Abstract
The opportunistic infections with Gram-negative bacilli are frequently reported. The clinical studies are focused on the course of human infectious and very often the source of infection remain unclear. We aim to see if the Gram-negative bacilli isolated from a non-contaminated environment—the caves—are reported in human infections. Eleven samples were collected from six Romanian caves. We used the standard procedure used in our clinical laboratory for bacterial identification and for antibiotic susceptibility testing of the cave isolates. Out of the 14 bacterial strains, three isolates are Gram-negative bacilli—one isolate belong to Hafnia alvei and two strains belong to Sphingomonas paucimobilis. We screened for the published studies—full-text original articles or review articles—that reported human infections with S. paucimobilis and H. alvei. Data sources—PubMed and Cochrane library. We retrieved 447 cases from 49 references—262 cases (58.61%) are S. paucimobilis infections and 185 cases (41.39%) are H. alvei infections. The types of infections are diverse but there are some infections more frequent; there are 116 cases (44.27%) and many infections of the bloodstream with S. paucimobilius (116 cases) and 121 cases (65.41%) are urinary tract infections with H. alvei. The acquired source of the bloodstream infections is reported for 93 of S. paucimobilis bloodstream infections—50 cases (43%) are hospital-acquired, and 40 cases (37%) are community-acquired. Most of the infections are reported in patients with different underlying conditions. There are 80 cases (17.9%) are reported of previously healthy persons. Out of the 72 cases of pediatric infections, 62 cases (86.11%) are caused by S. paucimobilis. There are ten death casualties—three are H. alvei infections, and seven are S. paucimobilis infections.
1. Introduction
Opportunistic infections are challenging issues [1,2]. The infections with multi-drug resistant strains are spreading in the hospital environment and cause serious diseases, mainly in immune-compromised persons [3,4]. It is difficult to establish the source of opportunistic infection, mainly in the hospital environment [5]. The need for the discovery of new antibiotics has galvanized researchers to focus on microorganisms that resist antibiotics [6]. A recent report of the complete genome of the Hafnia alvei A23BA isolated from plant rhizosphere demonstrates that environmental strains could advance the discovery of antibiotic-producing environmental strains [7].
In addition, the complete genome sequence of S. paucimobilis strain Kira was recently published. The strain consists of 3,917,410 bp, with a G + C content of 65.7% [8]. The organisms have to adapt to a large panel of factors that are not constant—temperature, carbon sources, pollutants, etc. [9,10]. The cave environment is unique for its lack of light and constant climate, and where the contaminations with pollutants can be minimal in its deepest parts [11,12,13,14]. As opposed to the relatively pristine cave environment, hospital environments are highly contaminated with and antibiotics. In addition, the specific cleaning procedures in hospitals greatly influence the microbial community [15]. Antibiotic resistance is the most striking phenotypic feature that evolved in hospital environment. In a balanced environment, the microorganisms are in a dynamic and complex process of adaptation. The innate antibiotic resistance is an important aspect of the competition between the microorganisms and is of great interest because some antibiotics, such as colistin, are the last resort for the treatment of multi-drug resistant bacterial infections [16,17,18,19].
In a complex project where different aspects of caves ecosystems have been analyzed, the presence of bacterial species involved in human infections was of interest. This review aimed to evaluate the potential Gram-negative bacilli implicated in human infections from non-polluted cave environments. Gram-negative environmental bacilli could cause opportunistic infections [20,21,22]. We isolated two Gram-negative opportunistic species, Hafnia alvei (H. alvei) and Sphingomonas paucimobilis (S. paucimobilis), in samples collected from six caves.
H. alvei is a Gram-negative rod that belongs to Enterobacterales and until 1978 it was placed in the genus Enterobacter—Enterobacter alvei and Enterobacter hafniae [23]. A brewery variety of H. alvei biogroup 1 is Obesumbacterium proteus [24]. H. alvei habits the intestine of humans and other animals and is found in sewage, soil, water, and dairy products. H. alvei is considered a potential pathogen in patients with underlying diseases [25,26].
S. paucimobilis is a strictly aerobic, non-fermentative Gram-negative rod that belongs to the genus Pseudomonas until 1977 [27]. S. paucimobilis produces a yellow pigment and could be confused with flavobacteria because its mobility is difficult to demonstrate. It was isolated from the environment and solutions used for cleaning wounds [28].
The clinical samples with S. paucimobilis or H. alvei are no longer considered contaminated samples or an indicator of non-conformity. The S. paucimobilis is intrinsically resistant to the action of polymyxin antibiotics (polymyxin B and colistin) [29,30]. Some structural modifications of the lipopolysaccharide of S. paucimobilis may be the results of the adaptation of these species to environmental conditions [31].
The present study aims to connect the biological studies with the clinical reports of opportunistic infections. The biological studies are focused on the accurate identification of the microorganisms by high-standards methods like DNA sequencing by the Sanger method [32]. The clinical reports of the infections rely on clinical laboratory identification methods. Most clinical laboratories use standard identification methods. Only well-equipped microbiological laboratories from clinical facilities could routinely perform MALDI-TOF mass spectrometry (MS) [33].
To link biological research to clinical trials, one approach is to use the same method for microorganisms’ identification. The other approach is to confirm the species’ identification by the MALDI-TOF MS or DNA sequencing by the Sanger method [9]. The second approach is hampering the availability of the clinical isolates. In clinical laboratories, the isolates are usually not preserved for future studies. However, the confirmation of the environmental species isolated from human infections is crucial to identify the source of contamination [34]. This study emphasized the need for a connection between the fundamental research and the clinical outcomes. In the present review, we address the type of infections according to age, underlying conditions, and course of infection with S. paucimobilis or H. alvei. We sought to have comparable outcomes with the clinical studies that reported human infectious with Gram-negative bacilli isolated from our cave samples. Therefore, we used the standard techniques methods used in clinical laboratories—Gram staining, routine culture media for primary isolation, and Vitek2 Biomerieux System for identification and antibiotic susceptibility testing. We sought the treatment of the infections with S. paucimobilis or H. alvei described in the literature. The present study is an update of the case reports and the reviews that concern the human infections with two environmental Gram-negative bacilli organisms isolated from the pristine cave—S. paucimobilis or H. alvei. We made a deep analysis of the reported cases in the literature, and we highlight the importance of accurate bacterial identification in the case of human infections with opportunistic microorganisms.
2. Materials and Methods
2.1. Cave Samples Collection and Bacterial Identification
2.1.1. Cave Samples Collection
Eleven samples were collected from six Romanian caves: Topolniţa, Cloşani, Muierilor (southern Romania), Apă din Valea Leșului, Ferice (northwestern Romania), and Tăuşoare (northern Romania) during the spring of 2019. The collected samples were Ursus spealaeus (the extinct cave bear), bones (Muierilor Cave) and sediments on the floor from all the caves. Samples were collected in sterile Falcon vials or plastic bags, transported on ice, and kept at −60 °C in the laboratory until analysis.
2.1.2. Bacteriological Identification and Antibiotic Phenotype of the Cave Isolates
We used the standard procedure used in our clinical laboratory for bacterial identification and for antibiotic susceptibility testing.
Routine culture media were used for bacterial isolation: nutrient broth, blood agar, MacConkey agar, Chapman agar, Sabouraud agar, and Chromogenic Modified URI-COLOUR LAB-AGARTM (culture media are from BioMaxima). The microbial identification and antibiotic susceptibility testing were made by Vitek2 Biomérieux System. The Vitek2 card types for identification were: BCL, CBC, GP, and GN. Only the S. paucimobilis and H. alvei strains were subject to antimicrobial susceptibility testing using the Vitek2 card type N222. The interpretation of antimicrobial testing was made according to the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines for H. alvei. The interpretation of antimicrobial testing for S. paucimobilis was made according to CLSI guideline for “Other Non-Enterobacterales” category. the EUCAST-based therapeutic guideline does not provide interpretative standards for S. paucimobilis. Colistin resistance was defined as MIC >2 μg/mL (EUCAST guidelines for H. alvei and Acinetobacter baumannii)(EUCAST—Colistin Breakpoints—guidance document 2021) [35,36,37].
2.2. Search Strategy for Literature Review
The search was done in PubMed database (https://www.ncbi.nlm.nih.gov/pubmed/, accessed on 12 May 2021 and Cochrane Library database (http://www.cochranelibrary.com/) (accessed on 12 May 2021). The search terms were “Sphingomonas paucimobilis” or “Hafnia alvei”.
