Patellar and Achilles Tendon Thickness Differences among Athletes with Different Numbers of Meals per Day: A Cross-Sectional Study
Abstract
:1. Introduction
2. Material and Methods
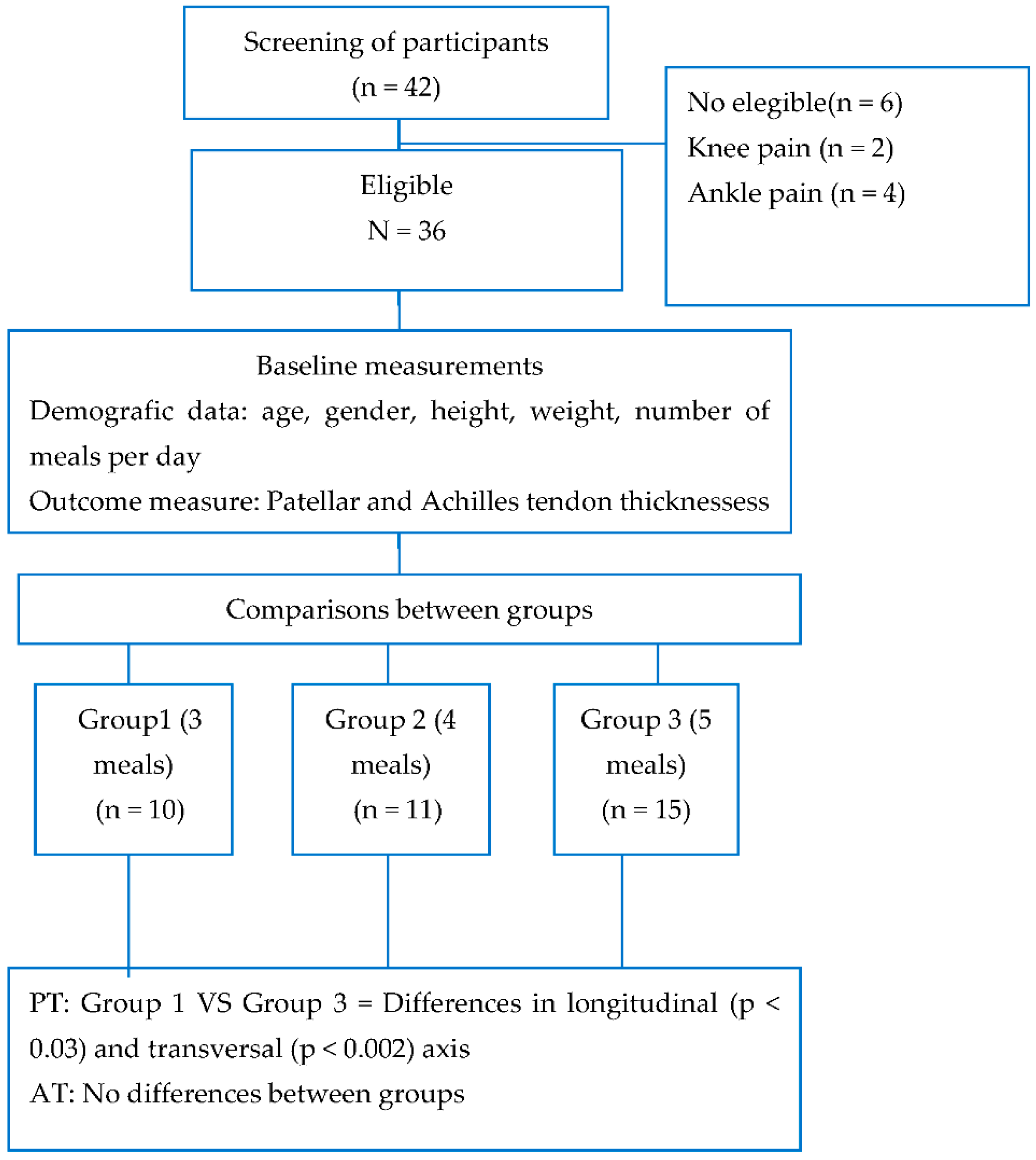
2.1. Sample Size
2.2. Study Protocol
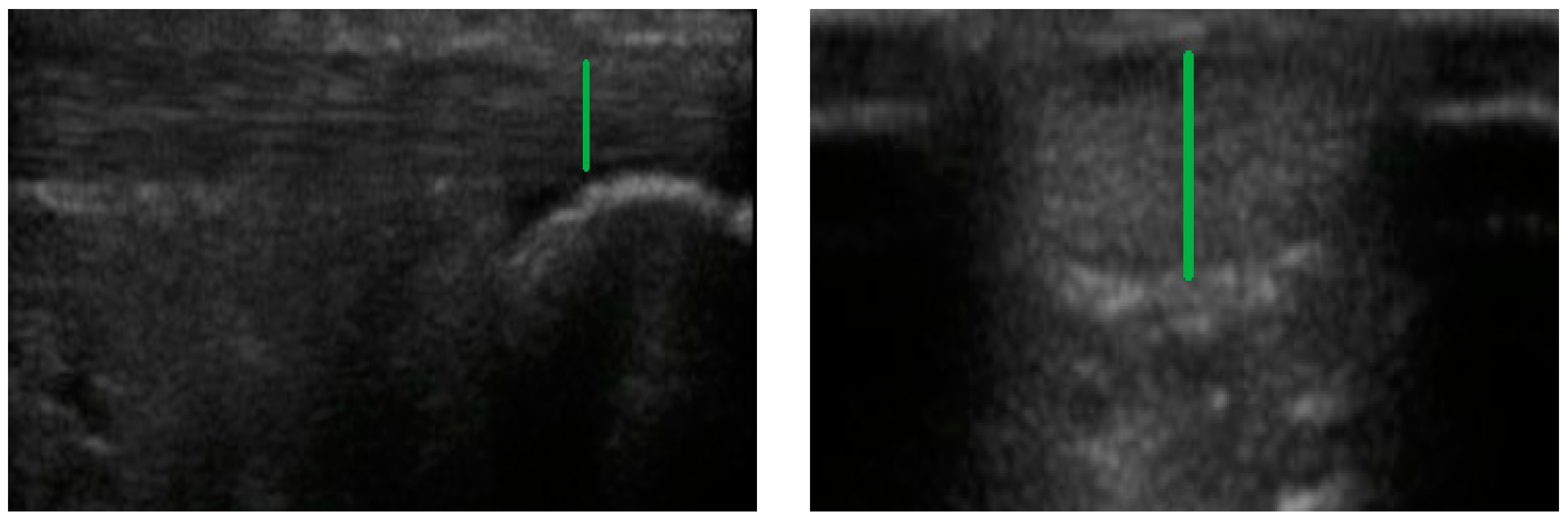
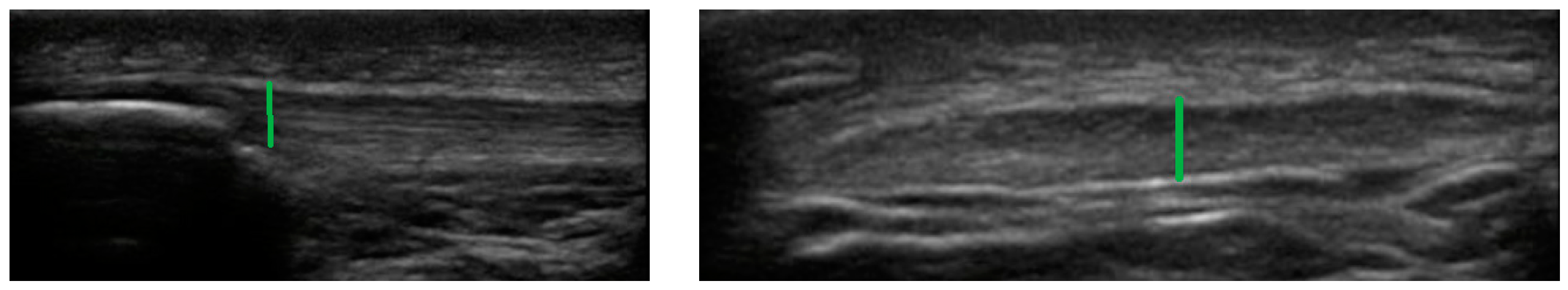
2.3. Ultrasound Measurements
2.4. Achilles Tendon
2.5. Patellar Tendon
2.6. Statistical Analysis
3. Results
Sample Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Achilles Tendon | (AT) |
| Patellar tendon | (PT) |
| Ultrasound imaging | (US) |
| Hypothalamic–pituitary–adrenal | (HPA) |
References
- Winter, S.C.; Gordon, S.; Brice, S.M.; Lindsay, D.; Barrs, S. A Multifactorial Approach to Overuse Running Injuries: A 1-Year Prospective Study. Sports Health 2020, 12, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Videbæk, S.; Bueno, A.M.; Nielsen, R.O.; Rasmussen, S. Incidence of Running-Related Injuries Per 1000 h of running in Different Types of Runners: A Systematic Review and Meta-Analysis. Sport. Med. 2015, 45, 1017–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarantino, D.; Palerm, S.; Sirico, F.; Balato, G.; D’addona, A.; Corrado, B. Achilles tendon pathologies: How to choose the best treatment. J. Hum. Sport Exerc. 2020, 15, 1300–1321. [Google Scholar] [CrossRef]
- Lopes, A.D.; Hespanhol, L.C.; Yeung, S.S.; Costa, L.O.P. What are the Main Running-Related Musculoskeletal Injuries? Sport. Med. 2012, 42, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, A.S.; Yin, A.; Hunt, K.J. Foot and Ankle Injuries in Runners. Phys. Med. Rehabil. Clin. N. Am. 2016, 27, 121–137. [Google Scholar] [CrossRef]
- Kulig, K.; Oki, K.C.; Chang, Y.J.; Bashford, G.R. Achilles and patellar tendon morphology in dancers with and without tendon pain. Med. Probl. Perform. Art. 2014, 29, 221–228. [Google Scholar] [CrossRef]
- SIRICO, F.; PALERMI, S.; MASSA, B.; CORRADO, B. Tendinopathies of the hip and pelvis in athletes: A narrative review. J. Hum. Sport Exerc. 2020, 15, S748–S762. [Google Scholar] [CrossRef]
- Lewis, T.; Zeisig, E.; Gaida, J.E. Does glucocorticoid exposure explain the association between metabolic dysfunction and tendinopathy? Endocr. Connect. 2020, 9, R36–R46. [Google Scholar] [CrossRef] [Green Version]
