Association of Occupational Noise Exposure and Incidence of Metabolic Syndrome in a Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Subjects
2.2. Covariate Definition and Measurement
2.3. Statistical Analysis
3. Results
3.1. Association between Noise Exposure and Metabolic Syndrome
3.1.1. Basic Demographic Characteristics
3.1.2. Prevalence and Incidence of Metabolic Syndrome
3.1.3. Analysis of Risk Factors of Noise Exposure for the Incidence of Metabolic Syndrome
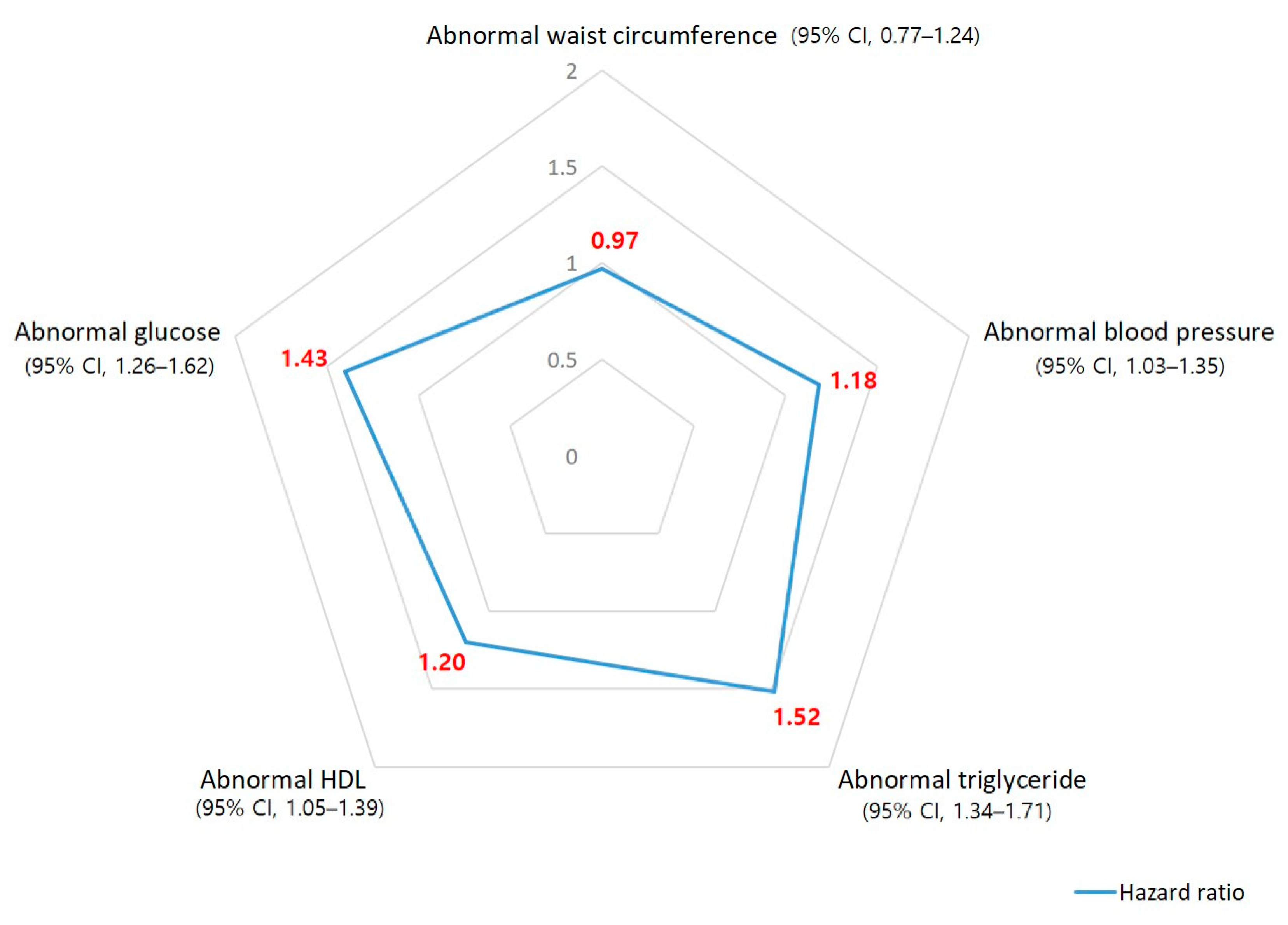
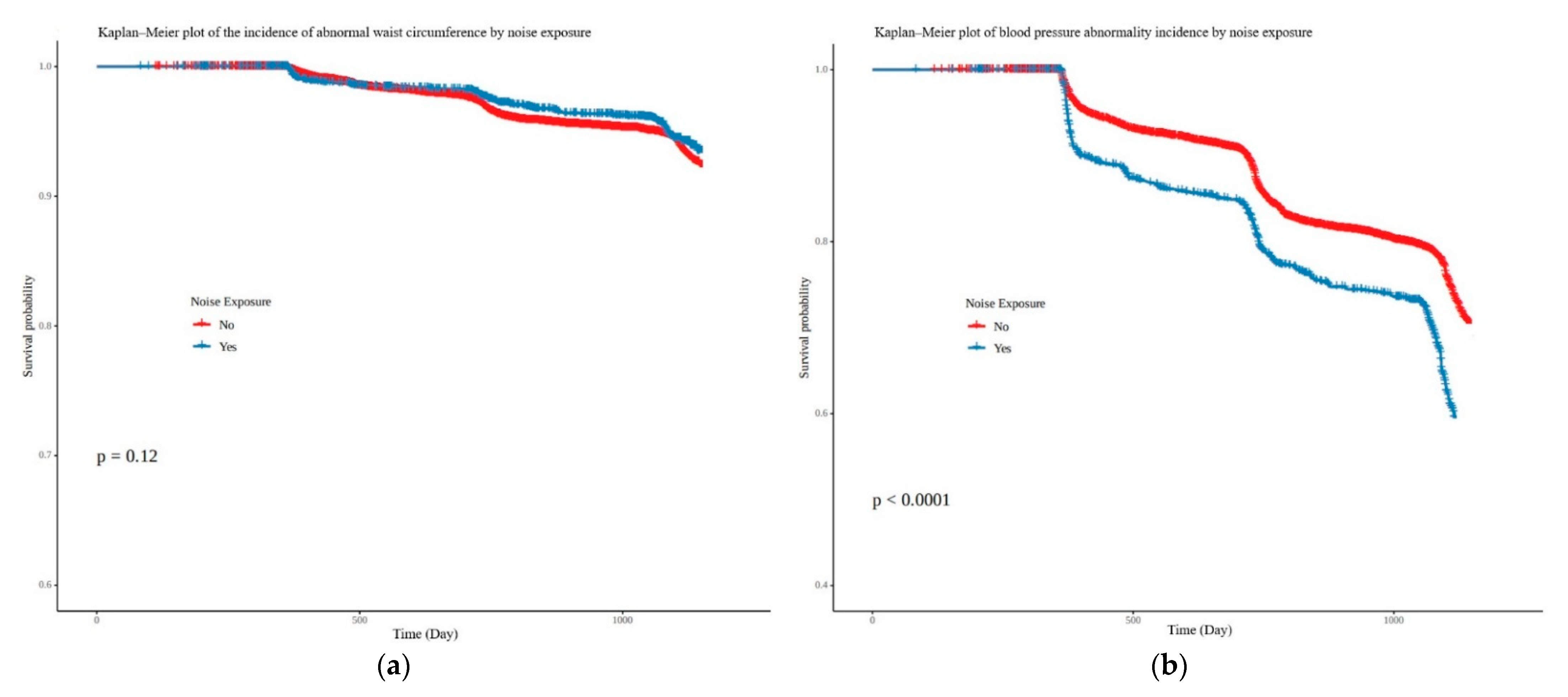
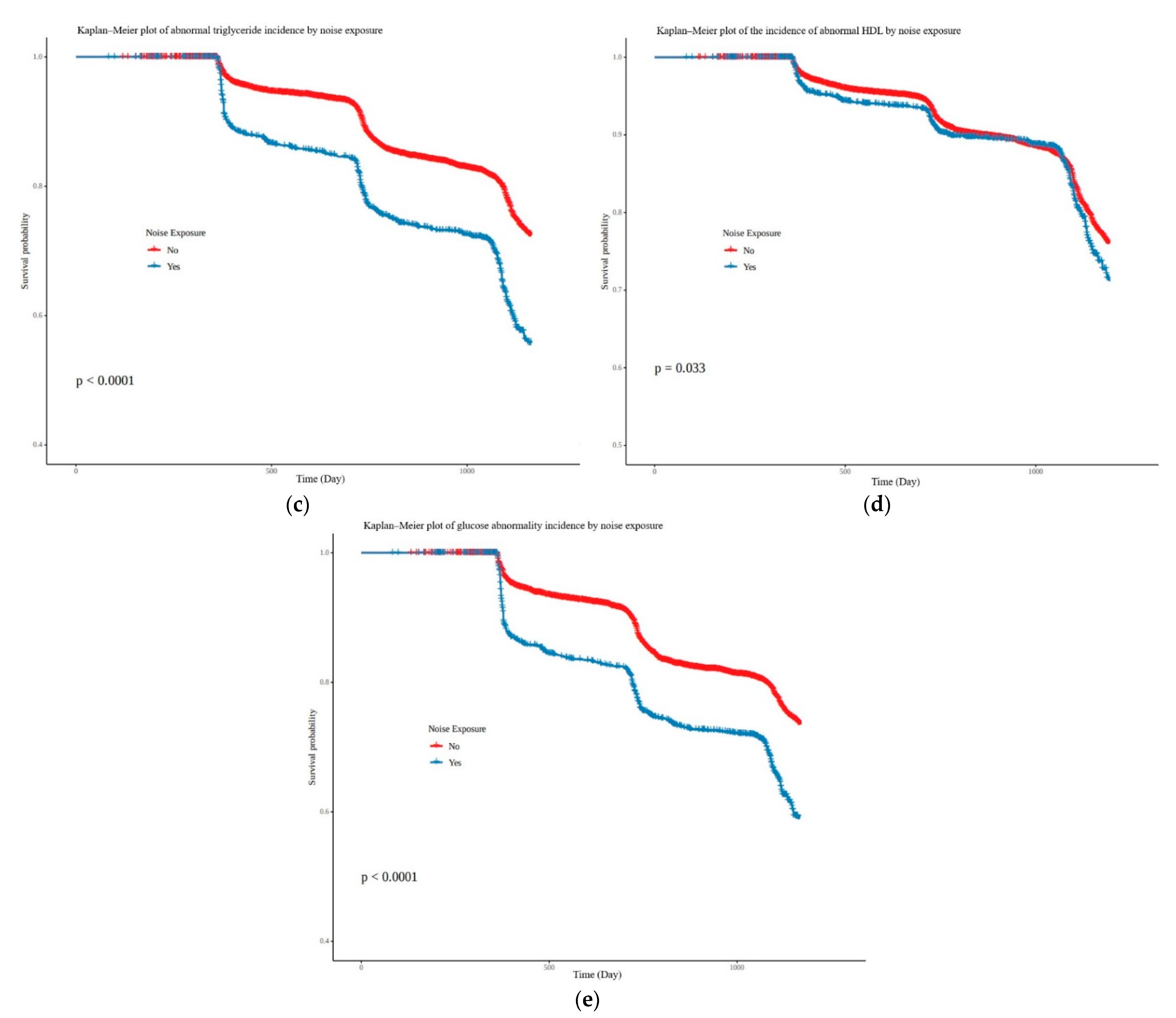
3.2. Correlation between Noise Exposure and Five Subgroups of Metabolic Syndrome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| WHO: Insulin Resistance Obligatory + Two Other Components | EGIR: Hyperinsulinemia (Plasma Insulin > 75th Percentile) + Two Other Components | Modified NCEP: Three of the Following | IDF: Waist Obligatory + Two Other Components |
|---|---|---|---|
| Insulin resistance (IGT, IFG, T2 D) | Fasting plasma glucose ≥ 100 mg/dL or treatment for elevated glucose | Fasting plasma glucose ≥ 100 mg/dL or treatment for elevated glucose | |
| Waist/hip ratio > 0.9 (men) or > 0.85 (women) or BMI > 30 kg/m2 | Waist ≥ 94 cm (men), or ≥80 cm (women) | Waist ≥ 102 cm (men), or ≥88 cm (women) | Waist ≥ 94 cm (men), or ≥80 cm (women) |
| TG > 150 mg/dL or HDL cholesterol < 35 mg/dL (men) or <39 mg/dL (women) | TG > 177 mg/dL or HDL cholesterol <39 mg/dL | TG > 150 mg/dL or treatment for this lipid abnormality | TG > 150 mg/dL or treatment for this lipid abnormality |