References Collection, Screening, and Selection
Inclusion criteria: The free full-text original articles or review articles; only English written articles; human infections.
Exclusion criteria: Conference papers; proceedings papers; comments; book chapters; non-English written articles; animal infections; the articles with no full text available
The screening and evaluation for eligibility of the references retrieved were made with the Covidence software (www.covidence.org) (accessed on 12 May 2021) (Figure 1).
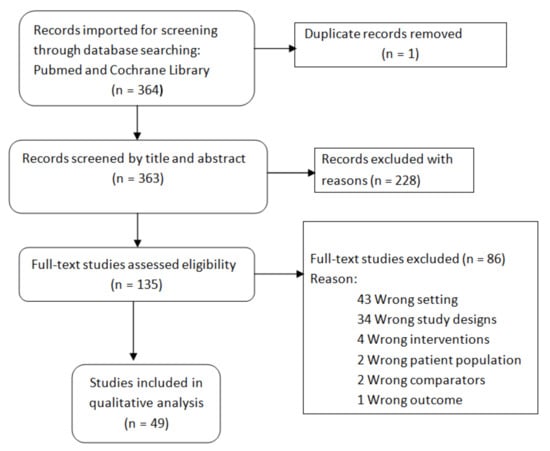
Figure 1.
Flowchart of references selection.
2.3. Statistical Analysis
The results were analyzed in Excel from the Microsoft Office package. The Student’s t-test was used to evaluate the relationship between the samples. The p-value < 0.05 threshold was considered to reject the null hypothesis. Review Manager 5.4.1 was used to evaluate the forest plot. The analysis of variance (ANOVA) was used for analyses the relationship between more groups of data (F-value).
3. Results
3.1. The Cave Isolates
We collected eleven samples from six Romania caves. Fourteen bacterial strains were isolated and identified according to morphology, culture and biochemical properties. Out of the 14 bacterial strains, three were Gram-negative bacilli (Table 1).

Table 1.
The bacterial strains were isolated from cave samples.
The present study aims to review the Gram-negative bacilli isolated from human infections. We selected the Gram-negative bacilli S. paucimobilis and H. alvei. The Gram-staining showed the Gram-negative rods, non-spore-forming. The S. paucimobilis produces convex, smooth, round transparent colonies, lactose-negative with yellowish pigment. On URI Chromogenic agar the S. paucimobilis produced small greenish-violet colonies. S. paucimobilis grows slowly on the nutrient broth and produces weak turbidity.
H. alvei organisms are small Gram-negative rods that form small, smooth, transparent, lactose-negative colonies. On URI Chromogenic agar, the H. alvei produced small greenish colonies. H. alvei produces uniform turbidity of the nutrient broth.
We selected card type N222 for antibiotic susceptibility testing of the S. paucimobilis and H. alvei strain isolated from caves. The CLSI and EUCAST MIC interpretation guidelines were compared (Table 2). For S. paucimobilis there are no interpretative standards in CLSI and EUCAST guidelines. We characterize the S. paucimobilis according to the CLSI standards for “Other Non-Enterobacterales” and according to the EUCAST standards for the non-fermentative bacilli Acinetobacter baumannii [35,36].

Table 2.
The antibiotic resistance phenotype of the S. paucimobilis and H. alvei strains.
3.2. The Results of the Literature Review
3.2.1. Type of Studies
Because the type of infections with S. paucimobilis and H. alvei are rare, most of the studies are case reports. Twenty-eight of the 49 references included in this review are case reports; four are case report and literature reviews, and four are case report reviews (Figure 2).
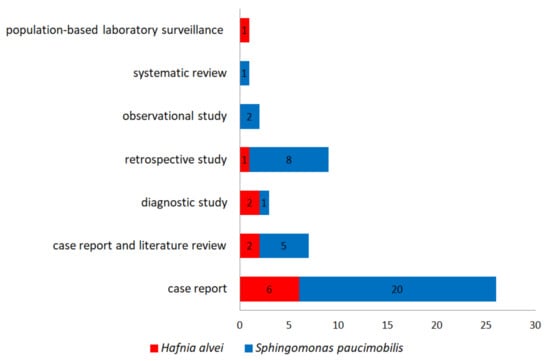
Figure 2.
The number of the references (study types) included in the present review.
3.2.2. The Case Report and Literature Reviews
There are seven references for case reports and literature reviews included in our study (Figure 2) [38,39,40,41,42,43,44]. We analyzed all the references cited in these seven references. We eliminate the duplicate case reports when count the total number of cases (Figure 3). When the literature review was not clearly systematized, we considered only the case report of the study [42]. We retrieved for further analyses 89 cases from the seven case reports and literature review references [38,39,40,41,42,43,44]. After screening the rest of the 42 references, we retrieved 358 cases. No duplicates were found. We included in our analysis 447 cases—262 S. paucimobilis infections and 185 H. alvei infections (Figure 3) [34,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84].
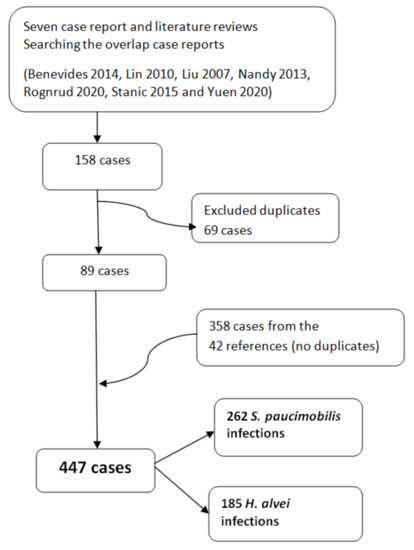
Figure 3.
The number of case reports/infections with S. paucimobilis and H. alvei retrieved from the 49 references included in the present review. The duplicated were excluded.
3.2.3. Type of Infections
The infections with S. paucimobilis and H. alvei are diverse and are reported both in immune-competent persons and in persons with underlying conditions. There are some differences in the type of infections reported with S. paucimobilis and compared to H. alvei. The most frequent infections with S. paucimobilis are bloodstream infections (BSI). The infections with H. alvei most frequently reported are urinary tract infections (UTI). The one-tailed two independent t-Test was made to compare the infections with S. paucimobilis and H. alvei. There was no statistical difference (p = 0.3) between the S. paucimobilis infections (M = 29.11, SD = 35.51) and H. alvei infections (M = 20.56, SD = 38.79) (Table 3).

Table 3.
The overall comparison of the infections with S. paucimobilis and H. alvei.
In most of the cases, the outcome of S. paucimobilis and H. alvei infections is favorable. There are ten death casualties reported despite the antibiotic treatment (Figure 4) [34,40,43,47,48,65,70,71,75].
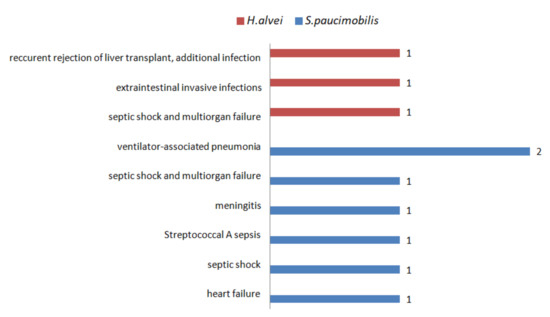
Figure 4.
The number of cases with lethal outcomes of S. paucimobilis and H. alvei infections.
3.2.4. The Bloodstream Infections
Out of the 447 cases included in the present review, 136 (30.42%) are bloodstream infections (BSIs). Out of 136 BSIs, 116 (85.29%) are S. paucimobilis infections and 20 (14.71%) are H. alvei infections. We further proceed with a deeper analysis of the BSIs because the studies provide comparable details about the origin of infection, the acquired source, and the underlying conditions associated with BSIs (Table 3, Figure 5 and Figure 6).
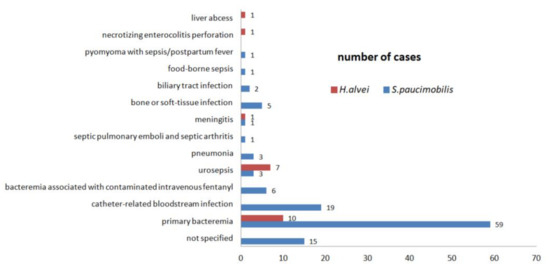
Figure 5.