- Sahlani, L.; Thompson, L.; Vira, A.; Panchal, A.R. Bedside ultrasound procedures: Musculoskeletal and non-musculoskeletal. Eur. J. Trauma Emerg. Surg. 2016, 42, 127–138. [Google Scholar] [CrossRef]
- Fredberg, U.; Bolvig, L.; Andersen, N.T. Prophylactic training in asymptomatic soccer players with ultrasonographic abnormalities in Achilles and patellar tendons: The Danish super league study. Am. J. Sports Med. 2008, 36, 451–460. [Google Scholar] [CrossRef]
- Di Meglio, F.; Sacco, A.M.; Belviso, I.; Romano, V.; Sirico, F.; Loiacono, C.; Palermi, S.; Pempinello, C.; Montagnani, S.; Nurzynska, D.; et al. Influence of supplements and drugs used for the treatment of musculoskeletal disorders on adult human tendon-derived stem cells. Muscles. Ligaments Tendons J. 2020, 10, 376–384. [Google Scholar] [CrossRef]
- Comin, J.; Cook, J.L.; Malliaras, P.; McCormack, M.; Calleja, M.; Clarke, A.; Connell, D. The prevalence and clinical significance of sonographic tendon abnormalities in asymptomatic ballet dancers: A 24-month longitudinal study. Br. J. Sports Med. 2013, 47, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Oster, H.; Challet, E.; Ott, V.; Arvat, E.; de Kloet, E.R.; Dijk, D.J.; Lightman, S.; Vgontzas, A.; Van Cauter, E. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr. Rev. 2017, 38, 3–45. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; Mcklveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Comprehensive Physiology. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Couppé, C.; Svensson, R.B.; Kongsgaard, M.; Kovanen, V.; Grosset, J.F.; Snorgaard, O.; Bencke, J.; Larsen, J.O.; Bandholm, T.; Christensen, T.M.; et al. Human Achilles tendon glycation and function in diabetes. J. Appl. Physiol. 2016, 120, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G. Bone endocrine regulation of energy metabolism and male reproduction. Comptes Rendus Biol. 2011, 334, 720–724. [Google Scholar] [CrossRef]