| Microalbuminuria | HDL cholesterol < 40 mg/dL (men) or <50 mg/dL (women) or treatment for this lipid abnormality | HDL cholesterol < 40 mg/dL (men) or <50 mg/dL (women) or treatment for this lipid abnormality | |
| Blood pressure > 140/90 mmHg | Blood pressure > 140/90 mmHg or antihypertensive medication | Blood pressure > 130/85 mmHg or antihypertensive medication | Blood pressure > 130/85 mmHg or antihypertensive medication |

References
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Huang, P.L. A Comprehensive Definition for Metabolic Syndrome. Dis. Model Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [Green Version]
- He, Y.N.; Zhao, W.H.; Zhao, L.Y.; Yu, D.M.; Zhang, J.; Yang, X.G.; Ding, G.G. Prevalence of metabolic syndrome in Chinese adults in 2010–2012. Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi 2017, 38, 212–215. [Google Scholar]
- Shin, D.; Kongpakpaisarn, K.; Bohra, C. Trends in the prevalence of metabolic syndrome and its components in the United States 2007–2014. Int. J. Cardiol. 2018, 259, 216–219. [Google Scholar] [CrossRef]
- Kim, M.H.; Lee, S.H.; Shin, K.S.; Son, D.Y.; Kim, S.H.; Joe, H.; Yoo, B.W.; Hong, S.H.; Cho, C.Y.; Shin, H.S.; et al. The change of metabolic syndrome prevalence and its risk factors in korean adults for decade. Korea national health and nutrition examination survey for 2008–2017. Korean J. Fam. Pract. 2020, 10, 44–52. [Google Scholar] [CrossRef]
