Predictors of Adverse Pregnancy Outcomes in Pregnant Women Living with Obesity: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Electronic Literature Search
2.2. Study Eligibility Criteria
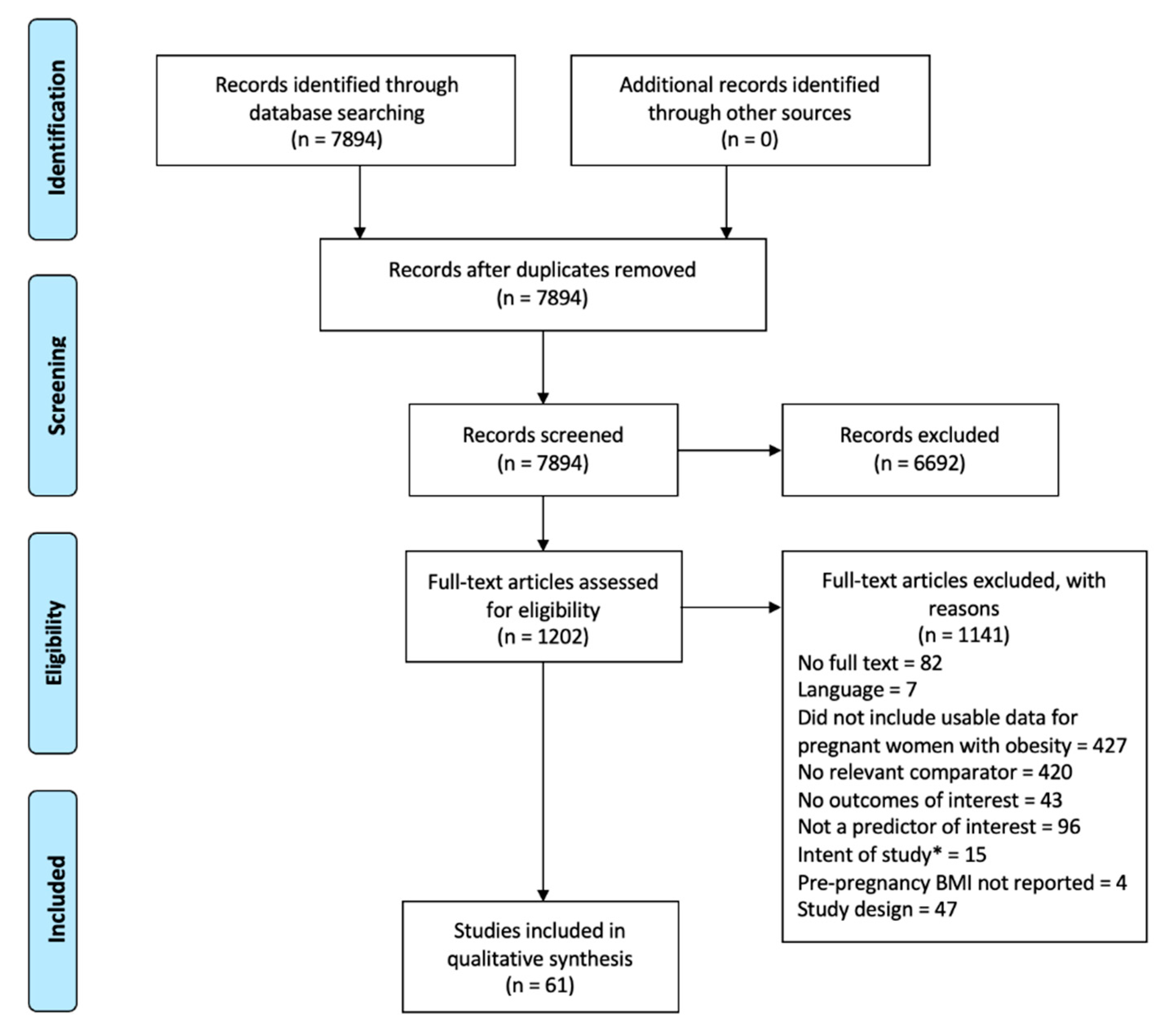
2.3. Process of Study Selection
2.4. Data Extraction Process
2.5. Summarizing Study Findings
3. Results
3.1. Extent of Literature Found
3.2. Primary Study Characteristics
3.3. Clinical Outcomes Evaluated
3.4. Demographics Evaluated and Approaches to Statistical Analysis
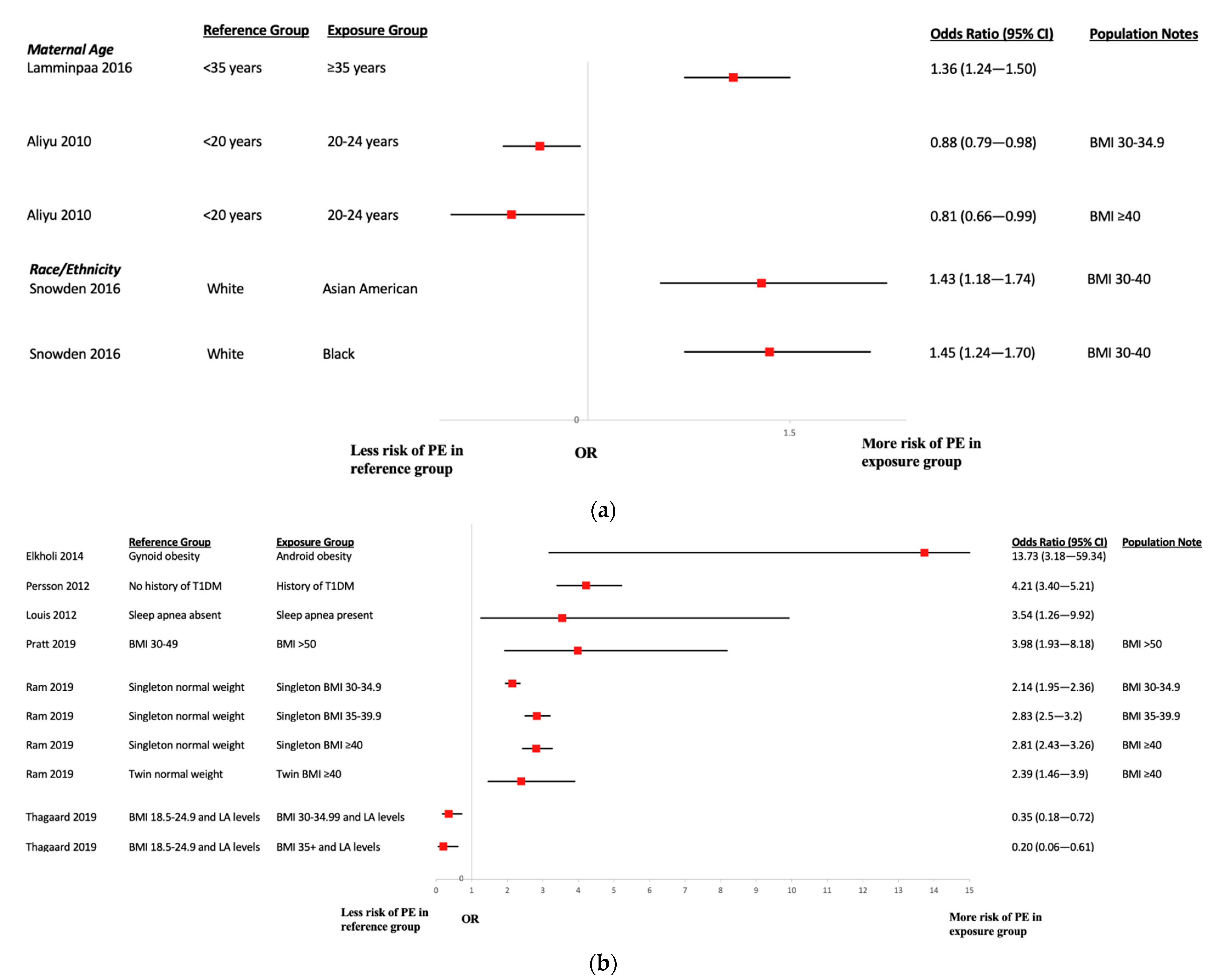
3.5. Risk Factors for Preeclampsia
3.6. Risk Factors for Low-Birth-Weight Newborns
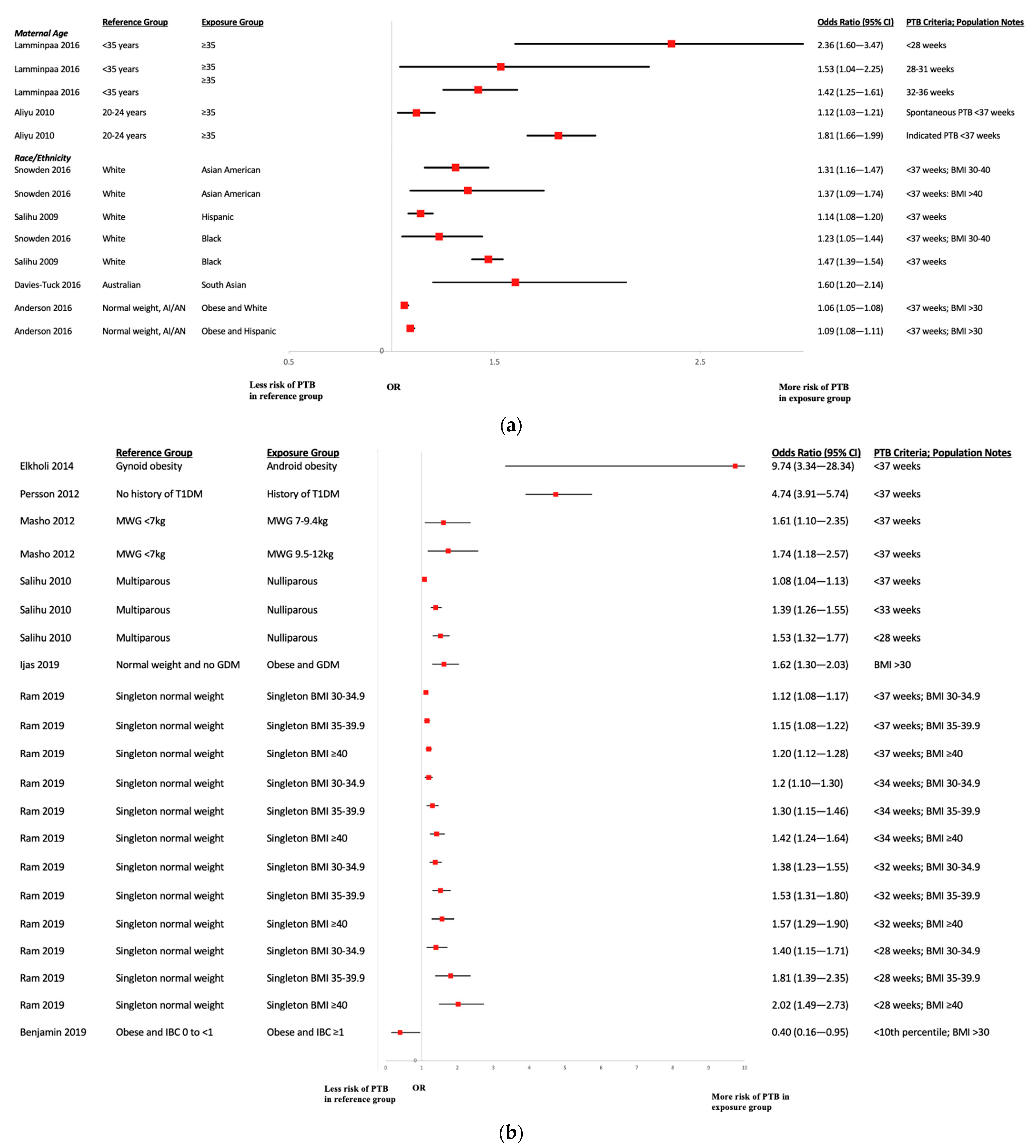
3.7. Risk Factors for Preterm Birth
3.8. Risk Factors for Gestational Diabetes Mellitus
3.9. Risk Factors for Stillbirth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef]
- Marchi, J.; Berg, M.V.D.; Dencker, A.; Olander, E.; Begley, C. Risks associated with obesity in pregnancy, for the mother and baby: A systematic review of reviews. Obes. Rev. 2015, 16, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.A.L.; Maxwell, C.; McLeod, L.; Maternal Fetal Medicine, C.; Clinical Practice, O. Obesity in pregnancy. J. Obstet. Gynaecol. Can. 2010, 32, 165–173. [Google Scholar] [CrossRef]
- Health Nexus. Obesity in Preconception and Pregnancy; Best Start Resource Centre: Toronto, ON, Canada, 2013. [Google Scholar]
- Davey Smith, G.; Steer, C.; Leary, S.; Ness, A. Is there an intrauterine influence on obesity? Evidence from parent child associations in the Avon Longitudinal Study of Parents and Children (ALSPAC). Arch. Dis. Child 2007, 92, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Devaux, M.G.S.; Goryakin, Y.; Cecchini, M.; Huber, H.; Colombo, F. OECD Obesity Update 2017; OECD: Paris, France, 2017. [Google Scholar]
- Feig, D.S.; Hwee, J.; Shah, B.R.; Booth, G.L.; Bierman, A.S.; Lipscombe, L.L. Trends in incidence of diabetes in pregnancy and serious perinatal outcomes: A large, population-based study in Ontario, Canada, 1996–2010. Diabetes Care 2014, 37, 1590–1596. [Google Scholar] [CrossRef]
