Microbial Electrochemical Technologies for Sustainable Nitrogen Removal in Marine and Coastal Environments
Abstract
:1. Introduction
1.1. Nitrogen Cycle Disturbance: A Big Silent Problem
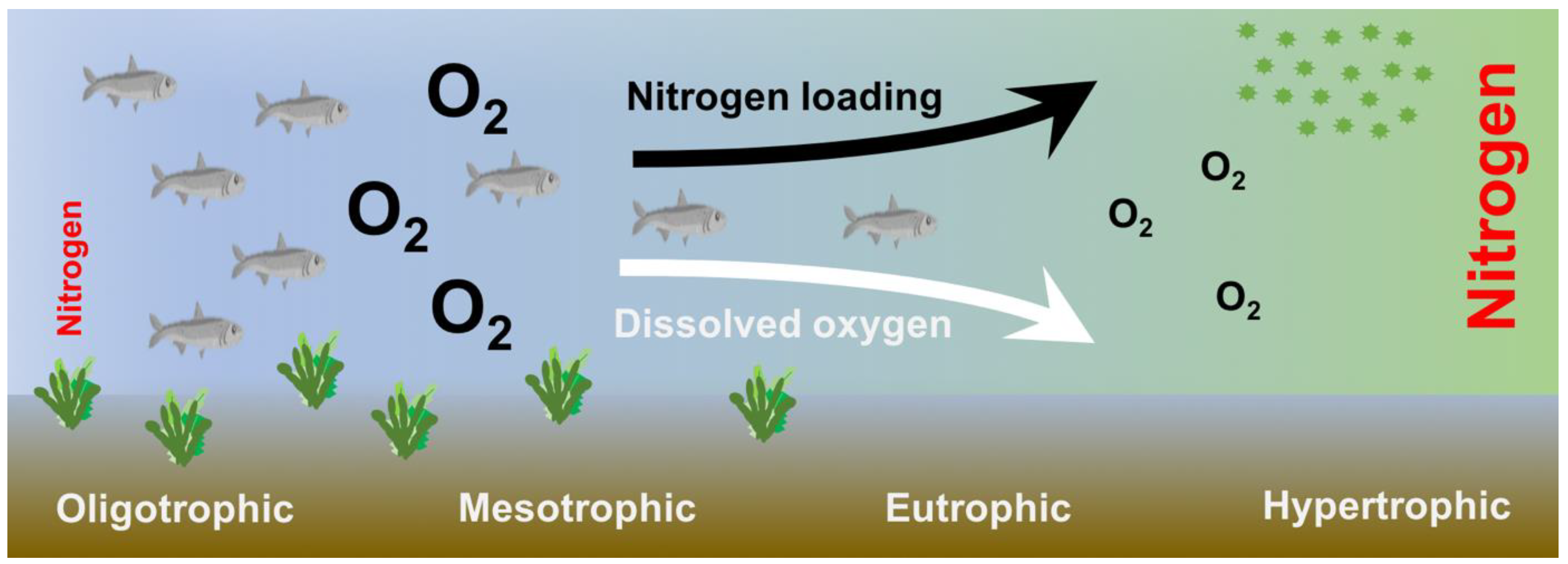
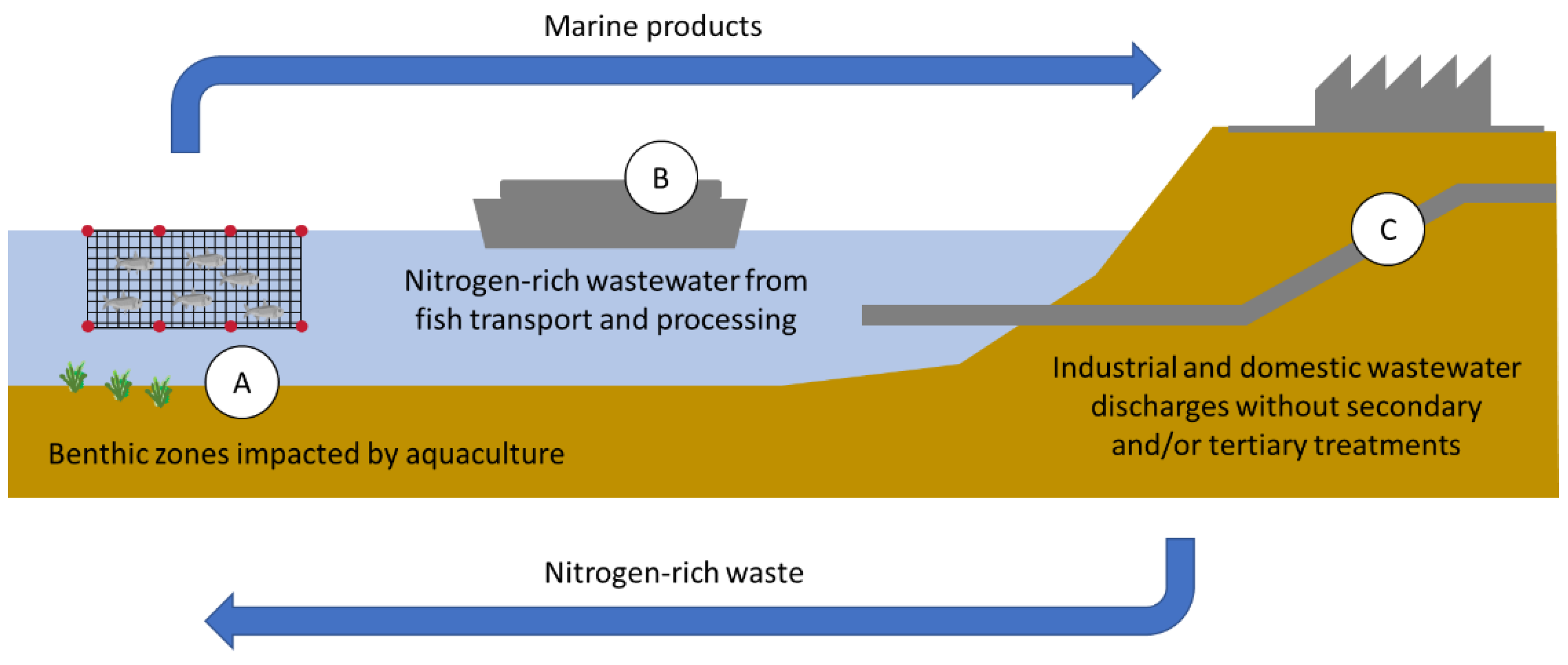
1.2. Sources and Effects of Nitrogen Excess on Coastal Marine Ecosystems
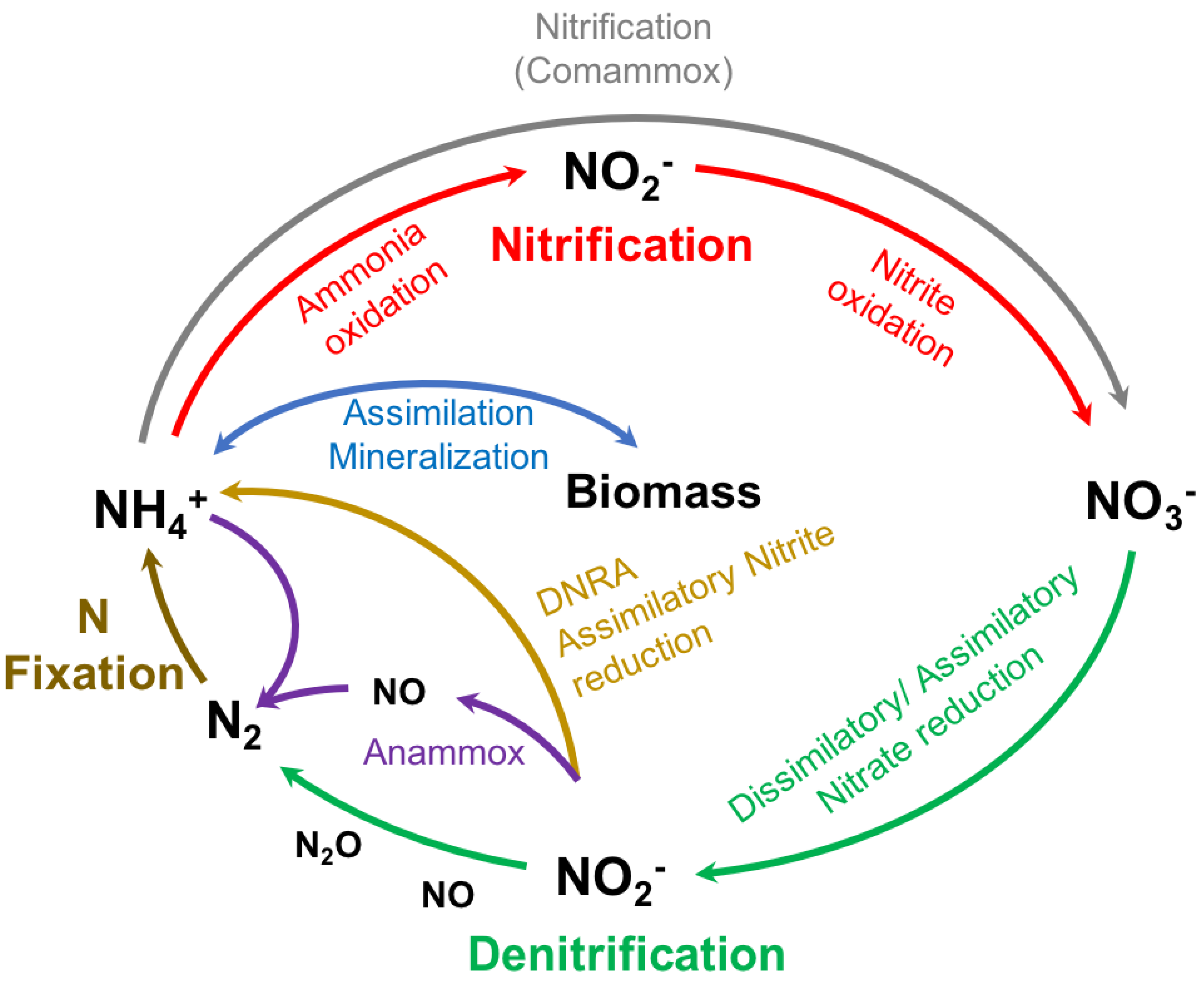
1.3. Microorganisms Responsible for the Natural Metabolization of Nitrogen
2. Technologies for Nitrogen Removal
3. Microbial Electrochemical Technologies (METs) for Sustainable Nitrogen Removal
3.1. Principles of METs
3.2. MET as a Promising Nitrogen and Carbon Removal Strategy
3.3. MET in Marine and Coastal Environments
3.4. Electrochemical Overpotentials as a Microbial Enrichment Technique
3.5. Future Opportunities for Applying MET in Coastal Environments
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S.; Lambin, E.; Lenton, T.M.; Scheffer, M.; Folke, C.; Joachim, H.; et al. Planetary Boundaries: Exploring the Safe Operating Space for Humanity. Ecol. Soc. 2009, 14, 1–33. [Google Scholar] [CrossRef]
- Sutton, M.A.; Bleeker, A.; Howard, C.M.; Erisman, J.W.; Abrol, Y.P.; Bekunda, M.; Datta, A.; Davidson, E.; de Vries, W.; Oenema, O.; et al. Our Nutrient World: The Challenge to Produce More Food and Energy with Less Pollution. Global Overview of Nutrient Management. Centre for Ecology and Hydrology, Edinburgh on behalf of the Global Partnership on Nutrient Management and the International Nitrogen Initiative, 2013. Available online: http://nora.nerc.ac.uk/id/eprint/500700/ (accessed on 17 January 2022).
