham, L.L.; Teitelbaum, S.; Wolff, M.S.; López-Carrillo, L. Personal care product use and urinary levels of phthalate metabolites in Mexican women. Environ. Int. 2011, 37, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Just, A.C.; Williams, P.L.; Smith, K.W.; Calafat, A.M.; Hauser, R. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 459–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parlett, L.E.; Calafat, A.M.; Swan, S.H. Women’s exposure to phthalates in relation to use of personal care products. J. Expo. Sci. Environ. Epidemiol. 2012, 23, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Langer, S.; Bekö, G.; Weschler, C.J.; Brive, L.M.; Toftum, J.; Callesen, M.; Clausen, G. Phthalate metabolites in urine samples from Danish children and correlations with phthalates in dust samples from their homes and daycare centers. Int. J. Hyg. Environ. Health 2014, 217, 78–87. [Google Scholar] [CrossRef] [PubMed]

| Chemical Name | Abbreviated Name | Frequency of Detection (n = 262) | Primary Chemical Structure Classification | Predicted Functional Use ** |
|---|---|---|---|---|
| Benzophenone | BP | 64 | Benzophenone | Photoinitiator, UV absorber, crosslinker, heat stabilizer, catalyst |
| Benzyl butyl phthalate | BBP | 66 | Phthalate | Fragrance, preservative, catalyst, flavorant |
| Benzyl salicylate | BS | 73 | Salicylic acid benzyl ester | UV absorber, preservative, fragrance, antioxidant, hair dye, skin conditioner, flavorant |
| Bis(2-ethylhexyl) phthalate | DEHP | 84 | Phthalate | Fragrance, emollient, preservative, UV absorber, catalyst |
| Butylated hydroxytoluene | BHT | 79 | Phenol | Antioxidant, UV absorber, heat stabilizer, fragrance, preservative, catalyst |
| Diethyl phthalate | DEP | 95 | Phthalate | Fragrance, preservative, UV absorber, catalyst, crosslinker, skin conditioner |
| Diisobutyl phthalate | DIBP | 85 | Phthalate | Fragrance, preservative, crosslinker |
| Di-n-butyl phthalate | DBP | 93 | Phthalate | Fragrance, preservative, UV absorber, catalyst, emollient |
| Dinonyl phthalate | DNP | 82 | Phthalate | Fragrance, emollient, preservative, skin conditioner |
| Galaxolide | HHCB | 94 | Polycyclic Musk | Fragrance |
| Lilial | Lilial | 75 | Aromatic Aldehyde | Fragrance |
| N,N-diethyl-m-toluamide | DEET | 52 | Monocarboxylic acid amide | Skin protectant, catalyst, antimicrobial, colorant |
| Tonalide | AHTN | 76 | Polycyclic Musk | Fragrance |
| Triphenyl phosphate | TPP | 52 | Organophosphate | Flame retardant, catalyst, buffer |
| Chemical Name | Abbreviated Name | CAS Number | Original Supplier * | Purity (%) |
|---|---|---|---|---|
| Benzophenone | BP | 119-61-9 | Sigma Aldrich | 99 |
| Butyl benzyl phthalate | BBP | 85-68-7 | Sigma Aldrich | 98 |
| Benzyl salicylate | BS | 118-58-1 | AccuStandard | 100 |
| Bis(2-ethylhexyl) phthalate | DEHP | 117-81-7 | AccuStandard | 99.6 |
| Butylated hydroxytoluene | BHT | 128-37-0 | AccuStandard | 99.8 |
| Diethyl phthalate | DEP | 84-66-2 | AccuStandard | 97.0 |
| Diisobutyl phthalate | DIBP | 84-69-5 | AccuStandard | 100 |
| Di-n-butyl phthalate | DBP | 84-74-2 | CDN Isotopes | 99.8 |
| Di-n-nonyl phthalate | DNP | 84-76-4 | Chem Service Inc. | 99.5 |
| Galaxolide | HHCB | 1222-05-5 | Sigma Aldrich | 87.5 |
| Lilial | Lilial | 80-54-6 | Sigma Aldrich | 98.4 |
| N,N-diethyl-m-toluamide | DEET | 134-62-3 | AccuStandard | 97.2 |
| Tonalide | AHTN | 21145-77-7 | Sigma Aldrich | 97.4 |
| Triphenyl phosphate | TPP | 115-86-6 | Sigma Aldrich | 99.8 |
| Chemical | NHBE Exposure Concentrations (µM) |
|---|---|
| * AHTN | 10, 25, 50, 75, 100, 200, 400 |
| BBP | 10, 25, 50, 75, 100, 200, 400 |
| BP | 10, 25, 50, 75, 100, 200, 400 |
| BS | 10, 25, 50, 75, 100, 200, 400 |
| * BHT | 10, 25, 50, 75, 100, 200, 400 |
| DBP | 10, 25, 50, 75, 100, 200, 400 |
| DEET | 10, 25, 50, 75, 100, 200, 400 |
| DEHP | 10, 25, 50, 75, 100, 200, 400 |