- Mendrick, D.L.; Diehl, A.M.; Topor, L.S.; Dietert, R.R.; Will, Y.; La Merrill, M.A.; Bouret, S.; Varma, V.; Hastings, K.L.; Schug, T.T.; et al. Metabolic syndrome and associated diseases: From the bench to the clinic. Toxicol. Sci. 2018, 162, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Farooqui, A.A.; Farooqui, T.; Panza, F.; Frisardi, V. Metabolic syndrome as a risk factor for neurological disorders. Cell. Mol. Life Sci. 2012, 69, 741–762. [Google Scholar] [CrossRef]
- Baffi, C.W.; Wood, L.; Winnica, D.; Strollo, P.J., Jr.; Gladwin, M.T.; Que, L.G.; Holguin, F. Metabolic syndrome and the lung. Chest 2016, 149, 1525–1534. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef]
- Stančáková, A.; Laakso, M. Genetics of metabolic syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 243–252. [Google Scholar] [CrossRef]
- Huang, T.; Chan, T.-C.; Huang, Y.-J.; Pan, W.-C. The association between noise exposure and metabolic syndrome: A longitudinal cohort study in Taiwan. Int. J. Environ. Res. Public Health 2020, 17, 4236. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ruan, W.; Yi, G.; Chen, Z.; Wang, D. Association of noise exposure with risk of metabolic syndrome: Evidence from 44,698 individuals. Diabetes Res. Clin. Pract. 2021, 178, 108944. [Google Scholar] [CrossRef]
- Sakhvidi, M.J.Z.; Sakhvidi, F.Z.; Mehrparvar, A.H.; Foraster, M.; Dadvand, P. Association between noise exposure and diabetes: A systematic review and meta-analysis. Environ. Res. 2018, 166, 647–657. [Google Scholar] [CrossRef]