- Sanchez-Lozada, L.G.; Andres-Hernando, A.; Garcia-Arroyo, F.E.; Cicerchi, C.; Li, N.; Kuwabara, M.; Roncal-Jimenez, C.A.; Johnson, R.J.; Lanaspa, M.A. Uric acid activates aldose reductase and the polyol pathway for endogenous fructose and fat production causing development of fatty liver in rats. J. Biol. Chem. 2019, 294, 4272–4281. [Google Scholar] [CrossRef]
- Kahleova, H.; Lloren, J.I.; Mashchak, A.; Hill, M.; Fraser, G.E. Meal Frequency and Timing Are Associated with Changes in Body Mass Index in Adventist Health Study 2. J. Nutr. 2017, 147, 1722–1728. [Google Scholar] [CrossRef] [Green Version]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef] [Green Version]
- Damsted, C.; Parner, E.T.; Sørensen, H.; Malisoux, L.; Nielsen, R.O. Design of ProjectRun21: A 14-week prospective cohort study of the influence of running experience and running pace on running-related injury in half-marathoners. Inj. Epidemiol. 2017, 4, 30. [Google Scholar] [CrossRef]
- Nadeau, M.-J.; Desrochers, A.; Lamontagne, M.; Larivière, C.; Gagnon, D.H. Quantitative ultrasound imaging of Achilles tendon integrity in symptomatic and asymptomatic individuals: Reliability and minimal detectable change. J. Foot Ankle Res. 2016, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Reina-Martin, I.; Navarro-Ledesma, S.; Ortega-Avila, A.B.; Deschamps, K.; Martinez-Franco, A.; Luque-Suarez, A.; Gijon-Nogueron, G. Morphological characteristics of passive and active structures of the foot across populations with different levels of physical activity. J. Sport Rehabil. 2021, 30, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Murley, G.S.; Tan, J.M.; Edwards, R.M.; De Luca, J.; Munteanu, S.E.; Cook, J.L. Foot posture is associated with morphometry of the peroneus longus muscle, tibialis anterior tendon, and Achilles tendon. Scand. J. Med. Sci. Sports 2014, 24, 535–541. [Google Scholar] [CrossRef]
- Schmidt, W.A.; Schmidt, H.; Schicke, B.; Gromnica-Ihle, E. Standard reference values for musculoskeletal ultrasonography. Ann. Rheum. Dis. 2004, 63, 988–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portney, L.G.; Watkins, M.P. Statistical measures of reliability. In Foundations of Clinical Research: Applications to Practice; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2000. [Google Scholar]