The source of infection is associated with bloodstream infections. Standard deviation s. S. paucimobilis s = 15.67; H. alvei s = 3.08.
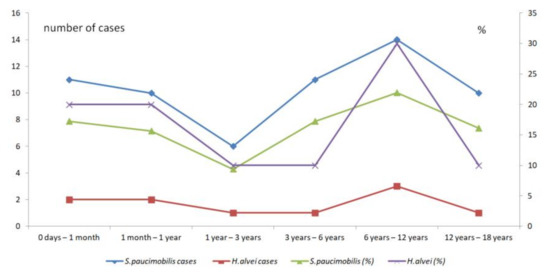
Figure 6.
The comparison of the pediatric S. paucimobilis and H. alvei infections according to age.
Six references provide details about the acquired source of S. paucimobilis BSIs (Figure 6) [39,41,56,59,65,71]. Out of the 116 S. paucimobilis BSIs, 50 cases (43%) are hospital-acquired BSIs, and 43 (37%) cases are community-acquired BSIs. For the H. alvei BSIs, there are no details available regarding the acquired source of infection (Table 4). The acquired source of infection is an important indicator when analyzing the environmental species. The accurate bacterial identification by routine laboratory procedures is crucial in order to establish the source of infection and the treatment options.

Table 4.
The acquired source of BSIs.
The underlying condition of the patients with BSIs is an important indicator when analyzing the clinical outcomes. Most of the patients have severe underlying conditions—malignancies or diabetes mellitus (Table 5).

Table 5.
The underlying conditions of the patients with BSIs.
3.2.5. The Urinary Tract Infections
Out of the 125 UTIs, 121 (97.58%) are H. alvei UTIs (Table 3). Laupland et al., in a population-based laboratory surveillance study conducted in the Calgary Health Region during 2000–2005, show the urine was the most common focus of H. alvei isolation—112 (81.16%) cases from a total of 138 patients. The identification and the antibiotic susceptibility testing were performed using Vitek Biomérieux System [51]. The Rahman et al., in a retrospective study about the UTIs in female subjects, shows that the H. alvei are less frequent (2%) in urine samples compared with E. coli (69%) or other bacterial species. The bacterial identification was made by conventional biochemical tests and antibiotic susceptibility testing was performed by disk diffusion method [61]. Toh et al., in a retrospective study, reported two S. paucimobilis UTIs that were healthcare-associated. The identification was made with Bactec or API 20NE Biomérieux System and antibiotic susceptibility testing was performed by disk diffusion method [71]. Demir et al. and Hassan et al. reported two cases of S. paucimobilis UTIs reported in immune-deficient patients with multiple underlying conditions [50,83]. The sepsis combined with UTI was described in immune-deficient patients. The H. alvei urosepsis is more frequent than S. paucimobilis urosepsis (Figure 5).
3.2.6. The Respiratory Tract Infections
There are 28 cases of respiratory tract infections—17 S. paucimobilis infections and 11 H. alvei infections (Table 3). It is described a case of S. paucimobilis ventilator-associated pneumonia with significant dysbiosis associated COVID-19 [80]. These demonstrate the interest in clinical identification tests for environmental species that could be responsible for infections in immune-deficient patients. The decision about the presence of environmental species in a sample greatly depends on the response of the following question. Is it contamination or infection? Laupland et al., in a population-based laboratory surveillance study, reported ten (7.25%) lower respiratory infections with H. alvei from a total of 138 patients—seven of the lower respiratory infections were hospital infections [51]. Toh et al. reported 12 S. paucimobilis pneumonia/empyema infections, ten of them ventilator-associated pneumonia [71]. Apart from the lower respiratory infections reported in tertiary care units, a rare case of Yuan et al. reported a case of S. paucimobilis empyema secondary to foreign body aspiration [77]. Eckrich et al. reported a case of S. paucimobilis involvement of small airway disease in a patient with cystic fibrosis. The study includes a healthy control group [84].
3.2.7. The Pediatric Infections
A particular characteristic of opportunistic infections is their occurrence in children. Out of the 447 cases included in the present review, 72 (16.11%) are pediatric infections. The studies concerning pediatrics offer accurate data about the age range, type of infections, and underlying conditions. We could make a detailed analysis of the 72 cases S. paucimobilis and H. alvei infections in children identified in the references included in the present review. Out of the 72 infections in children, 62 (86.11%) are S. paucimobilis infections. Most infections have been described in school-aged children (six years to 12 years). There is a significant difference between the pediatric S. paucimobilis infections (M = 10.33, SD = 2.58) compared with pediatric H. alvei infections according to age (M = 1.67, SD = 0.82) (Table 6).

Table 6.
The overall comparison of the pediatric infections with S. paucimobilis and H. alvei according to age.
However, when compares the percentages of pediatric infections according to ages there is no significant difference (t(10) = - 0.12, p = 0.45) between the S. paucimobilis infections (M = 16.23, SD = 4.03) and H. alvei infections (M = 16.67, SD = 8.16). More, the peak of infections is between 6 years to 12 years for both species (Figure 6).
The detailed comparison of the S. paucimobilis and H. alvei reveals that there is no difference between the types of infections in pediatric and adult cases. However, the panel of types of infection is more diversified in adult infections (Table 7).

Table 7.
The comparisons of the type of pediatric infections with adult infections.
The underlying conditions associated with S. paucimobilis and H. alvei infections are diverse. The most frequent underlying conditions are associated with the impairment of immunity and neutropenia, consequently of malignant diseases. We noticed the infections resulted as direct or indirect contamination—trauma, ventilatory-associated pneumonia, peritoneal dialysis-associated peritonitis, or bacteremia associated with contaminated intravenous fentanyl. There are infections in patients with no underlying conditions—in our study, we identified 80 (17.9%) cases reported in previously healthy patients (Table 8). However, in many cases, the source of infection remains elusive. This is an important indicator that could advance the understanding of these types of infections.

Table 8.
The comparisons of the underlying conditions associated with the pediatric infections and adult infections.
Malignant diseases were reported both in adult and pediatric infections with S. paucimobilis and H. alvei. However, there were notable differences about the type of malignancy when there were details about the nature of the malignancy.
In adult infections, the most frequent are the solid cancers—oral cancer, hepatocellular carcinoma, colon cancer, hypopharyngeal cancer, esophageal cancer, bladder carcinoma, breast cancer, cholangiocarcinoma, and ovarian cancer.
In pediatric infections, the most frequent are the blood and bone marrow cancers—acute lymphoblastic leukemia, aplastic anemia, lymphoma, non-Hodgkin’s lymphoma, acute myeloid leukemia, and acute non-lymphocytic leukemia after allogenic bone marrow transplantation. In addition, the solid cancers associated with pediatric infections are different from those reported in adult infections—neuroblastoma, anaplastic ependymoma, localized osteosarcoma, and Ewing sarcoma.
The types of infections are various and not always explained by underlying conditions. Out of 72 of the pediatric cases, 27 (37.5%) involved children with no underlying conditions. In this respect, there are open questions about the S. paucimobilis and H. alvei infections in children regarding the infections with environmental species in healthy children. What could other host factors be responsible for the initiation and evolution of these infections? Out of the 72 pediatric cases, 23 (31.94%) were reported in children with malignancy—one case is H. alvei infection, and 22 cases were S. paucimobilis infections (Table 4).
3.2.8. The Studies with Healthy-Control Groups
There are two studies that enrolled healthy-control groups—the Eckrich et al. study involving small airway disease in mild cystic fibrosis and the Ridell et al. study about the association of H. alvei with diarrhea [63,84]. Eckrich et al. conclude that the sputum neutrophils is the most informative indicator to prevent lung damage and identify Pseudomonas aeruginosa and Staphylococcus aureus, the most frequent species that colonize the airways in cystic fibrosis. Out of 32 cystic fibrosis cases, one case is S. paucimobilis infection [84]. The Ridell et al. study stressed that the H.alvei involvement in diarrhea is due to a mechanism that differs from the attachment–effacement mechanism [63]. We selected the S. paucimobilis and H. alvei cases and in a forest plot, we observed the probability of a healthy person to be infected with these two bacterial species (Figure 7). Because of the limited number of studies with healthy-control groups available, the interpretation of the results is not accurate.