- Wallis, A.B.; Saftlas, A.F.; Hsia, J.; Atrash, H.K. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am. J. Hypertens. 2008, 21, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Neiger, R. Long-Term Effects of Pregnancy Complications on Maternal Health: A Review. J. Clin. Med. 2017, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimons, K.J.; Modder, J. Setting maternity care standards for women with obesity in pregnancy. Semin. Fetal Neonatal Med. 2010, 15, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Sampson, M.; McGowan, J.; Cogo, E.; Grimshaw, J.; Moher, D.; Lefebvre, C. An evidence-based practice guideline for the peer review of electronic search strategies. J. Clin. Epidemiol. 2009, 62, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Rifas-Shiman, S.L.; Shivappa, N.; Wirth, M.D.; Hebert, J.R.; Gold, D.R.; Gillman, M.W.; Oken, E. Dietary Inflammatory Potential during Pregnancy Is Associated with Lower Fetal Growth and Breastfeeding Failure: Results from Project Viva. J. Nutr. 2016, 146, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Davies-Tuck, M.; Mockler, J.C.; Stewart, L.; Knight, M.; Wallace, E.M. Obesity and pregnancy outcomes: Do the relationships differ by maternal region of birth? A retrospective cohort study. BMC Pregnancy Childbirth 2016, 16, 288. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marshall, N.E.; Guild, C.; Cheng, Y.W.; Caughey, A.B.; Halloran, D.R. Racial disparities in pregnancy outcomes in obese women. J. Matern. Fetal Neonatal. Med. 2014, 27, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Machtinger, R.; Zera, C.; Racowsky, C.; Missmer, S.; Gargiulo, A.; Schiff, E.; Wilkins-Haug, L. The effect of mode of conception on obstetrical outcomes differs by body mass index. Reprod. Biomed. Online 2015, 31, 531–537. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Snowden, J.M.; Mission, J.F.; Marshall, N.E.; Quigley, B.; Main, E.; Gilbert, W.M.; Chung, J.H.; Caughey, A.B. The Impact of maternal obesity and race/ethnicity on perinatal outcomes: Independent and joint effects. Obesity 2016, 24, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Elkholi, D.G.E.Y.; Nagy, H.M. The effects of adipocytokines on the endocrino-metabolic features and obstetric outcome in pregnant obese women with polycystic ovary syndrome. Middle East Fertil. Soc. J. 2014, 19, 293–302. [Google Scholar] [CrossRef]
- Parker, M.H.; Berghella, V.; Nijjar, J.B. Bariatric surgery and associated adverse pregnancy outcomes among obese women. J. Matern. Fetal Neonatal. Med. 2016, 29, 1747–1750. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Pasupathy, D.; Hanson, U.; Westgren, M.; Norman, M. Pre-pregnancy body mass index and the risk of adverse outcome in type 1 diabetic pregnancies: A population-based cohort study. BMJ Open 2012, 2, e000601. [Google Scholar] [CrossRef] [PubMed]