- Milgrom, Y.; Milgrom, C.; Altaras, T.; Globus, O.; Zeltzer, E.; Finestone, A.S. Achilles tendons hypertrophy in response to high loading training. Foot Ankle Int. 2014, 35, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Schütz, U.H.W.; Schmidt-Trucksäss, A.; Knechtle, B.; Machann, J.; Wiedelbach, H.; Ehrhardt, M.; Freund, W.; Gröninger, S.; Brunner, H.; Schulze, I.; et al. The Transeurope Footrace Project: Longitudinal data acquisition in a cluster randomized mobile MRI observational cohort study on 44 endurance runners at a 64-stage 4,486km transcontinental ultramarathon. BMC Med. 2012, 10, 78. [Google Scholar] [CrossRef] [Green Version]
- Pruimboom, L.; Raison, C.L.; Muskiet, F.A.J. Physical Activity Protects the Human Brain against Metabolic Stress Induced by a Postprandial and Chronic Inflammation. Behav. Neurol. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yu, J.T.; Zhang, W.; Cui, W.Z.; Wu, Z.C.; Zhang, Q.; Tan, L. Genetic association of SLC2A14 polymorphism with Alzheimer’s disease in a Han Chinese population. J. Mol. Neurosci. 2012, 47, 481–484. [Google Scholar] [CrossRef]
- Abdul Muneer, P.M.; Alikunju, S.; Szlachetka, A.M.; Murrin, L.C.; Haorah, J. Impairment of brain endothelial glucose transporter by methamphetamine causes blood-brain barrier dysfunction. Mol. Neurodegener. 2011, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Núñez, B.; Kuipers, R.S.; Luxwolda, M.F.; De Graaf, D.J.; Breeuwsma, B.B.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. Saturated fatty acid (SFA) status and SFA intake exhibit different relations with serum total cholesterol and lipoprotein cholesterol: A mechanistic explanation centered around lifestyle-induced low-grade inflammation. J. Nutr. Biochem. 2014, 25, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: Potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanaspa, M.A.; Ishimoto, T.; Li, N.; Cicerchi, C.; Orlicky, D.J.; Ruzycki, P.; Rivard, C.; Inaba, S.; Roncal-Jimenez, C.A.; Bales, E.S.; et al. Endogenous Fructose Production and Metabolism in the Liver Contributes to the Development of Metabolic Syndrome. Nat. Commun. 2013, 4, 2434. