The Effect of Deep Micro Vibrotactile Stimulation on Cognitive Function of Mild Cognitive Impairment and Mild Dementia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure for the Assessment
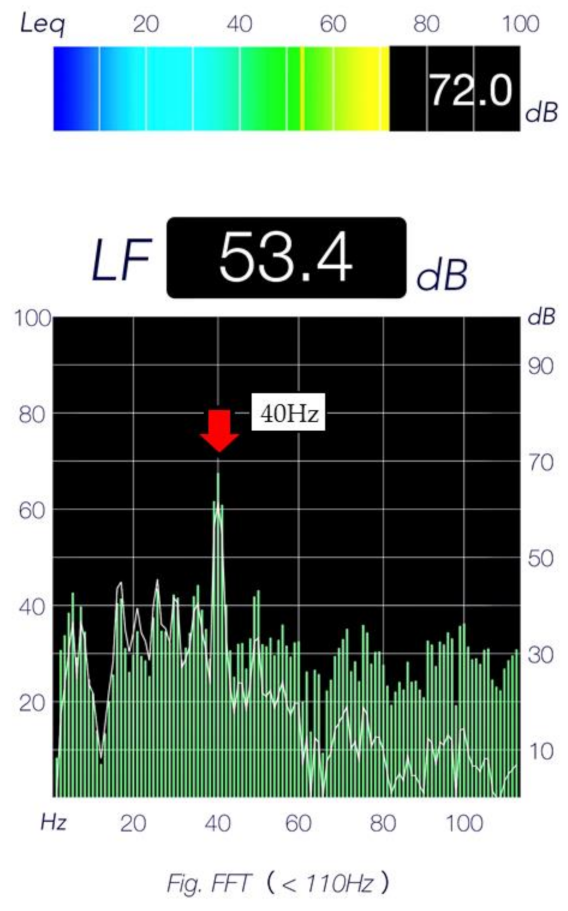
2.3. Treatment
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lancioni, G.E.; Perilli, V.; Singh, N.N.; O’Reilly, M.F.; Cassano, G. A man with severe Alzheimer’s disease stops wandering during a picture colouring activity. Dev. Neurorehabilit. 2011, 14, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Perilli, V.; Lancioni, G.E.; Singh, N.N.; O’Reilly, M.F.; Sigafoos, J.; Cassano, G.; Noemi, C.; Katia, P.; Mauro, G.M.; Doretta, O. Persons with Alzheimer’s disease make phone calls independently using a computer-aided telephone system. Res. Dev. Disabil. 2012, 33, 1014–1020. [Google Scholar] [CrossRef]
- Passini, R.; Pigot, H.; Rainville, C.; Te’treault, M. Wayfinding in a nursing home for advanced dementia of the Alzheimer’s type. Environ. Behav. 2000, 32, 684–710. [Google Scholar] [CrossRef]
- Rainville, C.; Passini, R.; Marchand, N. A multiple case study of wayfinding in dementia of the Alzheimer’s type: Decision making. Aging Neuropsychol. Cogn. 2001, 8, 54–71. [Google Scholar] [CrossRef]
- Verret, L.; Mann, E.O.; Hang, G.B.; Barth, A.M.; Cobos, I.; Ho, K.; Nino, D.; Eliezer, M.; Anatol, C.K.; Istavan, M. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 2012, 149, 708–721. [Google Scholar] [CrossRef] [Green Version]
- Palop, J.J.; Chin, J.; Roberson, E.D.; Wang, J.; Thwin, M.T.; Bien-Ly, N.; Yoo, J.; Ho, K.O.; Yu, G.-Q.; Kreitzer, A.; et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 2007, 55, 697–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canter, R.G.; Penney, J.; Tsai, L.H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature 2016, 539, 187–196. [Google Scholar] [CrossRef]
- Bero, A.W.; Yan, P.; Roh, J.H.; Cirrito, J.R.; Stewart, F.R.; Raichle, M.E.; Lee, J.-M.; Holtzman, D.M. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat. Neurosci. 2011, 14, 750–756. [Google Scholar] [CrossRef] [Green Version]
- Iaccarino, H.F.; Singer, A.C.; Martorell, A.J.; Rudenko, A.; Gao, F.; Gillingham, T.Z.; Mathys, H.; Seo, J.; Kritskiy, O.; Abdurrob, F.; et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016, 540, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.; Dolgin, E. Failed Alzheimer’s trial does not kill leading theory of disease. Nat. News 2016, 540, 15–16. [Google Scholar] [CrossRef]
- Ballard, C.; Khan, Z.; Clack, H.; Corbett, A. Nonpharmacological treatment of Alzheimer Disease. Can. J. Psychiatry 2011, 56, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Marla, B.W. Non-Pharmacologic Interventions Persons with Dementia. Mo. Med. 2017, 114, 116–119. [Google Scholar]
- Vieira, M.A.M. Efeitos de um Programa Conjugado da Metodologia de Luz e som e Prática Motriz Complexa em Funções de Memória em Indivíduos Portadores da Doença de Alzheimer Relações Com Hemisfericidade. Master’s Thesis, 151f. Rio de Janeiro Universidade Castelo Branco, Rio de Janeiro City, Brazil, 2008. [Google Scholar]