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; De Vries, W.; De Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen cycles: Past, present and future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Galloway, J.N.; Trends, R.; Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; et al. Transformation of the Nitrogen Cycle: Potential Solutions. Science 2013, 320, 889–892. [Google Scholar] [CrossRef] [Green Version]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. Signaling global human impact on marine ecosystems. Science 2008, 319, 948–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halpern, B.S.; Selkoe, K.A.; Micheli, F.; Kappel, C. V Evaluating and Ranking the Vulnerability of Global Marine Ecosystems to Anthropogenic Threats. Conserv. Biol. 2007, 21, 1301–1315. [Google Scholar] [CrossRef]
- Smith, V.H. Eutrophication of Freshwater and Coastal Marine Ecosystems A Global Problem. Environ. Sci. Pollut. Res. 2003, 10, 126–139. [Google Scholar] [CrossRef]
- Khan, A.F.; Naushin, F.; Rehman, F.; Masoodi, A.; Irfan, M.; Hashmi, F.; Ansari, A.A. Eutrophication: Causes, Consequences and Control, 2nd ed.; Springer Science & Business Media: Tabuk, Saudi Arabia, 2010. [Google Scholar]
- Soto, D.; Norambuena, F. Evaluation of salmon farming effects on marine systems in the inner seas of southern Chile: A large-scale mensurative experiment. J. Appl. Ichthyol. 2004, 20, 493–501. [Google Scholar] [CrossRef]
- Abessa, D.M.S.; Hortelani, M.A.; Sarkis, J.E. Influence of a Brazilian sewage outfall on the toxicity and contamination of adjacent sediments. Mar. Pollut. Bull. 2005, 50, 875–885. [Google Scholar] [CrossRef]
- Powley, H.R.; Du, H.H.; Lima, A.T.; Krom, M.D.; Cappellen, P. Van Direct Discharges of Domestic Wastewater are a Major Source of Phosphorus and Nitrogen to the Mediterranean Sea. Environ. Sci. Technol. 2016, 50, 8722–8730. [Google Scholar] [CrossRef] [Green Version]
- Roberts, P.J.W.; Salas, H.J.; Reiff, F.M.; Libhaber, M.; Labbe, A.; Thomson, J.C. Marine Wastewater Outfalls and Treatment Systems; IWA Publishing: London, UK, 2010. [Google Scholar]
- dos Santos, D.M.; Buruaem, L.; Gonçalves, R.M.; Williams, M.; Abessa, D.M.S.; Kookana, R.; de Marchi, M.R.R. Multiresidue determination and predicted risk assessment of contaminants of emerging concern in marine sediments from the vicinities of submarine sewage outfalls. Mar. Pollut. Bull. 2018, 129, 299–307. [Google Scholar] [CrossRef] [Green Version]
- De-La-Ossa-Carretero, J.A.; Del-Pilar-Ruso, Y.; Giménez-Casalduero, F.; Sánchez-Lizaso, J.L. Assessing reliable indicators to sewage pollution in coastal soft-bottom communities. Environ. Monit. Assess. 2012, 184, 2133–2149. [Google Scholar] [CrossRef] [PubMed]
- Superintendencia de Servicios Sanitarios Tratamiento de Aguas Servidas, 2016. Available online: http://www.siss.gob.cl (accessed on 20 May 2020).
- De-la-Ossa-Carretero, J.A.; Del-Pilar-Ruso, Y.; Gimenez-Casalduero, F.; Sánchez-Lizaso, J.L. Monitoring the effects of wastewater treatment strategies. Environ. Monit. Assess. 2016, 188, 110. [Google Scholar] [CrossRef] [PubMed]
- Feitosa, R.C.; Rosman, P.C.C.; Carvalho, J.L.B.; Côrtes, M.B.V.; Wasserman, J.C. Comparative study of fecal bacterial decay models for the simulation of plumes of submarine sewage outfalls. Water Sci. Technol. 2013, 68, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Arroyave Gómez, D.M.; Gallego Suárez, D.; Bartoli, M.; Toro-Botero, M. Spatial and seasonal variability of sedimentary features and nitrogen benthic metabolism in a tropical coastal area (Taganga Bay, Colombia Caribbean) impacted by a sewage outfall. Biogeochemistry 2020, 150, 85–107. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Cabello, F.; Young, K.; Carvajal, J.; Varela, D.A.; Henrı, L. Ocean & Coastal Management Salmon aquaculture and coastal ecosystem health in Chile: Analysis of regulations, environmental impacts and bioremediation systems. Ocean. Coast. Manag. 2009, 52, 243–249. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustain. Action 2020. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Riquelme, V.A.; Hernández-González, M.C.; Varela, D.; Jiménez, J.E.; Henríquez, L.A.; Vergara, P.A.; Guíñez, R.; Filún, L. A review of the impacts of salmonid farming on marine coastal ecosystems in the southeast Pacific. ICES J. Mar. Sci. 2006, 63, 1338–1345. [Google Scholar] [CrossRef]
- Naylor, R.L.; Goldburg, R.J.; Primavera, J.H.; Kautsky, N.; Beveridge, M.C.M.; Clay, J.; Folke, C.; Lubchenco, J.; Mooney, H.; Troell, M. Effect of aquaculture on world fish supplies. Nature 2000, 405, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Stigebrandt, A.; Aure, J.; Ervik, A.; Kupka, P. Regulating the local environmental impact of intensive marine fish farming III. A model for estimation of the holding capacity in the Modelling—Ongrowing fish farm—Monitoring system. Aquaculture 2004, 234, 239–261. [Google Scholar] [CrossRef]
- Ervik, A.; Hansen, P.K.; Aure, J.; Stigebrandt, A.; Johannessen, P.; Jahnsen, T. Regulating the local environmental impact of intensive marine fish farming I. The concept of the MOM system. Aquac. Eng. 1997, 158, 85–94. [Google Scholar] [CrossRef]
- Hansen, P.K.; Ervik, A.; Schaanning, M.; Johannessen, P.; Aure, J.; Jahnsen, T.; Stigebrandt, A. Regulating the local environmental impact of intensive, marine fish farming II. The monitoring programme of the MOM system ž Modelling—Ongrowing fish farms—Monitoring. Aquaculture 2001, 194, 75–92. [Google Scholar] [CrossRef]