- Dzhambov, A.M. Long-term noise exposure and the risk for type 2 diabetes: A meta-analysis. Noise Health 2015, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Bolm-Audorff, U.; Hegewald, J.; Pretzsch, A.; Freiberg, A.; Nienhaus, A.; Seidler, A. Occupational noise and hypertension risk: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2020, 17, 6281. [Google Scholar] [CrossRef] [PubMed]
- Kheirandish, A.; Mehrparvar, A.; Abou-Bakre, A.; Zare Sakhvidi, M.J. Association between long-term occupational noise exposure and obesity. Environ. Sci. Pollut. Res. 2021, 1–10. [Google Scholar] [CrossRef]
- NIOSH Criteria for a Recommended Standard: Occupational Exposure to Heat and Hot Environments. Available online: https://books.google.com.hk/books?hl=zh-CN&lr=&id=KxD6HGC86S8C&oi=fnd&pg=PR25&dq=Health,+Control+CfD,+Prevention,+Dept+HS.+NIOSH+criteria+for+a+recommended+standard:+Occupational+exposure+to+heat+and+hot+environments:+National+Institute+on+Drug+Abuse&ots=-4YDagm9Kt&sig=2HXvSTqmCsXwfo38FxEJbZREH58&redir_esc=y (accessed on 7 January 2022).
- Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices. Available online: https://www.cdc.gov/niosh/topics/noise/preventhearingloss/hearlosspreventprograms.html (accessed on 7 January 2022).
- Seo, M.H.; Lee, W.Y.; Kim, S.S.; Kang, J.H.; Kim, K.K.; Kim, B.Y.; Kim, Y.H.; Kim, W.J.; Kim, E.M.; Kim, H.S.; et al. Committee of clinical practice guidelines, Korean society for the study of obesity (KSSO). 2018 Korean society for the study of obesity guideline for the management of obesity in korea. J. Obes. Metab. Syndr. 2019, 28, 40–45. [Google Scholar] [CrossRef]