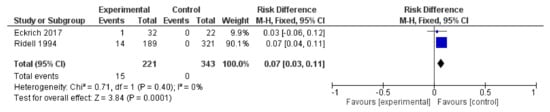
Figure 7.
The comparison of health-control groups with the S. paucimobilis small airway disease (Eckrick et al.) and H. alvei diarrhea (Ridell et al.), respectively.
3.2.9. The Microbiological Diagnostic Methods
The accurate bacterial species is crucial to the diagnostic process. The treatment greatly depends on laboratory findings. However, routine diagnostic tests are designed mainly for bacterial species that are often isolated from human infections. The environmental species are rarely reported—in our study, these case reports. Yet, the environmental species are isolated not only from immune-compromised persons but from healthy persons. In addition, very often the environmental species inherit the gene of antibiotic resistance. It is of great interest to accurately establish the antibiotic resistance phenotype. The laboratory diagnostic methods and antimicrobial susceptibility were specified in the references and are presented in Figure 8. When specified, the bacteriological methods mostly rely on conventional methods.
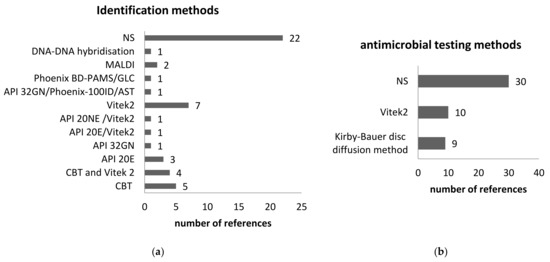
Figure 8.
The bacteriological diagnostic was reported on the 49 references included in the study. (a) Identification methods; (b) antimicrobial susceptibility testing. NS—not specified, Phoenix BD-PAMS—BD Phoenix Automated Microbiology System and gas-liquid chromatography, GLC—gas-liquid chromatography; CBT- conventional biochemical tests.
3.3. The Innate or Natural Antibiotic Resistance
The environmental bacterial species are susceptible to most classes of antibiotics. The innate or natural resistance to some antibiotics is described for S. paucimobilis and H. alvei. The EUCAST guidelines reported the innate resistance of H. alvei and H. paraalvei at aminopenicillins, aminopenicillins and beta-lactamases inhibitors, cephalosporin’s first generations, and colistin. The CLSI guidelines reported the innate resistance of H. alvei at ampicillin, amoxicillin-clavulanic acid, ampicillin-sulbactam, cephalosporins I (cefazolin, cephalothin), and cephamycins (cefoxitin, cefotetan) [35,36]. Holmes B. et al. described the resistance of Hafnia isolates to cephalosporins and penicillin [24]. The CLSI and EUCAST guidelines are focused on the bacterial species of medical interest. There are no indications of the innate resistance for S. paucimobilis. Pitt T.L. mentioned that most of the S. paucimobilis are resistant to ureidopenicillins and ‘earlier’ cephalosporins [28].
The three bacterial species isolated from caves have an inherent resistance to colistin. Although the commercial cards Vitek2 for antimicrobial testing do not accurately determine the resistance at colistin, we further analyzed the results. It is well-known that S. paucimobilis has inherent resistance to colistin and the H. alvei do not have an inherent resistance to colistin. However, Jayol et al. suggest that the colistin resistance is underestimated by conventional antibiotic testing methods, and Hafnia is a naturally colistin-resistant enterobacterial genus [85].
3.4. The Limitations of the Study
The reports of opportunistic infections with environmental species are rare, and the characteristics are not constantly reported.
The commercial Vitek2 identification cards are suitable for the identification of clinically significant bacterial species. However, the environmental species require slightly different growing conditions—e.g., lower temperature and a prolonged time of incubation. The actual EUCAST guidelines recommend the micro-dilution broth method for colistin, which is not a routine method in clinical laboratories.
3.5. The Strength of the Study
The study links the research aspects of the environmental bacterial species with the clinical characterization of human infections. We started our study by screening a balanced environment—the caves—in order to isolate the Gram-negative bacilli that are already reported in the literature in opportunistic infections. The use of the identification method and antimicrobial testing method currently used in clinical laboratories permits an overview of human infections with environmental Gram-negative bacilli.
4. Discussion
In our study, we isolated 14 bacterial strains from 11 samples collected from six Romanian caves; some of these species could cause human infections. Aerococcus viridian is a rarely reported Gram-positive cocci, opportunistic organism in endocarditis or urinary tract infections [86,87]. Rhodococcus coprophilus is a Gram-positive aerobic bacteria that is a fecal indicator of freshwater [88]. Bacillus cereus is a Gram-positive spore-forming rod associated with food poisoning and rarely with local and severe systemic infections [89]. Bacillus smithii is a thermophilic Gram-positive rod recently evaluated as a probiotic candidate in inflammatory bowel disease treatment [90]. Geobacillus thermoleovorans is a recently sequenced Gram-positive thermophilic bacteria [91]. Geobacillus species are evaluated for applications in biotechnology [92]. Corynebacterium afermentans is a Gram-positive rod rarely isolated from brain and liver abscesses and orthopedic infections [93,94,95,96]. In the present study, we selected the Gram-negative bacilli reported in human infections—H. alvei and S. paucimobilis.
There are many studies attempting to respond to the question about the relevance of the presence of an environmental bacterial species in clinical samples. The lack of uniformity in reported data does not allow advancing the analysis in many aspects—the source of infections, the clinical data, the treatment, and the identification methods. Laupland et al. admitted that, in their population-based study, the H. alvei infections are overestimated for two potential reasons—lack of clinical data and the lack of confirmation of the residence status of the patients included in the study [51]. We identified another risk of bias—the accuracy of the identification method. The standard tests used in clinical laboratories are designed to identify the most encountered bacterial species isolated in human infections. In our study, the H. alvei cave isolate was identified with 86% probability according to Vitek2 Biomérieux System.
In a recent study, Yu et al., using a 16S rRNA gene sequence analysis, demonstrated that a Vogesella perlucida isolate was misidentified as H. alvei by traditional microbiological testing [97]. The H. alvei isolate exhibits colistin resistance, which is unusual for an environmental isolate. Even though human infections with environmental species are rare, the present study shows that S. paucimobilis and H. alvei infections are reported in a large panel of samples both in children and adults.
The source of infection is of great interest in terms of isolated opportunistic species. Effective treatment depends on the decision about the nature of the presence of bacterial specie isolated. However, even when the possibility of contamination is very unlikely, it is impossible to determine the source of infection [47]. Saboor et al. hypothesized that drinking water was a source of S. paucimobilis BSI in an immune-competent 10-year-old boy based on the ubiquity of this bacterial species [65].
Ventilator-associated pneumonia is very often life-threatening. In the context of the ongoing COVID-19 pandemic, Cutuli et al. reported a H. alvei pneumonia in patients that need mechanical ventilation. The authors stressed the importance of monitoring the microbiota to early diagnose infectious diseases [80]. A very recent case report highlighted that the rapid and accurate diagnosis of H. alvei infection elucidated the diagnosis of suspicious pulmonary masses [98]. The analysis of opportunistic species in their natural environment could advance the understanding of their involvement in human infections. Very often, opportunistic infections are reported in an immune-compromised host with underlying conditions. Our study revealed that in practice infections with S. paucimobilis and H. alvei are reported in immune-compromised and immune-competent patients.
Some authors highlighted that they do not notice an apparent immune suppression [38,39,41,47,56,65]. However, because the opportunistic infections are the consequence of a lack of equilibrium with a host-microorganism, it is relevant to analyze both sides of the balance. This was the main reason for our study, from searching the Gram-negative species from samples collected from a non-contaminated habitat—the caves. The competition of the microorganisms that inhabit a specific environment is a complex and dynamic process that depends on many factors. The analysis of the factors that act in concert in a balanced environment is beyond the aim of the present paper, but the present review revealed that the infections with S. paucimobilis and H. alvei are reported worldwide and there are many open questions about the source of contamination and about the relation host-microorganism.
The source of infections is an important indicator to distinguish between hospital-acquired infections and community-acquired infections. In the clinical reports, this indicator is reported mainly for BSIs. The samples are taken on admission from the previously hospitalized patients, but it is not always easy to determine the origin of the infection. Ryan et al. reported the BSIs due to S. paucimobilis in patients with an underlying disease or condition that determines an unfavorable clinical outcome [99].