- Ribary, U.; Ioannidest, A.A.; Singht, K.D.; Hassont, R.; Bolton, P.J.; Lado, F.; Mogilner, A.; Llinás, R. Magnetic fieldtomography of coherent thalamocortical 40-Hz oscillationsin humans. Proc. Natl. Acad. Sci. USA 1991, 88, 11037–11041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jefferys, J.G.R.; Traub, R.D.; Whittington, M.A. Neuronalnetworks for induced ‘40Hz’ Rhythms. Trends Neurosci. 1996, 19, 202–208. [Google Scholar] [CrossRef]
- Clements, C.A.; Ahonen, H.; Evans, M.; Freedman, M.; Bartel, L. Short-Term Effects of Rhythmic Sensory Stimulation in Alzheimer’s Disease: An Exploratory Pilot Study. J. Alzheimer’s Dis. 2016, 52, 651–660. [Google Scholar] [CrossRef]
- Ayuto, K.; Yasuhiro, S.; Yu, K.; Hidetaka, O. Examination of the effe4ct of Deep Micro Vibrotactile stimulation on cognitive function for elderly with Alzheimer’s Disease. Ann. Alzheimer’s Dement. Care 2021, 5, 1–3. [Google Scholar]
- Sainsbury, A.L.; Seebass, G.; Bansal, A.; Young, J.B. Reliability of the Barthel Index when used with older people. Age Ageing 2005, 34, 228–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuidema, S.U.; Buursema, A.L.; Gerritsen, M.G.; Oosterwal, K.C.; Smits, M.M.; Koopmans, R.T.; de Jonghe, J.F. Assessing neuropsychiatric symptoms in nursing home patients with dementia: Reliability and Reliable Change Index of the Neuropsychiatric Inventory and the Cohen-Mansfield Agitation Inventory. Int. J. Geriatr. Psychiatry 2011, 26, 127–134. [Google Scholar] [CrossRef]
- Cummings, J.L.; Mega, M.; Gray, K.; Rosenberg, T.S.; Carusi, D.A.; Gornbein, J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994, 44, 2308–2314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, J.L. The Neuropsychiatric Inventory: Assessing psychopathology in dementia patients. Neurology 1997, 48 (Suppl. 6), S10–S16. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C.; McKeel, D.W.; Fulling, K., Jr.; Torack, R.M.; Berg, L. Validation of clinical diagnostic criteria for Alzheimer’s disease. Ann. Neurol. 1998, 24, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Fillenbaum, G.G.; Peterson, B.; Morris, J.C. Estimating the validity of the clinical dementia rating scale: The CERAD experience. Aging Clin. Exp. Res. 1996, 8, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Marilyn, S.A.; Steven, T.D.; Dennis, D.; Buruno, D.; Howard, H.F. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar]
- Suzuki, Y. Method of Extracting Sensibility from Time Series Data and Converting it to Vibrotactile. J. Robot. Netw. Artif. Life 2020, 7, 142–145. [Google Scholar] [CrossRef]
- Makizako, H.; Shimada, H.; Park, H.; Doi, T.; Yoshida, D.; Uemura, K.; Tsutsumimoto, K.; Suzuki, T. Evaluation of multidimensional neurocognitive function using a tablet personal computer: Test-retest reliability and validity in community-dwelling older adults. Geriatr. Gerontol. 2013, 13, 860–866. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Tombaugh, T.N.; McIntyre, N.J. The mini-mental state examination: A comprehensive review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef]
- Clements, C.A.; Bartel, L.; Ahonen, H.; Freedman, M.; Evans, M. Can rhythmic sensory stimulation decrease cognitive decline in Alzheimer’s disease?: A clinical case study. Music Med. 2017, 9, 174–177. [Google Scholar] [CrossRef]
- Llinas, R.; Ribary, U. 40-Hz oscillation characterizes dream state in humans. Proc. Natl. Acad. Sci. USA 1993, 90, 2078–2081. [Google Scholar] [CrossRef] [Green Version]