- Korea Centers for Disease Control & Prevention National Health Information Portal. 2022. Available online: https://health.kdca.go.kr/healthinfo/biz/health/gnrlzHealthInfo/gnrlzHealthInfo/gnrlzHealthInfoView.do (accessed on 7 January 2022).
- Oh, J.Y.; Yang, Y.J.; Kim, B.S.; Kang, J.H. Validity and reliability of Korean version of International Physical Activity Questionnaire (IPAQ) short form. J. Korean Acad. Fam. Med. 2007, 28, 532–541. [Google Scholar]
- Babisch, W. The noise/stress concept, risk assessment and research needs. Noise Health 2002, 4, 1. [Google Scholar]
- Babisch, W. Cardiovascular effects of noise. Noise Health 2011, 13, 201. [Google Scholar] [CrossRef]
- Yu, Y.; Paul, K.; Arah, O.A.; Mayeda, E.R.; Wu, J.; Lee, E.; Shih, I.F.; Su, J.; Jerrett, M.; Haan, M.; et al. Air pollution, noise exposure, and metabolic syndrome-A cohort study in elderly Mexican-Americans in Sacramento area. Environ. Int. 2020, 134, 105269. [Google Scholar] [CrossRef] [PubMed]

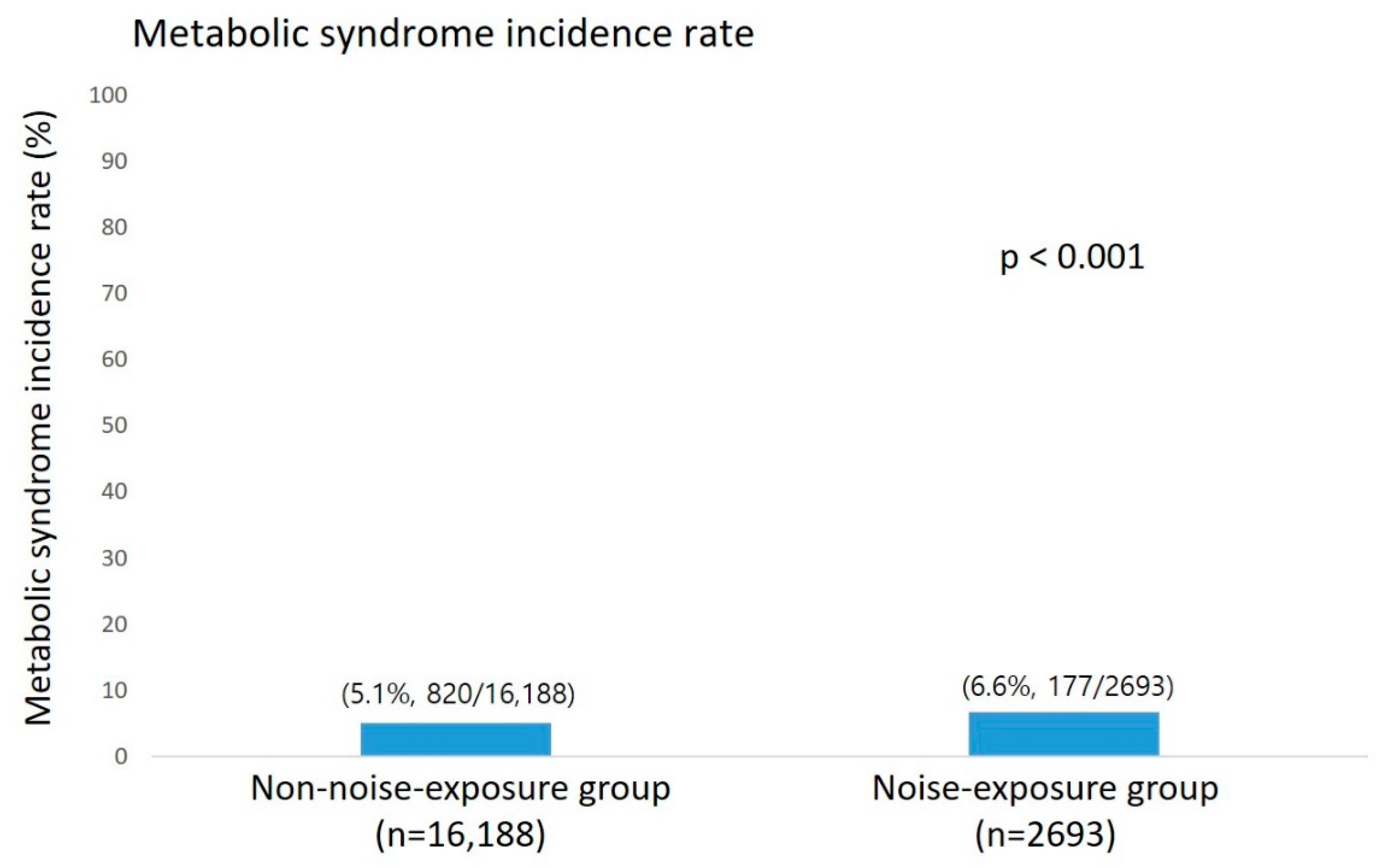

| Characteristics | Total, No. (%) (n = 18,881) | Non-Exposure (n = 16,188) | Noise Exposure (n = 2693) | p-Value |
|---|---|---|---|---|
| Age (year), mean (SD) | 41.04 (11.06) | 41.27 (11.06) | 39.66 (1.97) | <0.001 |
| Age (year) | <0.001 | |||
| <30 | 3369 (17.8) | 2758 (17.0) | 611 (22.7) | |
| 30–39 | 5987 (31.7) | 5174 (32.0) | 813 (30.2) | |
| 40–49 | 4544 (24.1) | 3895 (24.1) | 649 (24.1) | |
| ≥50 | 4981 (26.4) | 4361 (26.9) | 620 (23.0) | |