- Kamjunke, N.; Nimptsch, J.; Harir, M.; Herzsprung, P.; Schmitt-Kopplin, P.; Neu, T.R.; Graeber, D.; Osorio, S.; Valenzuela, J.; Carlos Reyes, J.; et al. Land-based salmon aquacultures change the quality and bacterial degradation of riverine dissolved organic matter. Sci. Rep. 2017, 7, 43739. [Google Scholar] [CrossRef]
- Holan, A.B.; Wold, P.A.; Leiknes, T.O. Intensive rearing of cod larvae (Gadus morhua) in recirculating aquaculture systems (RAS) implementing a membrane bioreactor (MBR) for enhanced colloidal particle and fine suspended solids removal. Aquac. Eng. 2014, 58, 52–58. [Google Scholar] [CrossRef]
- Ahmed, N.; Turchini, G.M. Recirculating aquaculture systems (RAS): Environmental solution and climate change adaptation. J. Clean. Prod. 2021, 297, 126604. [Google Scholar] [CrossRef]
- Yogev, U.; Gross, A. Reducing environmental impact of recirculating aquaculture systems by introducing a novel microaerophilic assimilation reactor: Modeling and proof of concept. J. Clean. Prod. 2019, 226, 1042–1050. [Google Scholar] [CrossRef]
- Guldhe, A.; Ansari, F.A.; Singh, P.; Bux, F. Heterotrophic cultivation of microalgae using aquaculture wastewater: A biorefinery concept for biomass production and nutrient remediation. Ecol. Eng. 2017, 99, 47–53. [Google Scholar] [CrossRef]
- Bahroun, S.; Bousnoubra, H.; Drouiche, N.; Kherici, N. Analysis of wastewater discharges to the Wadi Kebir East River by the environmental discharge objectives (EDO) method. Desalination Water Treat. 2016, 57, 24750–24754. [Google Scholar] [CrossRef]
- Orhon, D.; Ates, E.; Sözen, S.; Çokgör, E.U. Characterizacion and COD fractionation of domestic wastewaters. Environ. Pollut. 1997, 95, 191–204. [Google Scholar] [CrossRef]
- Lananan, F.; Hajar, S.; Hamid, A.; Nur, W.; Din, S.; Khatoon, H.; Jusoh, A.; Endut, A. International Biodeterioration & Biodegradation Symbiotic bioremediation of aquaculture wastewater in reducing ammonia and phosphorus utilizing Effective Microorganism (EM-1) and microalgae (Chlorella sp.). Int. Biodeterior. Biodegrad. 2014, 95, 127–134. [Google Scholar] [CrossRef]
- Libes, S.M. Introduction to Marine Biogeochemistry; Elsevier’s Science & Technology: Conway, AR, USA, 2009. [Google Scholar]
- Quinn, P.K.; Charlson, R.J.; Bates, T.S. Simultaneous observations of ammonia in the atmosphere and ocean. Nature 1988, 335, 336–338. [Google Scholar] [CrossRef]
- Glibert, P.M. Eutrophication, harmful algae and biodiversity—Challenging paradigms in a world of complex nutrient changes. Mar. Pollut. Bull. 2017, 124, 591–606. [Google Scholar] [CrossRef]
- Granéli, E.; Turner, J.T. Ecology of Harmful Algae; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful Algal Blooms and Eutrophication Nutrient Sources, Composition, and Consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Díaz, P.A.; Álvarez, G.; Varela, D.; Pérez-Santos, I.; Díaz, M.; Molinet, C.; Seguel, M.; Aguilera-Belmonte, A.; Guzmán, L.; Uribe, E.; et al. Impacts of harmful algal blooms on the aquaculture industry: Chile as a case study. Perspect. Phycol. 2019, 6, 39–50. [Google Scholar] [CrossRef]
- Zehr, J.P. Nitrogen fixation by marine cyanobacteria. Trends Microbiol. 2011, 19, 162–173. [Google Scholar] [CrossRef]
- Zehr, J.P.; Kudela, R.M. Nitrogen Cycle of the Open Ocean: From Genes to Ecosystems. Annu. Rev. Mar. Sci. 2011, 3, 197–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bock, E.; Wagner, M. Oxidation of Inorganic Nitrogen Compounds as an Energy Source. In The Prokaryotes; Springer Science+Business Media: New York, NY, USA, 2006; pp. 457–495. [Google Scholar]
- Hu, H.W.; He, J.Z. Comammox—A newly discovered nitrification process in the terrestrial nitrogen cycle. J. Soils Sediments 2017, 17, 2709–2717. [Google Scholar] [CrossRef]
- Daims, H.; Lücker, S.; Wagner, M. A New Perspective on Microbes Formerly Known as Nitrite-Oxidizing Bacteria. Trends Microbiol. 2016, 24, 699–712. [Google Scholar] [CrossRef]
- Kuenen, J.G. Anammox bacteria: From discovery to application. Nat. Rev. Microbiol. 2008, 6, 320–326. [Google Scholar] [CrossRef]
- Allen, A.E.; Howard-jones, M.H.; Booth, M.G.; Frischer, M.E.; Verity, P.G.; Bronk, D.A.; Sanderson, M.P. Importance of heterotrophic bacterial assimilation of ammonium and nitrate in the Barents Sea during summer. J. Mar. Syst. 2002, 38, 93–108. [Google Scholar] [CrossRef]
- Rezvani, F.; Sarrafzadeh, M.; Ebrahimi, S. Nitrate removal from drinking water with a focus on biological methods: A review. Environ. Sci. Pollut. Res. 2019, 26, 1124–1141. [Google Scholar] [CrossRef] [PubMed]
- Mook, W.T.; Chakrabarti, M.H.; Aroua, M.K.; Khan, G.M.A.; Ali, B.S.; Islam, M.S.; Abu Hassan, M.A. Removal of total ammonia nitrogen (TAN), nitrate and total organic carbon (TOC) from aquaculture wastewater using electrochemical technology: A review. Desalination 2012, 285, 1–13. [Google Scholar] [CrossRef]
- Kalaruban, M.; Loganathan, P.; Shim, W.G.; Kandasamy, J.; Naidu, G.; Nguyen, T.V.; Vigneswaran, S. Removing nitrate from water using iron-modified Dowex 21K XLT ion exchange resin: Batch and fluidised-bed adsorption studies. Sep. Purif. Technol. 2016, 158, 62–70. [Google Scholar] [CrossRef]
- Bellona, C.; Drewes, J.E.; Oelker, G.; Luna, J.; Filteau, G.; Amy, G. Comparing nanofiltration and reverse osmosis for drinking water augmentation. J. Am. Water Work. Assoc. 2008, 100, 102–116. [Google Scholar] [CrossRef]
- Nataraj, S.K.; Hosamani, K.M.; Aminabhavi, T.M. Electrodialytic removal of nitrates and hardness from simulated mixtures using ion-exchange membranes. J. Appl. Polym. Sci. 2006, 99, 1788–1794. [Google Scholar] [CrossRef]