- Fries, P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn. Sci. 2005, 9, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.M. Synchronous neural oscillations and cognitive processes. Trends Cogn. Sci. 2003, 7, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Koike, Y.; Iwamoto, S.; Kimata, Y.; Nohno, T.; Hiragami, F.; Kawamura, K.; Kawamura, K.; Numata, K.; Murai, H.; Okisima, K. Low-frequency vibratory sound induces neurite outgrowth in PC12m3 cells in which nerve growth factor-induced neurite outgrowth is impaired. Tissue Cult. Res. Commun. 2004, 23, 81–90. [Google Scholar]
- Patel, A. A new approach to the cognitive neuro-science of melody. In The Cognitive Neuroscience of Music; Peretz, I., Zatorre, R., Eds.; Oxford University Press: Oxford, UK, 2003; pp. 325–345. [Google Scholar]
- Lee, J.; Ryu, S.; Kim, H.J.; Jung, J.; Lee, B.; Kim, T. 40 Hz acoustic stimulation decreases amyloid beta and modulates brain rhythms in a mouse model of Alzheimer’s disease. bioRxiv 2018. [Google Scholar] [CrossRef] [Green Version]
- Martorell, A.J.; Paulson, A.L.; Suk, H.J.; Abdurrob, F.; Drummond, G.T.; Guan, W.; Young, J.Z.; Kim, D.N.; Kritskiy, O.; Barker, S.J. Multi-sensory gamma stimulation ameliorates alzheimer’s Associated pathology and improves cognition. Cell 2019, 177, 256–271. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.; McDermott, B.; Oliveira, B.L.; O’Brien, A.; Coogan, D.; Lang, M.; Moriarty, N.; Dowd, E.; Quinlan, L.; Mc Ginley, B. Gamma band light stimulation in human case studies: Groundwork for potential alzheimer’s disease treatment. J. Alzheimers Dis. 2019, 70, 171–185. [Google Scholar] [CrossRef] [Green Version]
- Ngo, H.V.; Miedema, A.; Faude, I.; Martinetz, T.; Mölle, M.; Born, J. Driving sleep slow oscillations by auditory closed-loop stimulation–A self-limiting process. J. Neurosci. 2015, 35, 6630–6638. [Google Scholar] [CrossRef] [Green Version]
- Leminen, M.M.; Virkkala, J.; Saure, E.; Paajanen, T.; Zee, P.C.; Santostasi, G.; Hublin, C.; Müller, K.; Porkka-Heiskanen, T.; Huotilainen, M. Enhanced memory consolidation via automatic sound stimulation during Non-REM sleep. Sleep 2017, 40, zsx003. [Google Scholar] [CrossRef] [Green Version]
- Brianner, M.B.; Dan, M.; Nihar, P.; Jonathan, E.; Shubir, D.; Matthew, W.; Watson, C.L.; Melanie, S.; Walsh, C.M.; Kramer, J.H. Neuroanatomical substrates of executive functions: Beyond prefrontal structures. Neuropsychologia 2016, 85, 100–109. [Google Scholar]
- Hsu, C.L.; Best, J.R.; Wang, S.; Voss, M.W.; Hsiung, R.G.Y.; MUnkacsy, M.; Cheung, W.; Handy, T.C.; Liu-Ambrose, T. The impact of aerobic exercise on fronto-parietal network Connectivity and its relation to mobility: An exploratory analysis of a 6-month randomized controlled trial. Front. Hum. Neurosci. 2017, 11, 344. [Google Scholar] [CrossRef] [Green Version]
- Wajda, D.A.; Mirelman, A.; Hausdorff, J.M.; Sosnoff, J.J. Intervention modalities for targeting cognitive-motor interference in individuals with neurodegenerative disease: A systematic review. Expert Rev. Neurother. 2017, 17, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.R. Clocking the Mind: Mental Chronometry and Individual Differences; Elsevier Science: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Salthouse, T.A. What cognitive abilities are involved in trail-making performance? Intelligence 2011, 39, 222–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| MCI Group | Mild Dementia Group | |||||