| Gender | <0.001 | |||
| Male | 13,830 (73.2) | 11,280 (69.7) | 2550 (94.7) | |
| Female | 5051 (26.8) | 4908 (30.3) | 143 (5.3) | |
| Body mass index, kg/m2 | <0.001 | |||
| Normal,18.5–22.9 | 8726 (46.2) | 7498 (46.3) | 1228 (45.6) | |
| Underweight, <18.5 | 589 (3.1) | 550 (3.4) | 39 (1.4) | |
| Overweight, 23–24.9 | 5333 (28.3) | 4564 (28.2) | 769 (28.6) | |
| Obese, ≥25 | 4233 (22.4) | 3576 (22.1) | 657 (24.4) | |
| Smoking status | <0.001 | |||
| Non-smoker | 9792 (51.9) | 8972 (55.4) | 820 (30.5) | |
| Ex-smoker | 4091 (21.6) | 3566 (22.0) | 525 (19.5) | |
| Current smoker | 4998 (26.5) | 3650 (22.6) | 1348 (50.0) | |
| Alcohol habit status | <0.001 | |||
| No | 10,581 (56.0) | 9372 (57.9) | 1209 (44.9) | |
| Yes | 8300 (44.0) | 6816 (42.1) | 1484 (55.1) | |
| Physical activity | <0.001 | |||
| Non-exercise group | 2550 (13.5) | 2105 (13.0) | 445 (16.5) | |
| Exercise group | 16,331 (86.5) | 14,083 (87.0) | 2248 (83.5) | |
| Night-shift work | <0.001 | |||
| No | 17,232 (92.3) | 15,036 (92.9) | 2196 (81.5) | |
| Yes | 1649 (8.7) | 1152 (7.1) | 497 (18.5) | |
| Cardiovascular-related exposure | <0.001 | |||
| No | 17,377 (92.0) | 15,928 (98.4) | 1449 (53.8) | |
| Yes | 1504 (8.0) | 260 (1.6) | 1244 (46.2) |
| Metabolic Syndrome and Subgroups | Univariate Model 1 | Adjusted Model 2 | ||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Metabolic syndrome | 1.48 | (1.32–1.65) | 1.36 | (1.19–1.57) |
| Abnormal waist circumference | 0.85 | (0.70–1.04) | 0.97 | (0.77–1.24) |