- Soares, M.I.M. Biological denitrification of groundwater. Water Air Soil Pollut. 2000, 123, 183–193. [Google Scholar] [CrossRef]
- Aslan, S.; Türkman, A. Biological denitrification of drinking water using various natural organic solid substrates. Water Sci. Technol. 2003, 48, 58140. [Google Scholar] [CrossRef]
- Davis, M.L. Secondary treatment by suspended growth biological processes. In Water and Wastewater Engineering: Design Principles and Practice; The McGraw-Hill Companies: New York, NY, USA, 2010; pp. 1–115. [Google Scholar]
- Van Hulle, S.W.H.; Vandeweyer, H.J.P.; Meesschaert, B.D.; Vanrolleghem, P.A.; Dejans, P.; Dumoulin, A. Engineering aspects and practical application of autotrophic nitrogen removal from nitrogen rich streams. Chem. Eng. J. 2010, 162, 1–20. [Google Scholar] [CrossRef]
- Park, J.Y.; Yoo, Y.J. Biological nitrate removal in industrial wastewater treatment: Which electron donor we can choose. Appl. Microbiol. Biotechnol. 2009, 82, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Elvira, S.I.; Diez, P.N.; Fdz-Polanco, F. Sludge minimisation technologies. Rev. Environ. Sci. Bio/Tecnol. 2006, 5, 375–398. [Google Scholar] [CrossRef]
- Flemming, H.; Wingender, J. The biofilm matrix. Nat. Publ. Group 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, A.; Hossain, M.I.; Cheng, L. High-strength wastewater treatment using microbial biofilm reactor: A critical review. World J. Microbiol. Biotechnol. 2020, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- di Biase, A.; Kowalski, M.S.; Devlin, T.R.; Oleszkiewicz, J.A. Moving bed biofilm reactor technology in municipal wastewater treatment: A review. J. Environ. Manag. 2019, 247, 849–866. [Google Scholar] [CrossRef] [PubMed]
- Hassard, F.; Biddle, J.; Cartmell, E.; Jefferson, B.; Tyrrel, S.; Stephenson, T. Rotating biological contactors for wastewater treatment—A review. Process Saf. Environ. Prot. 2015, 94, 285–306. [Google Scholar] [CrossRef] [Green Version]
- Rittman, B.E. The membrane biofilm reactor: The natural partnership of membranes and biofilm. Water Sci. Technol. 2006, 53, 219–225. [Google Scholar] [CrossRef]
- Tsuneda, S.; Ohno, T.; Soejima, K.; Hirata, A. Simultaneous nitrogen and phosphorus removal using denitrifying phosphate-accumulating organisms in a sequencing batch reactor. Biochem. Eng. J. 2006, 27, 191–196. [Google Scholar] [CrossRef]
- Sun, H.; Xu, S.; Zhuang, G.; Zhuang, X. Performance and recent improvement in microbial fuel cells for simultaneous carbon and nitrogen removal: A review. J. Environ. Sci. 2016, 39, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Samatya, S.; Kabay, N.; Yu, M. Removal of nitrate from aqueous solution by nitrate selective ion exchange resins. React. Funct. Polym. 2006, 66, 1206–1214. [Google Scholar] [CrossRef]
- Darbi, A.; Viraraghavan, T.; Butler, R.; Corkal, D. Pilot-Scale Evaluation of Select Nitrate Removal Technologies. J. Environ. Sci. Health 2006, 4529. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, J.J.; Steyn, A. Nitrate removal with reverse osmosis in a rural area in South Africa. Desalination 2003, 5, 15–26. [Google Scholar] [CrossRef]
- Hell, F.; Lahnsteiner, J.; Frischherz, H.; Baumgartner, G. Experience with full-scale electrodialysis for nitrate and hardness removal. Desalination 1998, 7, 173–180. [Google Scholar] [CrossRef]
- Monsalvo, V.M.; Mohedano, A.F.; Rodriguez, J.J. Activated carbons from sewage sludge Application to aqueous-phase adsorption of 4-chlorophenol. Desalination 2011, 277, 377–382. [Google Scholar] [CrossRef]
- Chaplin, B.P.; Reinhard, M.; Schneider, W.F.; Schu, C.; Shapley, J.R.; Strathmann, T.J.; Werth, C.J. Critical Review of Pd-Based Catalytic Treatment of Priority Contaminants in Water. Environ. Sci. Technol. 2012, 46, 3655–3670. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Zhang, T.C. Effects of low pH on nitrate reduction by iron powder. Water Res. 2004, 38, 2631–2642. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Z.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef]
- Lovley, D.R. Powering microbes with electricity: Direct electron transfer from electrodes to microbes. Environ. Microbiol. Rep. 2011, 3, 27–35. [Google Scholar] [CrossRef]
- Franks, A.E.; Nevin, K.P. Microbial Fuel Cells, A Current Review. Energies 2010, 3, 899–919. [Google Scholar] [CrossRef]
- Whitfield, M. The ion-association model and the buffer capacity of the carbon dioxide system in seawater at 25 °C and 1 atmosphere total pressure. Limnol. Oceanogr. 1974, 19, 235–248. [Google Scholar] [CrossRef]
- Logan, B.E.; Call, D.; Cheng, S.; Hamelers, H.V.M.; Sleutels, T.H.J.A.; Jeremiasse, A.W.; Rozendal, R.A. Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ. Sci. Technol. 2008, 42, 8630–8640. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rojas, F.; Muñoz, D.; Tapia, N.; Canales, C.; Vargas, I.T. Bioelectrochemical chlorate reduction by Dechloromonas agitata CKB. Bioresour. Technol. 2020, 315, 123818. [Google Scholar] [CrossRef]
- Jeremiasse, A.W.; Hamelers, H.V.M.; Buisman, C.J.N. Microbial electrolysis cell with a microbial biocathode. Bioelectrochemistry 2010, 78, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Zhang, L.; Li, D.; Su, W.; Tao, Y.; Qian, J. Autotrophic nitrogen removal from ammonium at low applied voltage in a single-compartment microbial electrolysis cell. Bioresour. Technol. 2012, 116, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, M.; Guo, J.; Sun, G. Bacterial extracellular electron transfer in bioelectrochemical systems. Process Biochem. 2012, 47, 1707–1714. [Google Scholar] [CrossRef]