| DEP | 10, 25, 50, 75, 100, 200, 400 |
| DIBP | 10, 25, 50, 75, 100, 200, 400 |
| DNP | 10, 25, 50, 75, 100, 200, 400 |
| * HHCB | 10, 25, 50, 75, 100, 200, 400 |
| Lilial | 10, 25, 50, 75, 100, 200, 400 |
| TPP | 10, 25, 50, 75, 100, 200, 400 |
| G14 Mix | 28, 70, 140, 210, 280 |
| NHBE Equi-Mix | 30, 75, 150, 225, 300 |
| NHBE BMC Mix | 11.5, 23, 57.6, 92.2, 115.2, 172.8, 230.4 |
| Chemical | Definitive Zebrafish Exposure Concentrations (µM) |
|---|---|
| AHTN | 0, 2, 10, 30, 50, 60, 75, 100 |
| * BBP | 0, 2.25, 3, 3.5, 4, 4.5, 5, 8 |
| BP | 0, 1, 2.54, 6.45, 16.4, 35, 74.8, 100 |
| BS | 0, 5, 10, 14, 18, 22, 30, 50 |
| BHT | 0, 1, 2.54, 6.45, 16.4, 35, 74.8, 100 |
| * DBP | 0, 1, 2, 3, 4, 5, 10, 20 |
| DEET | 0, 1, 2.54, 6.45, 16.4, 35, 74.8, 100 |
| DEHP | 0, 1, 3, 5, 10, 20, 40, 80 |
| DEP | 0, 1, 2.54, 6.45, 16.4, 35, 74.8, 100 |
| * DIBP | 0, 2, 4, 5, 6, 8, 10, 15 |
| DNP | 0, 2, 10, 30, 50, 70, 80, 100 |
| HHCB | 0, 10, 14, 16, 20, 24, 28, 32 |
| Lilial | 0, 20, 40, 60, 70, 80, 90, 100 |
| TPP | 0, 2, 3, 4, 5, 6, 8, 10 |
| G14 Mix | 0, 28, 56, 63, 70, 77, 84, 112 |
| ZF BMC Mix | 0, 0.51, 1.02, 2.04, 4.08, 6.13, 8.16, 10.2 |
| ZF Equi-Mix | 0, 1.5, 3, 3.6, 4.2, 5.1, 6, 7.5 |
| Zebrafish Morphological Endpoints | |
|---|---|
| 24 hpf | mortality, delayed progression, spontaneous movement |
| 120 hpf | mortality, edemas, bent axis, touch response, and craniofacial, muscular/cardiovascular, lower trunk, brain, skin, notochord malformations |
| Mixture Name | Model | Chemical Components | Concentration Determination |
|---|---|---|---|
| G14 Mix | NHBE and Zebrafish | All G14 chemicals | Equimolar |
| NHBE Equi-Mix | NHBE | AHTN, BHT, HHCB | Equimolar |
| NHBE BMC Mix | NHBE | AHTN, BHT, HHCB | Anchored to individual BMC50 |
| ZF Equi-Mix | Zebrafish | BBP, DBP, DIBP | Equimolar |
| ZF BMC Mix | Zebrafish | BBP, DBP, DIBP | Anchored to individual BMC50 |
| Model | Chemical | BMC10 (µM) | BMC50 (µM) | Regression Model |
|---|---|---|---|---|
| NHBE | HHCB | 3.61 | 42.2 | Gamma |
| NHBE | AHTN | 33.5 | 84.9 | Gamma |
| NHBE | BHT | 45.9 | 103 | Logistic |
| NHBE | G14 Mix | 1.25 | 47.7 | Log Logistic |
| NHBE | NHBE Equi-Mix | 8.29 | 54.0 | Weibull |
| NHBE | NHBE BMC Mix | 47.8 | 94.9 | Log Logistic |
| Zebrafish | BBP | 3.29 | 4.28 | Log Logistic |
| Zebrafish | DBP | 2.80 | 4.27 | Log Logistic |
| Zebrafish | DIBP | 3.48 | 5.32 | Logistic |
| Zebrafish | G14 Mix | 46.2 | 55.4 | Log Logistic |
| Zebrafish | ZF Equi-Mix | 3.58 | 5.36 | Gamma |
| Zebrafish | ZF BMC Mix | 3.58 | 6.41 | Weibull |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, B.N.; Wilson, L.B.; Kim, D.N.; Pande, P.; Anderson, K.A.; Tilton, S.C.; Tanguay, R.L. A Comparative Multi-System Approach to Characterizing Bioactivity of Commonly Occurring Chemicals. Int. J. Environ. Res. Public Health 2022, 19, 3829. https://doi.org/10.3390/ijerph19073829
Rivera BN, Wilson LB, Kim DN, Pande P, Anderson KA, Tilton SC, Tanguay RL. A Comparative Multi-System Approach to Characterizing Bioactivity of Commonly Occurring Chemicals. International Journal of Environmental Research and Public Health. 2022; 19(7):3829. https://doi.org/10.3390/ijerph19073829
Chicago/Turabian StyleRivera, Brianna N., Lindsay B. Wilson, Doo Nam Kim, Paritosh Pande, Kim A. Anderson, Susan C. Tilton, and Robyn L. Tanguay. 2022. "A Comparative Multi-System Approach to Characterizing Bioactivity of Commonly Occurring Chemicals" International Journal of Environmental Research and Public Health 19, no. 7: 3829. https://doi.org/10.3390/ijerph19073829
APA StyleRivera, B. N., Wilson, L. B., Kim, D. N., Pande, P., Anderson, K. A., Tilton, S. C., & Tanguay, R. L. (2022). A Comparative Multi-System Approach to Characterizing Bioactivity of Commonly Occurring Chemicals. International Journal of Environmental Research and Public Health, 19(7), 3829. https://doi.org/10.3390/ijerph19073829