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Kuwabara, M.; Andres-Hernando, A.; Li, N.; Cicerchi, C.; Jensen, T.; Orlicky, D.J.; Roncal-Jimenez, C.A.; Ishimoto, T.; Nakagawa, T.; et al. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 3138–3143. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Lozada, L.G.; Lanaspa, M.A.; Cristóbal-García, M.; García-Arroyo, F.; Soto, V.; Cruz-Robles, D.; Nakagawa, T.; Yu, M.A.; Kang, D.H.; Johnson, R.J. Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp. Nephrol. 2013, 121, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imaram, W.; Gersch, C.; Kim, K.M.; Johnson, R.J.; Henderson, G.N.; Angerhofer, A. Radicals in the reaction between peroxynitrite and uric acid identified by electron spin resonance spectroscopy and liquid chromatography mass spectrometry. Free Radic. Biol. Med. 2010, 49, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Qiu, L.; Lin, J.; Xu, F.; Gao, Y.; Zhang, C.; Liu, Y.; Luo, Y.; Yang, J.Y. Inhibition of aldose reductase activates hepatic peroxisome proliferator-activated receptor-α and ameliorates hepatosteatosis in diabetic db/db mice. Exp. Diabetes Res. 2012, 2012, 789730. [Google Scholar] [CrossRef] [Green Version]
- Palermi, S.; Massa, B.; Vecchiato, M.; Mazza, F.; De Blasiis, P.; Romano, A.M.; Di Salvatore, M.G.; Della Valle, E.; Tarantino, D.; Ruosi, C.; et al. Indirect structural muscle injuries of lower limb: Rehabilitation and therapeutic exercise. J. Funct. Morphol. Kinesiol. 2021, 6, 75. [Google Scholar] [CrossRef] [PubMed]
| Group 1 (3 Meals/Day) N = 10 | Group 2 (4 Meals/Day) N = 11 | Group 3 (5 Meals/Day) N = 15 | |
|---|---|---|---|
| Age (years; SD) | 40.5 (8.29) | 34.9 (9.35) | 31.6 (10.6) |
| Body mass index (SD) | 24.2 (2.26) | 23.3 (2.82) | 23 (3.59) |
| Number of kms/week (SD) | 58 (27.4) | 57.6 (30.3) | 53.1 (20.6) |
| Number of training hours/week (SD) | 10.1 | 10.2 | 12.5 |
| Group 1 | Group 2 | Group 3 | F | p Values | |
|---|---|---|---|---|---|
| PT (longitudinal) | 5.70 (4.10–7.40) | 4.91 (3.20–7.10) | 4.69 (3.40–6.00) | 3.659 | 0.047 * |
| PT (transversal) | 4.34 (3.70–5.70) | 4.10 (2.90–7.00) | 3.68 (2.90–4.90) | 4.022 | 0.036 * |
| AT (longitudinal) | 21.4 (17–28) | 22.2 (19.1–25.5) | 22.2 (18.7–27.6) | 0.234 | 0.793 |