| Abnormal blood pressure | 1.63 | (1.47–1.80) | 1.18 | (1.03–1.35) |
| Abnormal triglyceride | 1.96 | (1.79–2.16) | 1.52 | (1.34–1.71) |
| Abnormal HDL | 1.13 | (1.01–1.27) | 1.20 | (1.05–1.39) |
| Abnormal glucose | 1.79 | (1.62–1.97) | 1.43 | (1.26–1.62) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.; Kim, H.; Yun, B.; Sim, J.; Kim, C.; Oh, Y.; Yoon, J.; Lee, J. Association of Occupational Noise Exposure and Incidence of Metabolic Syndrome in a Retrospective Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 2209. https://doi.org/10.3390/ijerph19042209
Kim G, Kim H, Yun B, Sim J, Kim C, Oh Y, Yoon J, Lee J. Association of Occupational Noise Exposure and Incidence of Metabolic Syndrome in a Retrospective Cohort Study. International Journal of Environmental Research and Public Health. 2022; 19(4):2209. https://doi.org/10.3390/ijerph19042209
Chicago/Turabian StyleKim, Gwansic, Hanjun Kim, Byungyoon Yun, Juho Sim, Changyoung Kim, Yeonsuh Oh, Jinha Yoon, and Jiho Lee. 2022. "Association of Occupational Noise Exposure and Incidence of Metabolic Syndrome in a Retrospective Cohort Study" International Journal of Environmental Research and Public Health 19, no. 4: 2209. https://doi.org/10.3390/ijerph19042209
APA StyleKim, G., Kim, H., Yun, B., Sim, J., Kim, C., Oh, Y., Yoon, J., & Lee, J. (2022). Association of Occupational Noise Exposure and Incidence of Metabolic Syndrome in a Retrospective Cohort Study. International Journal of Environmental Research and Public Health, 19(4), 2209. https://doi.org/10.3390/ijerph19042209