- von Canstein, H.; Ogawa, J.; Shimizu, S.; Lloyd, J.R. Secretion of Flavins by Shewanella Species and Their Role in Extracellular Electron Transfer. Appl. Environ. Microbiol. 2008, 74, 615–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 6–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, E.; Cai, Y.; Luo, Y.; Piao, Z. Riboflavin-shuttled extracellular electron transfer from Enterococcus faecalis to electrodes in microbial fuel cells. Can. J. Microbiol. 2014, 60, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xiao, Y.; Wang, L.; Zheng, Y.; Chang, K.; Zheng, Z.; Yang, Z.; Varcoe, J.R.; Zhao, F. Extracellular electron transfer mediated by flavins in Gram-positive Bacillus sp. WS-XY1 and yeast Pichia stipitis. Electrochim. Acta 2014, 146, 564–567. [Google Scholar] [CrossRef] [Green Version]
- Torres, C.I.; Marcus, A.K.; Lee, H.; Parameswaran, P.; Krajmalnik-Brown, R.; Rittmann, B.E. A kinetic perspective on extracellular electron transfer by anode-respiring bacteria. FEMS Microbiol. Rev. 2010, 34, 3–17. [Google Scholar] [CrossRef] [Green Version]
- Rabaey, K.; Boon, N.; Höfte, M.; Verstraete, W. Microbial phenazine production enhances electron transfer in biofuel cells. Environ. Sci. Technol. 2005, 39, 3401–3408. [Google Scholar] [CrossRef]
- Pham, T.H.; Boon, N.; De Maeyer, K.; Höfte, M.; Rabaey, K.; Verstraete, W. Use of Pseudomonas species producing phenazine-based metabolites in the anodes of microbial fuel cells to improve electricity generation. Appl. Microbiol. Biotechnol. 2008, 80, 985–993. [Google Scholar] [CrossRef]
- Glasser, N.R.; Kern, S.E.; Newman, D.K. Phenazine redox cycling enhances anaerobic survival in Pseudomonas aeruginosa by facilitating generation of ATP and a proton-motive force. Mol. Microbiol. 2014, 92, 399–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chukwubuikem, A.; Berger, C.; Mady, A.; Rosenbaum, M.A. Role of phenazine-enzyme physiology for current generation in a bioelectrochemical system. Microb. Biotechnol. 2021, 14, 1613–1626. [Google Scholar] [CrossRef]
- Reguera, G.; Mccarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.Y.; Wanger, G.; Man, K.; Yuzvinsky, T.D.; Southam, G.; Yang, J. Electrical transport along bacterial nanowires from. Proc. Natl. Acad. Sci. USA 2010, 107, 18127–18131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anguita, J.M.; Rojas, C.; Pastén, P.A.; Vargas, I.T. A new aerobic chemolithoautotrophic arsenic oxidizing microorganism isolated from a high Andean watershed. Biodegradation 2018, 29, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Thrash, J.C.; Van Trump, J.I.; Weber, K.A.; Miller, E.; Achenbach, L.A.; Coates, J.D. Electrochemical stimulation of microbial perchlorate reduction. Environ. Sci. Technol. 2007, 41, 1740–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, H.; Fu, S.; Liu, G.; Zhang, R.; Bai, Y.; Luo, X. Autotrophic biocathode for high efficient sulfate reduction in microbial electrolysis cells. Bioresour. Technol. 2014, 167, 462–468. [Google Scholar] [CrossRef]
- De La Fuente, M.J.; De la Iglesia, R.; Farias, L.; Daims, H.; Lukumbuzya, M.; Vargas, I.T. Electrochemical Enrichment of Marine Denitrifying Bacteria to Enhance Nitrate Metabolization in Seawater. J. Environ. Chem. Eng. 2021, 21, 125452. [Google Scholar] [CrossRef]
- Virdis, B.; Rabaey, K.; Yuan, Z. Microbial fuel cells for simultaneous carbon and nitrogen removal. Water Res. 2008, 42, 3013–3024. [Google Scholar] [CrossRef]
- Xie, S.; Liang, P.; Chen, Y.; Xia, X.; Huang, X. Simultaneous carbon and nitrogen removal using an oxic / anoxic-biocathode microbial fuel cells coupled system. Bioresour. Technol. 2011, 102, 348–354. [Google Scholar] [CrossRef]
- Virdis, B.; Read, S.T.; Rabaey, K.; Rozendal, R.A.; Yuan, Z.; Keller, J. Biofilm stratification during simultaneous nitrification and denitrification (SND) at a biocathode. Bioresour. Technol. 2011, 102, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, H.; Ma, Y.; Yuan, G. Membrane filtration biocathode microbial fuel cell for nitrogen removal and electricity generation. Enzym. Microb. Technol. 2014, 60, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, H.; Zhang, C.; Zhang, G.; Yang, F.; Yuan, G.; Gao, F. Simultaneous nitrogen and carbon removal in a single chamber microbial fuel cell with a rotating biocathode. Process Biochem. 2013, 48, 893–900. [Google Scholar] [CrossRef]
- Virdis, B.; Rabaey, K.; Rozendal, R.A.; Yuan, Z.; Keller, J. Simultaneous nitrification, denitrification and carbon removal in microbial fuel cells. Water Res. 2010, 44, 2970–2980. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Lee, H.L.; Lee, Y.P.; Kim, T.S.; Kim, M.K.; Anh, D.T.N.; Tran, H.T.; Ahn, D.H. Simultaneous carbon and nitrogen removal from piggery wastewater using loop configuration microbial fuel cell. Process Biochem. 2013, 48, 1080–1085. [Google Scholar] [CrossRef]
- Zhu, G.; Huang, S.; Lu, Y.; Gu, X. Simultaneous nitrification and denitrification in the bio-cathode of a multi-anode microbial fuel cell. Environ. Technol. 2019, 42, 1260–1270. [Google Scholar] [CrossRef]
- Pous, N.; Korth, B.; Alvarez, M.O.; Dolors, M.; Harnisch, F. Electrifying biotrickling filters for the treatment of aquaponics wastewater. Bioresour. Technol. 2020, 319, 124221. [Google Scholar] [CrossRef]
- Sunagawa, S.; Coelho, L.P.; Chaffron, S.; Kultima, J.R.; Labadie, K.; Salazar, G.; Djahanschiri, B.; Zeller, G.; Mende, D.R.; Alberti, A.; et al. Structure and function of the global ocean microbiome. Science 2015, 348, 1261359. [Google Scholar] [CrossRef] [Green Version]