- Lamminpaa, R.; Vehvilainen-Julkunen, K.; Gissler, M.; Selander, T.; Heinonen, S. Pregnancy outcomes of overweight and obese women aged 35 years or older-A registry-based study in Finland. Obes. Res. Clin. Pract. 2016, 10, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Metsälä, J.; Stach-Lempinen, B.; Gissler, M.; Eriksson, J.; Koivusalo, S.B. Risk of Pregnancy Complications in Relation to Maternal Prepregnancy Body Mass Index: Population-Based Study from Finland 2006–10. Paediatr. Perinat. Epidemiol. 2016, 30, 28–37. [Google Scholar] [CrossRef]
- Houde, M.; Dahdouh, E.M.; Mongrain, V.; Dubuc, É.; Francoeur, D.; Balayla, J. The Effect of Adequate Gestational Weight Gain among Adolescents Relative to Adults of Equivalent Body Mass Index and the Risk of Preterm Birth, Cesarean Delivery, and Low Birth Weight. J. Pediatr. Adolesc. Gynecol. 2015, 28, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Masho, S.W.; Bishop, D.L.; Munn, M. Pre-pregnancy BMI and weight gain: Where is the tipping point for preterm birth? BMC Pregnancy Childbirth 2013, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Saraiva, C.; Curtis, M.; Wilson, H.G.; Troyan, J.; Sharma, A.J. Fraction of gestational diabetes mellitus attributable to overweight and obesity by race/ethnicity, California, 2007–2009. Am. J. Public Health 2013, 103, e65–e72. [Google Scholar] [CrossRef] [PubMed]
- Ducarme, G.; Parisio, L.; Santulli, P.; Carbillon, L.; Mandelbrot, L.; Luton, D. Neonatal outcomes in pregnancies after bariatric surgery: A retrospective multi-centric cohort study in three French referral centers. J. Matern. Fetal Neonatal. Med. 2013, 26, 275–278. [Google Scholar] [CrossRef]
- Halloran, D.R.; Marshall, N.E.; Kunovich, R.M.; Caughey, A.B. Obesity trends and perinatal outcomes in black and white teenagers. Am. J. Obstet. Gynecol. 2012, 207, e491–e497. [Google Scholar] [CrossRef] [PubMed]