In contrast, we identify the S. paucimobilis in patients with no underlying conditions reported—22 infections and one pediatric infection. Furthermore, for 76 S. paucimobilis infections (one adult infection and 75 pediatric infections) the presence of underlying conditions is unclear. Similarly, there were 57 H. alvei infections (five adult infections and 52 pediatric infections) reported from patients with no underlying conditions. There are 114 pediatric H. alvei infections for which the presence of underlying conditions is unclear. In our opinion, the clinical context of infections with low pathogens is crucial.
The infections with S. paucimobilis and H. alvei were successfully treated in most of the cases analyzed in the present review. In our study, we identified a few deaths reported in highly debilitated patients.
An important issue in clinical laboratories is the accurate identification of bacterial species isolated from human infection by routine laboratory tests. Even the environmental species are considered opportunistic—our study shows that there are many case reports that highlight a large panel of infections with two environmental species—H. alvei and S. paucimobilis. In order to have comparative methods with the clinical reports, we chose to maintain routine methods for identification. However, the present study is part of a larger study where a huge number of cave isolates will be identified by sequence-based bacterial analysis, which was 16S rRNA sequencing by the Sanger method [9].
We are interested in the antibiotic-resistance phenotype of bacterial species isolated from uncontaminated environments. The issue is relevant in the context of the treatment of opportunistic infection with these species. S. paucimobilis has an innate resistance to colistin, which is a reserve antibiotic. However, there are discussions about an adequate method for testing the polymyxin B/colistin and the critical breakpoints. CLSI recommended “intermediate” or “resistant” categories do not fit the EUCAST categories (EUCAST—Colistin Breakpoints—guidance document 2021). EUCAST guidelines recommend the micro-broth dilution method to detect the resistance to polymixinB/colistin and use colistin sulphate. Although polymixinB/colistin are reserve antibiotics that are not recommended in monotherapy, the emerging of multi-drug resistant species that exhibit the resistance to colistin need adequate interpretative guidelines [17,29,99,100]. A method suitable for the routine testing of polymixinB/colistin susceptibility is of great interest and there are studies that propose alternatives to the microbroth dilution method [101].
5. Conclusions
We isolated two Gram-negative bacilli, S. paucimobilis and H. alvei, from cave samples, which were identified by conventional bacteriological methods in order to have comparable outcomes with clinical reports. We sought to review the clinical reports with the environmental species. The environmental species should not be considered contaminants without a thorough analysis.
Human infections with S. paucimobilis and H. alvei are rare and reported mostly in debilitated patients with underlying diseases. However, our review that included 49 references and 447 cases stressed that S. paucimobilis and H. alvei were isolated from immune-deficient and immune-competent hosts and that the source of infections is not easily determined. A deep view of the opportunistic species in their natural habitat could advance the understanding of the infections mainly in immune-competent hosts.
Author Contributions
Conceptualization, M.I.I.; methodology, M.I.I., D.Ș.N.; writing—original draft preparation, M.I.I.; writing—review and editing, M.I.I., O.T.M.; supervision, O.T.M., A.M.C., M.I.I.; project administration, O.T.M., A.M.C.; funding acquisition, O.T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from the Ministry of Research and Innovation, CNCS—UEFISCDI, project number PN-IIIP4-ID-PCCF-2016-0016, within PNCDI III, contract nr. 2/2019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank the reviewers for their comments, which have greatly improved the quality of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Centers for Disease Control and Prevention; Infectious Disease Society of America; American Society of Blood and Marrow Transplantation. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients MMWR. Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2000, 49, 1–125, CE1-7. [Google Scholar]
- Kumar, R.; Ison, M.G. Opportunistic Infections in Transplant Patients. Infect. Dis. Clin. N. Am. 2019, 33, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Gao, J.; Tang, W. Nosocomial infection and its molecular mechanisms of antibiotic resistance. Biosci. Trends 2016, 10, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Rizi, K.S.; Hasanzade, S.; Soleimanpour, S.; Youssefi, M.; Jamehdar, S.A.; Ghazvini, K.; Safdari, H.; Farsiani, H. Phenotypic and molecular characterization of antimicrobial resistance in clinical species of Enterobacter, Serratia, and Hafnia in Northeast Iran. Gene Rep. 2021, 25, 101352. [Google Scholar] [CrossRef]
- Ta, C.; Wong, G.; Cole, W.; Medvedev, G. Scrub sink contamination and transmission to operating room personnel. New Microbes New Infect. 2020, 37, 100754. [Google Scholar] [CrossRef]
- Quinn, G.A.; Banat, A.M.; Abdelhameed, A.M.; Banat, I.M. Streptomyces from traditional medicine: Sources of new innovations in antibiotic discovery. J. Med. Microbiol. 2020, 69, 1040–1048. [Google Scholar] [CrossRef]
- Awolope, O.K.; O’Driscoll, N.H.; Di Salvo, A.; Lamb, A.J. The complete genome sequence of Hafnia alvei A23BA; a potential antibiotic-producing rhizobacterium. BMC Res. Notes 2021, 14, 20–23. [Google Scholar] [CrossRef]
- Nishimura, K.; Ikarashi, M.; Yasuda, Y.; Sato, M.; Guerrero, M.C.; Galipon, J.; Arakawa, K. Complete Genome Sequence of Sphingomonas paucimobilis Strain Kira, Isolated from Human Neuroblastoma SH-SY5Y Cell Cultures Supplemented with Retinoic Acid. Microbiol. Resour. Announc. 2021, 10, e01156-20. [Google Scholar] [CrossRef]
- Bercea, S.; Năstase-Bucur, R.; Mirea, I.C.; Măntoiu, D.Ş.; Kenesz, M.; Petculescu, A.; Baricz, A.; Andrei, A.-Ş.; Banciu, H.L.; Papp, B.; et al. Novel approach to microbiological air monitoring in show caves. Aerobiologia 2018, 34, 445–468. [Google Scholar] [CrossRef]
- Jardine, J.L.; Abia, A.L.; Mavumengwana, V.; Ubomba-Jaswa, E. Phylogenetic Analysis and Antimicrobial Profiles of Cultured Emerging Opportunistic Pathogens (Phyla Actinobacteria and Proteobacteria) Identified in Hot Springs. Int. J. Environ. Res. Public Health 2017, 14, 1070. [Google Scholar] [CrossRef]
- Moldovan, O.T.; Bercea, S.; Năstase-Bucur, R.; Constantin, S.; Kenesz, M.; Mirea, I.C.; Petculescu, A.; Robu, M.; Arghir, R.A. Management of water bodies in show caves – A microbial approach. Tour. Manag. 2020, 78. [Google Scholar] [CrossRef]
- Bercea, S.; Năstase-Bucur, R.; Moldovan, O.T.; Kenesz, M.; Constantin, S. Yearly microbial cycle of human exposed surfaces in show caves. Subterr. Biol. 2019, 31, 1–14. [Google Scholar] [CrossRef]