- Brailo, M.; Schreier, H.J.; McDonald, R.; Maršić-Lučić, J.; Gavrilović, A.; Pećarević, M.; Jug-Dujaković, J. Bacterial community analysis of marine recirculating aquaculture system bioreactors for complete nitrogen removal established from a commercial inoculum. Aquaculture 2019, 503, 198–206. [Google Scholar] [CrossRef]
- Li, W.-W.; Yu, H.-Q. Stimulating sediment bioremediation with benthic microbial fuel cells. Biotechnol. Adv. 2015, 33, 1–12. [Google Scholar] [CrossRef]
- Sajana, T.K.; Ghangrekar, M.M.; Mitra, A. In Situ Bioremediation Using Sediment Microbial Fuel Cell. J. Hazard. Toxic Radioact. Waste 2017, 21, 04016022. [Google Scholar] [CrossRef]
- Lu, L.; Huggins, T.; Jin, S.; Zuo, Y.; Ren, Z.J. Microbial metabolism and community structure in response to bioelectrochemically enhanced remediation of petroleum hydrocarbon-contaminated soil. Environ. Sci. Technol. 2014, 48, 4021–4029. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.P.; Risgaard-Petersen, N.; Fossing, H.; Christensen, P.B.; Sayama, M. Electric currents couple spatially separated biogeochemical processes in marine sediment. Nature 2010, 463, 1071–1074. [Google Scholar] [CrossRef]
- Umar, M.F.; Rafatullah, M.; Abbas, S.Z.; Mohamad Ibrahim, M.N.; Ismail, N. Advancement in benthic microbial fuel cells toward sustainable bioremediation and renewable energy production. Int. J. Environ. Res. Public Health 2021, 18, 3811. [Google Scholar] [CrossRef]
- Guzman, J.J.; Cooke, K.G.; Gay, M.O.; Radachowsky, S.E.; Girguis, P.R.; Chiu, M.A. Benthic microbial fuel cells: Long-term power sources for wireless marine sensor networks. Sens. Command. Control. Commun. Intell. (C3I) Technol. Homel. Secur. Homel. Def. IX 2010, 7666, 76662M. [Google Scholar] [CrossRef]
- Olias, L.G.; Di Lorenzo, M. Microbial fuel cells for in-field water quality monitoring. RSC Adv. 2021, 11, 16307–16317. [Google Scholar] [CrossRef]
- Erable, B.; Etcheverry, L.; Bergel, A. From microbial fuel cell (MFC) to microbial electrochemical snorkel (MES): Maximizing chemical oxygen demand (COD) removal from wastewater. Biofouling 2011, 27, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Nastro, R.A.; Gambino, E.; Toscanesi, M.; Arienzo, M.; Ferrara, L.; Trifuoggi, M. Microbial Fuel Cells (MFCs) remediation activity of marine sediments sampled at a dismissed industrial site: What opportunities? J. Clean. Prod. 2019, 235, 1559–1566. [Google Scholar] [CrossRef]
- Viggi, C.C.; Presta, E.; Bellagamba, M.; Kaciulis, S.; Balijepalli, S.K.; Zanaroli, G.; Papini, M.P.; Rossetti, S.; Aulenta, F. The “Oil-Spill Snorkel”: An innovative bioelectrochemical approach to accelerate hydrocarbons biodegradation in marine sediments. Front. Microbiol. 2015, 6, 881. [Google Scholar] [CrossRef]
- Zhang, Y.; Angelidaki, I. Bioelectrode-based approach for enhancing nitrate and nitrite removal and electricity generation from eutrophic lakes. Water Res. 2012, 46, 6445–6453. [Google Scholar] [CrossRef]
- Algar, C.K.; Howard, A.; Ward, C.; Wanger, G. Sediment microbial fuel cells as a barrier to sulfide accumulation and their potential for sediment remediation beneath aquaculture pens. Sci. Rep. 2020, 10, 13087. [Google Scholar] [CrossRef] [PubMed]
- Sajana, T.K.; Ghangrekar, M.M.; Mitra, A. Application of sediment microbial fuel cell for in situ reclamation of aquaculture pond water quality. Aquac. Eng. 2013, 57, 101–107. [Google Scholar] [CrossRef]
- Marx Sander, E.; Virdis, B.; Freguia, S. Bioelectrochemical denitrification for the treatment of saltwater recirculating aquaculture streams. ACS Omega 2018, 3, 4252–4261. [Google Scholar] [CrossRef] [PubMed]
- Commault, A.S.; Lear, G.; Novis, P.; Weld, R.J. Photosynthetic biocathode enhances the power output of a sediment-type microbial fuel cell. N. Z. J. Bot. 2014, 52, 48–59. [Google Scholar] [CrossRef]
- Sun, J.; Xu, W.; Cai, B.; Huang, G.; Zhang, H.; Zhang, Y.; Yuan, Y.; Chang, K.; Chen, K.; Peng, Y.; et al. High-concentration nitrogen removal coupling with bioelectric power generation by a self-sustaining algal-bacterial biocathode photo-bioelectrochemical system under daily light/dark cycle. Chemosphere 2019, 222, 797–809. [Google Scholar] [CrossRef]
- Kubota, K.; Watanabe, T.; Maki, H.; Kanaya, G.; Higashi, H.; Syutsubo, K. Operation of sediment microbial fuel cells in Tokyo Bay, an extremely eutrophicated coastal sea. Bioresour. Technol. Rep. 2019, 6, 39–45. [Google Scholar] [CrossRef]
- Yang, X.; Chen, S. Microorganisms in sediment microbial fuel cells: Ecological niche, microbial response, and environmental function. Sci. Total Environ. 2020, 756, 144145. [Google Scholar] [CrossRef]
- Reimers, C.E.; Li, C.; Graw, M.F.; Schrader, P.S.; Wolf, M. The identification of cable bacteria attached to the anode of a benthic microbial fuel cell: Evidence of long distance extracellular electron transport to electrodes. Front. Microbiol. 2017, 8, 2055. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Cheng, S.; Yu, Z.; Yang, J.; Huang, H.; Sun, Y. Enhancing bio-cathodic nitrate removal through anode-cathode polarity inversion together with regulating the anode electroactivity. Sci. Total Environ. 2021, 764, 142809. [Google Scholar] [CrossRef]
- Castro, H.F.; Williams, N.H.; Ogram, A. Phylogeny of sulfate-reducing bacteria 1. FEMS Microbiol. Ecol. 2000, 31, 1–9. [Google Scholar] [CrossRef]
- Emerson, D.; Fleming, E.J.; Mcbeth, J.M. Iron-Oxidizing Bacteria: An Environmental and Genomic Perspective. Annu. Rev. Microbiol. 2010, 64, 561–583. [Google Scholar] [CrossRef] [PubMed]