| AT (transversal) | 5.60 (4.20–7.20) | 5.00 (4.30–7.50) | 4.60 (3.30–6.10) | 2.986 | 0.073 |
| Group 1 vs. Group 2 | p Values | Group 1 vs. Group 3 | p Values | Group 2 vs. Group 3 | p Values | |
|---|---|---|---|---|---|---|
| PT (longitudinal) a | 0.791 b | 0.239 | 1.007 b | 0.038 * | 0.216 b | 0.844 |
| PT (transversal) a | 0.240 b | 0.832 | 0.660 b | 0.028 * | 0.420 b | 0.546 |
| AT (longitudinal) b | −0.831 a | 0.780 | −0.833 a | 0.751 | −0.002 a | 1 |
| AT (transversal) b | 0.317 a | 0.701 | 0.863 a | 0.062 | 0.546 a | 0.290 |
| Number of Meals Per Day | |
|---|---|
| PT (longitudinal) † (r) | −0.384 p = 0.02 * |
| PT (transversal) † (r) | −0.406 p = 0.01 * |
| AT (longitudinal) †† (r) | 0.118 p = 0.494 |
| AT (transversal) †† (r) | −0.386 p = 0.02 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Ledesma, S.; Gijon-Nogueron, G.; Reina-Martín, I.; Ortega-Avila, A.B.; Pruimboom, L. Patellar and Achilles Tendon Thickness Differences among Athletes with Different Numbers of Meals per Day: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2022, 19, 2468. https://doi.org/10.3390/ijerph19042468
Navarro-Ledesma S, Gijon-Nogueron G, Reina-Martín I, Ortega-Avila AB, Pruimboom L. Patellar and Achilles Tendon Thickness Differences among Athletes with Different Numbers of Meals per Day: A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2022; 19(4):2468. https://doi.org/10.3390/ijerph19042468
Chicago/Turabian StyleNavarro-Ledesma, Santiago, Gabriel Gijon-Nogueron, Inmaculada Reina-Martín, Ana Belen Ortega-Avila, and Leo Pruimboom. 2022. "Patellar and Achilles Tendon Thickness Differences among Athletes with Different Numbers of Meals per Day: A Cross-Sectional Study" International Journal of Environmental Research and Public Health 19, no. 4: 2468. https://doi.org/10.3390/ijerph19042468
APA StyleNavarro-Ledesma, S., Gijon-Nogueron, G., Reina-Martín, I., Ortega-Avila, A. B., & Pruimboom, L. (2022). Patellar and Achilles Tendon Thickness Differences among Athletes with Different Numbers of Meals per Day: A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 19(4), 2468. https://doi.org/10.3390/ijerph19042468