- Leuko, S.; Koskinen, K.; Sanna, L.; D’Angeli, I.M.; De Waele, J.; Marcia, P.; Moissl-Eichinger, C.; Rettberg, P. The influence of human exploration on the microbial community structure and ammonia oxidizing potential of the Su Bentu limestone cave in Sardinia, Italy. PLoS ONE 2017, 12, e0180700. [Google Scholar]
- Man, B.; Wang, H.; Xiang, X.; Wang, R.; Yun, Y.; Gong, L. Phylogenetic diversity of culturable fungi in the Heshang Cave, central China. Front. Microbiol. 2015, 6, 1158. [Google Scholar] [CrossRef] [PubMed]
- Paduano, S.; Marchesi, I.; Casali, M.E.; Valeriani, F.; Frezza, G.; Vecchi, E.; Sircana, L.; Romano Spica, V.; Borella, P.; Bargellini, A. Characterisation of Microbial Community Associated with Different Disinfection Treatments in Hospital hot Water Networks. Int. J. Environ. Res. Public Health 2020, 17, 2158. [Google Scholar] [CrossRef]
- Oikonomou, O.; Sarrou, S.; Papagiannitsis, C.C.; Georgiadou, S.; Mantzarlis, K.; Zakynthinos, E.; Dalekos, G.N.; Petinaki, E. Rapid dissemination of colistin and carbapenem resistant Acinetobacter baumannii in Central Greece: Mechanisms of resistance, molecular identification and epidemiological data. BMC Infect. Dis. 2015, 15, 559. [Google Scholar] [CrossRef]
- Lesho, E.; Yoon, E.-J.; McGann, P.; Snesrud, E.; Kwak, Y.; Milillo, M.; Onmus-Leone, F.; Preston, L.; St Clair, K.; Nikolich, M.; et al. Emergence of colistin-resistance in extremely drug-resistant Acinetobacter baumannii containing a novel pmrCAB operon during colistin therapy of wound infections. J. Infect. Dis. 2013, 208, 1142–1151. [Google Scholar] [CrossRef]
- Adams, M.D.; Nickel, G.C.; Bajaksouzian, S.; Lavender, H.; Murthy, A.R.; Jacobs, M.R.; Bonomo, R.A. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob. Agents Chemother. 2009, 53, 3628–3634. [Google Scholar] [CrossRef]
- Caneiras, C.; Calisto, F.; Jorge da Silva, G.; Lito, L.; Melo-Cristino, J.; Duarte, A.; Caneiras, C.; Calisto, F.; Jorge da Silva, G.; Lito, L.; et al. First Description of Colistin and Tigecycline-Resistant Acinetobacter baumannii Producing KPC-3 Carbapenemase in Portugal. Antibiotics 2018, 7, 96. [Google Scholar] [CrossRef]
- Handschuh, H.; Ryan, M.P.; O’Dwyer, J.; Adley, C.C. Assessment of the Bacterial Diversity of Aircraft Water: Identification of the Frequent Fliers. PLoS One 2017, 12, e0170567. [Google Scholar] [CrossRef]
- Kulakov, L.A.; McAlister, M.B.; Ogden, K.L.; Larkin, M.J.; O’Hanlon, J.F. Analysis of bacteria contaminating ultrapure water in industrial systems. Appl. Environ. Microbiol. 2002, 68, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.P.; Pembroke, J.T. Brevundimonas spp: Emerging global opportunistic pathogens. Virulence 2018, 9, 480–493. [Google Scholar] [CrossRef]
- Brenner, D.J. Characterization and clinical identification of Enterobacteriaceae by DNA hybridization. Prog. Clin. Pathol. 1978, 7, 71–117. [Google Scholar] [PubMed]
- Holmes, B.; Aucken, H.M. Citrobacter, Enterobacter, Klebsiella, Serratia and other members of the Enterobacteriaceae. In Topley & Wilson’s Microbiology an Microbial Infections, Systematic Bacteriology; Bawols, A., Duerden, B.I., Eds.; Arnold: London, UK, 1998; Volume 2, pp. 999–1020. ISBN 0340663170. [Google Scholar]
- Monnet, D.L.; Hansen, W.; Bollet, C.; Freney, J. Autres Enterobacteriaceae. In Manuel de Bactériologie Clinique; Elsevier: Paris, France, 1992; Volume 2, pp. 785–855. ISBN 2-906077-25-9. [Google Scholar]
- Klapholz, A.; Lessnau, K.D.; Huang, B.; Talavera, W.; Boyle, J.F. Hafnia alvei. Respiratory tract isolates in a community hospital over a three-year period and a literature review. Chest 1994, 105, 1098–1100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yabuuchi, E.; Yano, I.; Oyaizu, H.; Hashimoto, Y.; Ezaki, T.; Yamamoto, H. Proposals of Sphingomonas paucimobilis gen. nov. and comb. nov., Sphingomonas parapaucimobilis sp. nov., Sphingomonas yanoikuyae sp. nov., Sphingomonas adhaesiva sp. nov., Sphingomonas capsulata comb. nov., and two genospecies of the genus Sphingomonas. Microbiol. Immunol. 1990, 34, 99–119. [Google Scholar] [CrossRef]
- Pitt, T.L. Pseudomonas, Burkholderia and related genera. In Topley & Wilson’s Microbiology an Microbial Infections, Volume 2, Systematic Bacteriology; Bawols, A., Duerden, B.I., Eds.; Arnold: London, UK, 1999; pp. 1109–1138. ISBN 0340663170. [Google Scholar]
- Moffatt, J.H.; Harper, M.; Boyce, J.D. Mechanisms of Polymyxin Resistance. Adv. Exp. Med. Biol. 2019, 1145, 55–71. [Google Scholar]
- Olaitan, A.O.; Morand, S.; Rolain, J.-M. Mechanisms of polymyxin resistance: Acquired and intrinsic resistance in bacteria. Front. Microbiol. 2014, 5, 643. [Google Scholar] [CrossRef]
- Kawahara, K.; Kuraishi, H.; Zähringer, U. Chemical structure and function of glycosphingolipids of Sphingomonas spp and their distribution among members of the alpha-4 subclass of Proteobacteria. J. Ind. Microbiol. Biotechnol. 1999, 23, 408–413. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- CLSI; Wayne, P. M58: Methods for the Identification of Cultured Microorganisms Using Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry, 1st Edition Spectrometry; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017; ISBN 1-56238-817-7. [Google Scholar]
- Maragakis, L.L.; Chaiwarith, R.; Srinivasan, A.; Torriani, F.J.; Avdic, E.; Lee, A.; Ross, T.R.; Carroll, K.C.; Perl, T.M.; LL, M.; et al. Sphingomonas paucimobilis bloodstream infections associated with contaminated intravenous fentanyl. Emerg. Infect. Dis. 2009, 15, 12–18. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; M100-S20; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters. Version 11.0. CMI 2021, 12, P501–P503. [Google Scholar]
- Kahlmeter, G.; Brown, D.F.J.; Goldstein, F.W.; MacGowan, A.P.; Mouton, J.W.; Odenholt, I.; Rodloff, A.; Soussy, C.J.; Steinbakk, M.; Soriano, F.; et al. European Committee on Antimicrobial Susceptibility Testing (EUCAST) Technical Notes on antimicrobial susceptibility testing. Clin. Microbiol. Infect. 2006, 12, 501–503. [Google Scholar] [CrossRef]
- Benevides, G.N.; Hein, N.; Lo, D.S.; Ferronato, A.E.; Ragazzi, S.L.B.; Yoshioka, C.R.M.; Hirose, M.; Cardoso, D.M.; Regina Dos Santos, S.; Gilio, A.E. Otomastoiditis caused by Sphingomonas paucimobilis: Case report and literature review. Autops. Case Reports 2014, 4, 13–20. [Google Scholar] [CrossRef][Green Version]
- Lin, J.-N.; Lai, C.-H.; Chen, Y.-H.; Lin, H.-L.; Huang, C.-K.; Chen, W.-F.; Wang, J.-L.; Chung, H.-C.; Liang, S.-H.; Lin, H.-H. Sphingomonas paucimobilis bacteremia in humans: 16 case reports and a literature review. J. Microbiol. Immunol. Infect. 2010, 43, 35–42. [Google Scholar] [CrossRef]
- Liu, C.H.; Lin, W.J.; Wang, C.C.; Lee, K.L.; Tsai, M.C. Young-infant sepsis combined with urinary tract infection due to Hafnia alvei. J. Formos. Med. Assoc. 2007, 106, S39–S43. [Google Scholar] [CrossRef][Green Version]
- Nandy, S.; Dudeja, M.; Das, A.K.; Tiwari, R. Community Acquired Bacteremia by Sphingomonas paucimobilis: Two Rare Case Reports. J. Clin. Diagn. Res. 2013, 7, 2947–2949. [Google Scholar] [CrossRef] [PubMed]