- Louis, J.; Auckley, D.; Miladinovic, B.; Shepherd, A.; Mencin, P.; Kumar, D.; Mercer, B.; Redline, S. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet. Gynecol. 2012, 120, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Hedderson, M.; Ehrlich, S.; Sridhar, S.; Darbinian, J.; Moore, S.; Ferrara, A. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care 2012, 35, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Olivarez, S.A.; Ferres, M.; Antony, K.; Mattewal, A.; Maheshwari, B.; Sangi-Haghpeykar, H.; Aagaard-Tillery, K. Obstructive sleep apnea screening in pregnancy, perinatal outcomes, and impact of maternal obesity. Am. J. Perinatol. 2011, 28, 651–658. [Google Scholar] [CrossRef]
- Salihu, H.; Mbah, A.K.; Alio, A.P.; Kornosky, J.L.; Whiteman, V.E.; Belogolovkin, V.; Rubin, L.P. Nulliparity and preterm birth in the era of obesity epidemic. J. Matern. Fetal Neonatal. Med. 2010, 23, 1444–1450. [Google Scholar] [CrossRef]
- Aliyu, M.H.; Luke, S.; Wilson, R.E.; Saidu, R.; Alio, A.P.; Salihu, H.M.; Belogolovkin, V. Obesity in older mothers, gestational weight gain, and risk estimates for preterm phenotypes. Maturitas 2010, 66, 88–93. [Google Scholar] [CrossRef]
- Aliyu, M.; Luke, S.; Kristensen, S.; Alio, A.P.; Salihu, H.M. Joint effect of obesity and teenage pregnancy on the risk of preeclampsia: A population-based study. J. Adolesc. Health 2010, 46, 77–82. [Google Scholar] [CrossRef]
- Shachar, B.Z.; Mayo, J.A.; Lee, H.C.; Carmichael, S.L.; Stevenson, D.K.; Shaw, G.M.; Gould, J.B. Effects of race/ethnicity and BMI on the association between height and risk for spontaneous preterm birth. Am. J. Obstet. Gynecol. 2015, 213, e701–e709. [Google Scholar] [CrossRef] [PubMed]
- Salihu, H.M.; Luke, S.; Alio, A.P.; Wathington, D.; Mbah, A.K.; Marty, P.J.; Whiteman, V. The superobese mother and ethnic disparities in preterm birth. J. Natl. Med. Assoc. 2009, 101, 1125–1131. [Google Scholar] [CrossRef]
- Thrift, A.P.; Callaway, L.K. The effect of obesity on pregnancy outcomes among Australian Indigenous and non-Indigenous women. Med. J. Aust. 2014, 201, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.R.; Sibai, A.J.; Istwan, N.B.; Rhea, D.J.; Desch, C.N.; Sibai, B.M. Spontaneously conceived pregnancy after 40: Influence of age and obesity on outcome. Am. J. Perinatol. 2014, 31, 795–798. [Google Scholar]
- Kim, C.; Kim, S.Y.; Sappenfield, W.; Wilson, H.G.; Salihu, H.M.; Sharma, A.J. Are gestational diabetes mellitus and preconception diabetes mellitus less common in non-Hispanic black women than in non-Hispanic white women? Matern. Child Health J. 2014, 18, 698–706. [Google Scholar] [CrossRef]