|---|---|---|---|---|---|---|
| n = 17 | n = 18 | |||||
| Mean | SD | Mean | SD | p Value | 95% CI | |
| Age (years) | 84.6 | 9 | 88.3 | 6.4 | 0.171 | 80.23, 92.74 |
| Gender (% female) | 76 | 89 | 0.076 | |||
| Height (cm) | 140.4 | 6.9 | 140.8 | 7.9 | 0.893 | 136.71, 144.46 |
| Weight (kg) | 46.4 | 7.8 | 47.4 | 8.7 | 0.747 | 42.61, 51.20 |
| BMI (kg/m2) | 22.7 | 2.9 | 24.7 | 3.9 | 0.094 | 21.75, 25.76 |
| BI (score) | 68.5 | 16.7 | 58.1 | 26.4 | 0.227 | 54.76, 73.38 |
| NPI-NH (score) | 9.8 | 12.9 | 22.9 | 20 | 0.037 * | 10.85, 20.09 |
| Pre-Test | Post-Test | p Value | 95% CI | |||
|---|---|---|---|---|---|---|
| Mean | Mean | Mean | Mean | |||
| GS (kg) | 12.5 | 6.2 | 13.4 | 5.4 | 0.555 | 8.61, 18.48 |
| UWS (m/s) | 0.69 | 0.18 | 0.61 | 0.29 | 0.582 | 0.44, 0.71 |
| WM (score) | 3.8 | 2.6 | 4.7 | 2.5 | 0.021 * | 2.44, 5.97 |
| TMT-A (s) | 104.1 | 88.4 | 89.1 | 82.5 | 0.761 | 70.78, 123.53 |
| TMT-B (s) | 209.8 | 96.0 | 166.2 | 92.3 | 0.036 * | 125.32, 254.03 |
| SDST (score) | 9.4 | 7.6 | 11.6 | 7.0 | 0.006 ** | 4.46, 16.30 |
| MMSE (score) | 18.4 | 6.4 | 19.2 | 5.8 | 0.425 | 15.81, 21.79 |
| MCI Group | Mild Dementia Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Test | Post-Test | PRE-TEST | Post-Test | |||||||
| Mean | SD | Mean | SD | p Value | Mean | SD | Mean | SD | p Value | |
| GS (kg) | 13.7 | 7.2 | 14.8 | 6.4 | 0.658 | 10.9 | 4.5 | 11.5 | 2.8 | 0.670 |
| UWS (m/s) | 0.69 | 0.2 | 0.56 | 0.34 | 0.876 | 0.6 | 0.24 | 0.66 | 0.26 | 0.436 |
| WM (score) | 4.8 | 2.9 | 5.7 | 3 | 0.259 | 2.8 | 1.9 | 3.6 | 1.4 | 0.015 * |
| TMT-A (s) | 77.5 | 69.3 | 84.3 | 90.1 | 0.733 | 129.2 | 98.6 | 93.8 | 77.0 | 0.377 |
| TMT-B (s) | 219.8 | 102.9 | 167 | 105 | 0.037 * | 200.4 | 90.9 | 165.3 | 81.5 | 0.491 |
| SDST (score) | 11 | 9 | 13.9 | 8.6 | 0.003 ** | 7.8 | 5.8 | 9.2 | 4.0 | 0.354 |
| MMSE (score) | 21.5 | 5.2 | 22.3 | 3.8 | 0.878 | 14.4 | 5.7 | 14.7 | 5.4 | 0.229 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kodama, A.; Suzuki, Y.; Sakuraba, K.; Kume, Y.; Ota, H. The Effect of Deep Micro Vibrotactile Stimulation on Cognitive Function of Mild Cognitive Impairment and Mild Dementia. Int. J. Environ. Res. Public Health 2022, 19, 3803. https://doi.org/10.3390/ijerph19073803
Kodama A, Suzuki Y, Sakuraba K, Kume Y, Ota H. The Effect of Deep Micro Vibrotactile Stimulation on Cognitive Function of Mild Cognitive Impairment and Mild Dementia. International Journal of Environmental Research and Public Health. 2022; 19(7):3803. https://doi.org/10.3390/ijerph19073803
Chicago/Turabian StyleKodama, Ayuto, Yasuhiro Suzuki, Kazuki Sakuraba, Yu Kume, and Hidetaka Ota. 2022. "The Effect of Deep Micro Vibrotactile Stimulation on Cognitive Function of Mild Cognitive Impairment and Mild Dementia" International Journal of Environmental Research and Public Health 19, no. 7: 3803. https://doi.org/10.3390/ijerph19073803
APA StyleKodama, A., Suzuki, Y., Sakuraba, K., Kume, Y., & Ota, H. (2022). The Effect of Deep Micro Vibrotactile Stimulation on Cognitive Function of Mild Cognitive Impairment and Mild Dementia. International Journal of Environmental Research and Public Health, 19(7), 3803. https://doi.org/10.3390/ijerph19073803