- Miceli, J.F.; Parameswaran, P.; Kang, D.; Krajmalnik-Brown, R. Enrichment and Analysis of Anode-Respiring Bacteria from Diverse Anaerobic Inocula. Environ. Sci. Technol. 2012, 46, 10349–10355. [Google Scholar] [CrossRef] [PubMed]
- Zhan, G.; Zhang, L.; Tao, Y.; Wang, Y.; Zhu, X.; Li, D. Anodic ammonia oxidation to nitrogen gas catalyzed by mixed biofilms in bioelectrochemical systems. Electrochim. Acta 2014, 135, 345–350. [Google Scholar] [CrossRef]
- Rowe, A.R.; Chellamuthu, P.; Lam, B.; Okamoto, A.; Nealson, K.H. Marine sediments microbes capable of electrode oxidation as a surrogate for lithotrophic insoluble substrate metabolism. Front. Microbiol. 2015, 6, 784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Leary, D.H.; Malanoski, A.H.P.; Li, R.W.; Judson Hervey, W.; Eddie, B.J.; Tender, G.S.; Yanosky, S.G.; Vora, G.J.; Tender, L.M.; et al. A previously uncharacterized, nonphotosynthetic member of the Chromatiaceae is the primary CO2-fixing constituent in a self-regenerating biocathode. Appl. Environ. Microbiol. 2015, 81, 699–712. [Google Scholar] [CrossRef] [Green Version]
- Torres, C.I.; Krajmalnik-Brown, R.; Parameswaran, P.; Marcus, A.K.; Wanger, G.; Gorby, Y.A.; Rittmann, B.E. Selecting anode-respiring bacteria based on anode potential: Phylogenetic, electrochemical, and microscopic characterization. Environ. Sci. Technol. 2009, 43, 9519–9524. [Google Scholar] [CrossRef]
- Kondaveeti, S.; Lee, S.; Park, H.; Min, B. Bacterial communities in a bioelectrochemical denitrification system: The effects of supplemental electron acceptors. Water Res. 2014, 51, 25–36. [Google Scholar] [CrossRef]
- Vilajeliu-Pons, A.; Koch, C.; Balaguer, M.D.; Colprim, J.; Harnisch, F.; Puig, S. Microbial electricity driven anoxic ammonium removal. Water Res. 2018, 130, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Zhang, Y.; Bu, G.; Quan, X.; Liu, Y. Producing nitrite from anodic ammonia oxidation to accelerate anammox in a bioelectrochemical system with a given anode potential. Chem. Eng. J. 2016, 291, 184–191. [Google Scholar] [CrossRef] [Green Version]

| Technology | Advantages | Disadvantages | References |
|---|---|---|---|
| Physicochemical | |||
| Ion Exchange | Selective resins for different pollutants, common application, low production cost. | It requires the resin’s regeneration, brine production, and high use of chemicals (salt). | [50,66] |
| Reverse Osmosis | Remove multiple contaminants, low production cost, environmentally friendly. | Need for post-treatment to remove accumulated contaminants in brine, membrane fouling, high operating cost. | [51,67,68] |
| Electrodialysis | Multiple removals of pollutants, higher water recovery (less waste). | High energy consumption, complex construction, and operation skipping brine production as final waste. | [52,69] |
| Activated Carbon Absorption | It does not generate residues of brine or concentrates, high adsorption capacity, elimination of multiple contaminants. | High cost of material and the high price of regeneration. | [49,70] |
| Chemical | |||
| Chemical denitrification. | Does not generate residues of brine or concentrates, nitrate reduction instead of accumulation in residues, elimination of multiple pollutants. | Inconsistency in nitrate reduction, pH, and temperature dependence. Risk of ammonia or nitrite production in the nitrate removal process. | [71,72] |
| Biological | |||
| Conventional Biological nitrification and denitrification technologies. | No dangerous byproducts are generated, no additional treatment is required, removal of multiple pollutants, lower cost of operation than physicochemical treatments in general. | Constant oxygenation of the medium is necessary (nitrification), and the addition of organic or inorganic electron donor (denitrification) post-treatments is also required for turbidity and sludge removal. | [53,54] |
| Non-conventional biofilm-based technologies | High complex biomass concentration per volume of bioreactor. Chemical gradients coupled with oxygen gradient (oxic and anoxic zones) lead to increased carbon and nitrogen removal in the same compartment. | Possible high mass transfer resistance. Scaling-up problems such as biofouling, granular disintegration, and mechanical failures. It is highly affected by suspended solids. | [60,61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De La Fuente, M.J.; Gallardo-Bustos, C.; De la Iglesia, R.; Vargas, I.T. Microbial Electrochemical Technologies for Sustainable Nitrogen Removal in Marine and Coastal Environments. Int. J. Environ. Res. Public Health 2022, 19, 2411. https://doi.org/10.3390/ijerph19042411
De La Fuente MJ, Gallardo-Bustos C, De la Iglesia R, Vargas IT. Microbial Electrochemical Technologies for Sustainable Nitrogen Removal in Marine and Coastal Environments. International Journal of Environmental Research and Public Health. 2022; 19(4):2411. https://doi.org/10.3390/ijerph19042411
Chicago/Turabian StyleDe La Fuente, María José, Carlos Gallardo-Bustos, Rodrigo De la Iglesia, and Ignacio T. Vargas. 2022. "Microbial Electrochemical Technologies for Sustainable Nitrogen Removal in Marine and Coastal Environments" International Journal of Environmental Research and Public Health 19, no. 4: 2411. https://doi.org/10.3390/ijerph19042411
APA StyleDe La Fuente, M. J., Gallardo-Bustos, C., De la Iglesia, R., & Vargas, I. T. (2022). Microbial Electrochemical Technologies for Sustainable Nitrogen Removal in Marine and Coastal Environments. International Journal of Environmental Research and Public Health, 19(4), 2411. https://doi.org/10.3390/ijerph19042411