- Reeske, A.; Zeeb, H.; Razum, O.; Spallek, J. Differences in the Incidence of Gestational Diabetes between Women of Turkish and German Origin: An Analysis of Health Insurance Data from a Statutory Health Insurance in Berlin, Germany (AOK), 2005–2007. Geburtshilfe Frauenheilkd 2012, 72, 305–310. [Google Scholar] [CrossRef]
- Lynch, A.M.; Eckel, R.H.; Murphy, J.R.; Gibbs, R.S.; West, N.A.; Giclas, P.C.; Salmon, J.E.; Holers, V.M. Prepregnancy obesity and complement system activation in early pregnancy and the subsequent development of preeclampsia. Am. J. Obstet. Gynecol. 2012, 206, e421–e428. [Google Scholar] [CrossRef]
- Belogolovkin, V.; Salihu, H.M.; Weldeselasse, H.; Biroscak, B.J.; August, E.M.; Mbah, A.K.; Alio, A.P. Impact of prior bariatric surgery on maternal and fetal outcomes among obese and non-obese mothers. Arch. Gynecol. Obstet. 2012, 285, 1211–1218. [Google Scholar] [CrossRef]
- Høgh, S.; Wolf, H.T.; von Euler-Chelpin, M.; Huusom, L.; Pinborg, A.; Tabor, A.; Hegaard, H.K. Multivitamin use and risk of preeclampsia in a high-income population: A cohort study. Sex Reprod. Health. 2020, 24, 100500. [Google Scholar] [CrossRef]
- Njagu, R.; Adkins, L.; Tucker, A.; Gatta, L.; Brown, H.L.; Reiff, E.; Dotters-Katz, S. Maternal weight gain and neonatal outcomes in women with class III obesity. J. Matern. Fetal Neonatal. Med. 2020, 35, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Teh, J.L.; Lomanto, D.; Kim, G.; So, J.B.-Y.; Shabbir, A. Maternal and fetal outcomes of Asian pregnancies after bariatric surgery. Surg. Obes. Relat. Dis. 2020, 16, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Porteous, H.; de Jersey, S.; Palmer, M. Attendance rates and characteristics of women with obesity referred to the dietitian for individual weight management advice during pregnancy. Aust. N. Z. J. Obstet. Gynaecol. 2020, 60, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Pratt, A.; Howat, P.; Hui, L. Maternal and perinatal outcomes for women with body mass index ≥50 kg/m2 in a non-tertiary hospital setting. Aust. N. Z. J. Obs. Gynaecol. 2020, 60, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Dolin, C.D.; Chervenak, J.; Pivo, S.; Welcome, A.U.; Kominiarek, M.A. Association between time interval from bariatric surgery to pregnancy and maternal weight outcomes. J. Matern. Neonatal Med. 2019, 34, 3285–3291. [Google Scholar] [CrossRef] [PubMed]