- Rognrud, K.; Diaz, A.M.; Hill, C.; Kershaw, M.A. Bacterial Endocarditis Caused by Sphingomonas paucimobilis: A Case Report and Literature Review. Case Rep. Infect. Dis. 2020, 2020, 1–3. [Google Scholar] [CrossRef]
- Stanic, M.; Meusburger, E.; Hartmann, G.; Lhotta, K. Hafnia alvei Urosepsis in a Kidney Transplant Patient. Case Rep. Transpl. 2015, 2015, 863131. [Google Scholar]
- Yuen, L.C.; Jackson, T. A Case of Intra-abdominal abscess due to Sphingomonas paucimobilis in a patient on Peritoneal dialysis: A case report and review of literature. Indian J. Nephrol. 2020, 30, 196–200. [Google Scholar] [CrossRef]
- Albert, M.J.; Khorshed, A.; Islam, M.; Montanaro, J.; Rahaman, H.A.S.M.; Haider, K.; Hossain, M.A.; Kibriya, A.K.M.G.; Tzipori, S. Hafnia alvei, a probable cause of diarrhea in humans. Infect. Immun. 1991, 59, 1507–1513. [Google Scholar] [CrossRef]
- El Beaino, M.; Fares, J.; Malek, A.; Hachem, R. Sphingomonas paucimobilis-related bone and soft-tissue infections: A systematic review. Int. J. Infect. Dis. 2018, 77, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Göker, T.; Aşik, R.Z.; Yilmaz, M.B.; Çelik, İ.; Tekiner, A. Sphingomonas Paucimobilis: A Rare Infectious Agent Found in Cerebrospinal Fluid. J. Korean Neurosurg. Soc. 2017, 60, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Lugito, H.; Pratama, N.; Kurniawan, A. A Lethal Case of Sphingomonas paucimobilis Bacteremia in an Immunocompromised Patient. Case Rep. Infect. Dis. 2016, 2016, 3294639. [Google Scholar]
- Hassan, E.A.; Elsherbiny, N.M.; Abd El-Rehim, A.S.; Soliman, A.M.A.; Ahmed, A.O. Health care-associated infections in pre-transplant liver intensive care unit: Perspectives and challenges. J. Infect. Public Health 2018, 11, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, A.S.; Kelkar, J.A.; Barve, P.M.; Mulay, A.; Sharma, S.; Amoaku, W. Post-clear corneal phacoemulsification endophthalmitis: Profile and management outcomes at a tertiary eye care center in western India. J. Ophthalmic. Inflamm. Infect. 2016, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Laupland, K.B.; Church, D.L.; Ross, T.; Pitout, J.D.D. Population-based laboratory surveillance of Hafnia alvei isolates in a large Canadian health region. Ann. Clin. Microbiol. Antimicrob. 2006, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.C.-W.; Siao-Ping Ong, N.D. Food-borne bacteremic illnesses in febrile neutropenic children. Hematol. Rep. 2011, 3, e11. [Google Scholar] [CrossRef]
- Lee, J.U.; Kim, J.K.; Yun, S.H.; Park, M.S.; Lee, N.E.; Sun, I.O.; Lee, K.Y. A case of peritoneal dialysis-associated peritonitis caused by Sphingomonas paucimobilis. Kidney Res. Clin. Pract. 2013, 32, 78–80. [Google Scholar] [CrossRef][Green Version]
- Liliav, B.; Yakoub, D.; Kasabian, A. Necrotizing fasciitis following endoscopic harvesting of the greater saphenous vein for coronary artery bypass graft. JSLS 2011, 15, 90–95. [Google Scholar] [CrossRef][Green Version]
- Mancini, M.; Panasiti, V.; Devirgiliis, V.; Pietropaolo, V.; Fioriti, D.; Nicosia, R.; Curzio, M.; Roberti, V.; Gobbi, S.; Bottoni, U.; et al. Bromhidrosis induced by sphingomonas paucimobilis: A case report. Int. J. Immunopathol. Pharmacol. 2009, 22, 845–848. [Google Scholar] [CrossRef]
- Bayram, N.; Devrim, I.; Apa, H.; Gülfidan, G.; Türkyilmaz, H.N.; Günay, I.; HN, T.; Günay, I. Sphingomonas paucimobilis infections in children: 24 case reports. Mediterr J Hematol Infect Dis 2013, 5, e2013040. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, H.; Khan, N.; Ullah, S.; Ullah, A.; Marwat, A. A Rare Case of Sphingomonas paucimobilis Meningitis in the Absence of Cerebrospinal Fluid Pleocytosis. J. Investig. Med. High Impact Case Rep. 2018, 6, 2324709618756424. [Google Scholar]
- Mohan, D.; Railey, M. Sphingomonas paucimobilis peritonitis: A case report and review of the literature. Saudi J. Kidney Dis. Transpl. 2015, 26, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, M.; Pekcan, S.; Demircili, M.E.; Taşbent, F.E.; Feyzioğlu, B.; Pirinç, Ş.; Baykan, M. A rare cause of bacteremia in a pediatric patient with Down syndrome: Sphingomonas paucimobilis. Int. J. Med. Sci. 2011, 8, 537–539. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pascale, R.; Russo, E.; Esposito, I.; Leone, S.; Esposito, S. Sphingomonas paucimobilis osteomyelitis in an immunocompetent patient. A rare case report and literature review. New Microbiol. 2013, 36, 423–426. [Google Scholar]
- Rahman, S.R.; Ahmed, M.F.; Begum, A. Occurrence of urinary tract infection in adolescent and adult women of shanty town in Dhaka City, Bangladesh. Ethiop. J. Health Sci. 2014, 24, 145–152. [Google Scholar] [CrossRef]
- Refaat, M.; Zakka, P.; Khoury, M.; Chami, H.; Mansour, S.; Harbieh, B.; Abi-Saleh, B.; Bizri, A. Cardiac implantable electronic device infections: Observational data from a tertiary care center in Lebanon. Medicine 2019, 98, e14906. [Google Scholar] [CrossRef]
- Ridell, J.; Siitonen, A.; Paulin, L.; Mattila, L.; Korkeala, H.; Albert, M.J. Hafnia alvei in stool specimens from patients with diarrhea and healthy controls. J. Clin. Microbiol. 1994, 32, 2335–2337. [Google Scholar] [CrossRef]
- Roca, M.; García, A.; Peñas-Pardo, L.; Bosch-Aparicio, N.; Agustí, J. Sphingomonas paucimobilis keratitis in a patient with neurotrophic keratopathy and severe neurosensory hypoacusis: Treatment with penetrating keratoplasty and amniotic membrane grafting. Oman J. Ophthalmol. 2018, 11, 291–293. [Google Scholar] [CrossRef]
- Saboor, F.; Amin, F.; Nadeem, S. Community acquired sphingomonas paucimobilis in a child-a rare case. J. Pak. Med. Assoc. 2018, 68, 1714–1715. [Google Scholar]
- Seo, S.W.; Chung, I.Y.; Kim, E.; Park, J.M. A case of postoperative Sphingomonas paucimobilis endophthalmitis after cataract extraction. Korean J. Ophthalmol. 2008, 22, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Chawla, K.; Vishwanath, S.; Munim, F.C. Nonfermenting Gram-negative Bacilli other than Pseudomonas aeruginosa and Acinetobacter Spp. Causing Respiratory Tract Infections in a Tertiary Care Center. J. Glob. Infect. Dis. 2013, 5, 144–148. [Google Scholar]
- Sirkhazi, M.; Sarriff, A.; Aziz, N.A.; Almana, F.; Arafat, O.; Shorman, M. Bacterial Spectrum, Isolation Sites and Susceptibility Patterns of Pathogens in Adult Febrile Neutropenic Cancer Patients at a Specialist Hospital in Saudi Arabia. World J. Oncol. 2014, 5, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Souto, A.; Guinda, M.; Mera, A.; Pardo, F. Septic arthritis caused by Sphingomonas paucimobilis in an immunocompetent patient. Reumatol. Clin. 2012, 8, 378–379. [Google Scholar] [CrossRef]
- Tai, M.L.S.; Velayuthan, R.D. Sphingomonas paucimobilis: An unusual cause of meningitis-case report. Neurol. Med. Chir. 2014, 54, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Toh, H.S.; Tay, H.T.; Kuar, W.K.; Weng, T.C.; Tang, H.J.; Tan, C.K. Risk factors associated with Sphingomonas paucimobilis infection. J. Microbiol. Immunol. Infect. 2011, 44, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Vieira Colombo, A.P.; Magalhães, C.B.; Hartenbach, F.A.R.R.; Martins do Souto, R.; Maciel da Silva-Boghossian, C. Periodontal-disease-associated biofilm: A reservoir for pathogens of medical importance. Microb. Pathog. 2015, 94, 27–34. [Google Scholar] [CrossRef]