- Browne, K.; Park, B.Y.; Goetzinger, K.R.; Caughey, A.B.; Yao, R. The joint effects of obesity and pregestational diabetes on the risk of stillbirth. J. Matern. Neonatal Med. 2019, 34, 332–338. [Google Scholar] [CrossRef]
- Ijas, H.; Koivunen, S.; Raudaskoski, T.; Kajantie, E.; Gissler, M.; Vaarasmaki, M. Independent and concomitant associations of gestational diabetes and maternal obesity to perinatal outcome: A register-based study. PLoS ONE 2019, 14, e0221549. [Google Scholar] [CrossRef]
- Karadag, C.; Demircan, S.; Caliskan, E. Effects of laparoscopic sleeve gastrectomy on obstetric outcomes within 12 months after surgery. J. Obstet. Gynaecol. Res. 2020, 46, 266–271. [Google Scholar] [CrossRef]
- Ram, M.; Berger, H.; Lipworth, H.; Geary, M.; McDonald, S.D.; Murray-Davis, B.; Riddell, C.; Hasan, H.; Barrett, J.; Melamed, N.; et al. The relationship between maternal body mass index and pregnancy outcomes in twin compared with singleton pregnancies. Int. J. Obes. 2020, 44, 33–44. [Google Scholar] [CrossRef]
- Meghelli, L.; Vambergue, A.; Drumez, E.; Deruelle, P. Complications of pregnancy in morbidly obese patients: What is the impact of gestational diabetes mellitus? J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101628. [Google Scholar] [CrossRef]
- Fallatah, A.M.; Bahrawi, A.J.; Babatin, H.M.; Nassibi, K.M.; AlEdreesi, Y.; Abduljabbar, H.S. Pregnancy Outcomes among Obese Pregnant Women with Varying Levels of Vitamin D in King Abdulaziz University Hospital: A Single-center Retrospective Study. Cureus 2019, 11, e6220. [Google Scholar] [CrossRef] [PubMed]
- Bar-Zeev, Y.; Haile, Z.T.; Chertok, I.A. Association Between Prenatal Smoking and Gestational Diabetes Mellitus. Obstet. Gynecol. 2020, 135, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Nilsson, I.A.K.; Gissler, M.; Lavebratt, C. Associations of Maternal Diabetes and Body Mass Index with Offspring Birth Weight and Prematurity. JAMA Pediatr. 2019, 173, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Ukah, U.V.; Bayrampour, H.; Sabr, Y.; Razaz, N.; Chan, W.S.; Lim, K.I.; Lisonkova, S. Association between gestational weight gain and severe adverse birth outcomes in Washington State, US: A population-based retrospective cohort study, 2004–2013. PLoS Med. 2019, 16, e1003009. [Google Scholar] [CrossRef] [PubMed]