- Walayat, S.; Malik, A.; Hussain, N.; Lynch, T. Sphingomonas paucimobilis presenting as acute phlebitis: A case report. IDCases 2018, 11, 6–8. [Google Scholar] [CrossRef]
- Wiström, J.; Myrnäs, T.; Lundgren, C.; Monsen, T.; Wistrom, J.; Myrnas, T.; Lundgren, C.; Monsen, T.; Wiström, J.; Myrnäs, T.; et al. A case of acute cholecystitis due to Aeromonas sobria and Hafnia alvei from northern Europe. Clin. Microbiol. Infect. 1998, 4, 607–609. [Google Scholar] [CrossRef][Green Version]
- Yarlagadda, K.; Shrimanker, I.; Nookala, V.K. Catheter-associated Hafnia alvei-induced Urosepsis. Cureus 2019, 11, e6471. [Google Scholar] [CrossRef]
- Yozgat, Y.; Kilic, A.; Karadeniz, C.; Ozdemir, R.; Doksoz, O.; Gulfidan, G.; Mese, T. Sphingomonas paucimobilis bacteraemia and shock in a patient with rheumatic carditis. Indian J. Med. Microbiol. 2014, 32, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Treadwell, T. Sphingomonas paucimobilis empyema caused by remote foreign body aspiration. BMJ Case Rep. 2018, 2018, 2017–2019. [Google Scholar]
- Chowdhary, P.; Ranjan, R.; Pandey, A.; Kumar, R. Sphingomonas paucimobilis septicemia in a neonate: A rare case report. Indian J. Pathol. Microbiol. 2016, 59, 119–121. [Google Scholar]
- Zhang, Y.; Liu, Z.R.; Chen, H.; Dong, W.J.; Fan, Y.C.; Yu, H.; Wang, G.J.; Li, Y.C.; Cao, K. Comparative study of bacterial status from conjunctival sac of the elder Qiang minority and Han people with dry eye in Sichuan, China. Int J Ophthalmol 2012, 5, 343–347. [Google Scholar]
- Cutuli, S.L.; De Maio, F.; De Pascale, G.; Grieco, D.L.; Monzo, F.R.; Carelli, S.; Tanzarella, E.S.; Pintaudi, G.; Piervincenzi, E.; Cascarano, L.; et al. COVID-19 influences lung microbiota dynamics and favors the emergence of rare infectious diseases: A case report of Hafnia Alvei pneumonia. J. Crit. Care 2021, 64, 173–175. [Google Scholar] [CrossRef]
- Del Borgo, C.; Maneschi, F.; Belvisi, V.; Morelli, F.; Vetica, A.; Marocco, R.; Tieghi, T.; Lichtner, M.; Mastroianni, C.M. Postpartum fever in the presence of a fibroid: Sphingomonas paucimobilis sepsis associated with pyomyoma. BMC Infect. Dis. 2013, 13, 574. [Google Scholar] [CrossRef]
- Demir, T.; Dadali, M. Recurrent complicated urinary tract infection due to rare pathogen Sphingomonas paucimobilis: Contamination or real deal? Infez Med 2016, 24, 241–244. [Google Scholar] [PubMed]
- Droutsas, K.; Kalantzis, G.; Symeonidis, C.; Georgalas, I. Posttraumatic Sphingomonas paucimobilis Endophthalmitis. Case Rep. Ophthalmol. Med. 2015, 2015, 192864. [Google Scholar] [PubMed]
- Eckrich, J.; Zissler, U.M.; Serve, F.; Leutz, P.; Smaczny, C.; Schmitt-Grohé, S.; Fussbroich, D.; Schubert, R.; Zielen, S.; Eickmeier, O.; et al. Airway inflammation in mild cystic fibrosis. J. Cyst. Fibros. 2017, 16, 107–115. [Google Scholar] [CrossRef]
- Jayol, A.; Saly, M.; Nordmann, P.; Ménard, A.; Poirel, L.; Dubois, V. Hafnia, an enterobacterial genus naturally resistant to colistin revealed by three susceptibility testing methods. J. Antimicrob. Chemother. 2017, 72, 2507–2511. [Google Scholar] [CrossRef][Green Version]
- Yadav, K.; Sharma, M.; Agarwal, S.; Bhatia, N.; Yadav, N. Aortic pseudoaneurysm & endocarditis caused by Aerococcus viridans: A case report and literature review. Cardiovasc. Revasc. Med. 2018, 19, 201–203. [Google Scholar] [PubMed]
- Gutiérrez-Fernández, J.; Gámiz-Gámiz, A.; Navarro-Marí, J.M.; Santos-Pérez, J.L. Genitourinary tract infection in children due to Aerococcus other than Aerococcus viridans. Literature review and 3 case reports. Enfermedades Infecc. y Microbiol. Clin. 2021, 39, 156–158. (In English) [Google Scholar] [CrossRef]
- Oragui, J.I.; Mara, D.D. Investigation of the survival characteristics of Rhodococcus coprophilus and certain fecal indicator bacteria. Appl. Environ. Microbiol. 1983, 46, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Glasset, B.; Sperry, M.; Dervyn, R.; Herbin, S.; Brisabois, A.; Ramarao, N. The cytotoxic potential of Bacillus cereus strains of various origins. Food Microbiol. 2021, 98, 103759. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ai, F.; Ji, C.; Tu, P.; Gao, Y.; Wu, Y.; Yan, F.; Yu, T. A Rapid Screening Method of Candidate Probiotics for Inflammatory Bowel Diseases and the Anti-inflammatory Effect of the Selected Strain Bacillus smithii XY1. Front. Microbiol. 2021, 12, 760385. [Google Scholar] [CrossRef]
- Boonmak, C.; Takahasi, Y.; Morikawa, M. Draft Genome Sequence of Geobacillus thermoleovorans Strain B23. Genome Announc. 2013, 1. [Google Scholar] [CrossRef]
- Suzuki, H. Peculiarities and biotechnological potential of environmental adaptation by Geobacillus species. Appl. Microbiol. Biotechnol. 2018, 102, 10425–10437. [Google Scholar] [CrossRef]
- Dragomirescu, C.C.; Lixandru, B.E.; Coldea, I.L.; Corneli, O.N.; Pana, M.; Palade, A.M.; Cristea, V.C.; Suciu, I.; Suciu, G.; Manolescu, L.S.C.; et al. Antimicrobial Susceptibility Testing for Corynebacterium Species Isolated from Clinical Samples in Romania. Antibiotics 2020, 9, 31. [Google Scholar] [CrossRef]
- Kalt, F.; Schulthess, B.; Sidler, F.; Herren, S.; Fucentese, S.F.; Zingg, P.O.; Berli, M.; Zinkernagel, A.S.; Zbinden, R.; Achermann, Y. Corynebacterium Species Rarely Cause Orthopedic Infections. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef]
- Dykhuizen, R.S.; Douglas, G.; Weir, J.; Gould, I.M. Corynebacterium afermentans subsp. lipophilum: Multiple abscess formation in brain and liver. Scand. J. Infect. Dis. 1995, 27, 637–639. [Google Scholar] [CrossRef]
- Kumari, P.; Tyagi, A.; Marks, P.; Kerr, K.G. Corynebacterium afermentans spp. afermentans sepsis in a neurosurgical patient. J. Infect. 1997, 35, 201–202. [Google Scholar] [CrossRef]
- Yu, Z.; Zhu, F.; Tao, X.; Zhang, L.; Wu, S.; Dong, C.; Dong, Y.; Chen, G.; Zhou, X.; Fang, Y.; et al. Vogesella perlucida-induced bacteremia in an advanced-age patient: First case report. BMC Infect. Dis. 2020, 20, 687. [Google Scholar] [CrossRef] [PubMed]
- Briegel, I.; Trautnitz, M.; Behr, J. Rare Cause of Lung Tumour - Pulmonary Infection with Hafnia alvei: A Case Report. Pneumologie 2022, 1718–2521. [Google Scholar]
- Ryan, M.P.; Adley, C.C. Sphingomonas paucimobilis: A persistent Gram-negative nosocomial infectious organism. J. Hosp. Infect. 2010, 75, 153–157. [Google Scholar] [CrossRef]
- Kathayat, D.; Antony, L.; Deblais, L.; Helmy, Y.A.; Scaria, J.; Rajashekara, G. Small molecule adjuvants potentiate Colistin activity and attenuate resistance development in Escherichia coli by affecting pmrAB system. Infect. Drug Resist. 2020, 13, 2205. [Google Scholar] [CrossRef] [PubMed]
- Matuschek, E.; Brolund, A.; Karlsson Lindsjö, O.; Giske, C.G.; Byfors, S.; Kahlmeter, G. Revisiting colistin susceptibility testing: Will adding calcium to Mueller–Hinton agar improve the detection of colistin resistance? Clin. Microbiol. Infect. 2021, 27, 1172.e1–1172.e5. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).