- Feghali, M.N.; Catov, J.M.; Zantow, E.; Mission, J.; Caritis, S.N.; Scifres, C.M. Timing of Gestational Weight Gain and Adverse Perinatal Outcomes in Overweight and Obese Women. Obstet. Gynecol. 2019, 133, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Roussel, E.; Touleimat, S.; Ollivier, L.; Verspyck, E. Birthweight and pregnancy outcomes in obese class II women with low weight gain: A retrospective study. PLoS ONE 2019, 14, e0215833. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Kalwa, M.; Oleksy, P.; Marszalek, K.; Radon-Pokracka, M.; Huras, H. The relationship between pre-pregnancy BMI, gestational weight gain and neonatal birth weight: A retrospective cohort study. Ginekol Pol. 2019, 90, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.M.; Thompson, J.A. An evaluation of whether a gestational weight gain of 5 to 9 kg for obese women optimizes maternal and neonatal health risks. BMC Pregnancy Childbirth 2019, 19, 126. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, R.H.; Littlejohn, S.; Canfield, M.A.; Ethen, M.K.; Hua, F.; Mitchell, L.E. Interpregnancy change in body mass index and infant outcomes in Texas: A population-based study. BMC Pregnancy Childbirth 2019, 19, 119. [Google Scholar] [CrossRef] [PubMed]
- Thagaard, I.N.; Hedley, P.L.; Holm, J.-C.; Lange, T.; Larsen, T.; Krebs, L.; Christiansen, M. Leptin and Adiponectin as markers for preeclampsia in obese pregnant women, a cohort study. Pregnancy Hypertens. 2019, 15, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Grove, G.; Ziauddeen, N.; Harris, S.; Alwan, N.A. Maternal interpregnancy weight change and premature birth: Findings from an English population-based cohort study. PLoS ONE 2019, 14, e0225400. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, S.; Nur, U. Effect of prepregnancy maternal BMI on adverse pregnancy and neonatal outcomes: Results from a retrospective cohort study of a multiethnic population in Qatar. BMJ Open 2019, 9, e029757. [Google Scholar] [CrossRef] [PubMed]
- Moore Simas, T.A.; Waring, M.E.; Callaghan, K.; Leung, K.; Ward Harvey, M.; Buabbud, A.; Chasan-Taber, L. Weight gain in early pregnancy and risk of gestational diabetes mellitus among Latinas. Diabetes Metab. 2019, 45, 26–31. [Google Scholar] [CrossRef]
- Frankenthal, D.; Hirsh-Yechezkel, G.; Boyko, V.; Orvieto, R.; Ron-El, R.; Lerner-Geva, L.; Farhi, A. The effect of body mass index (BMI) and gestational weight gain on adverse obstetrical outcomes in pregnancies following assisted reproductive technology as compared to spontaneously conceived pregnancies. Obes. Res. Clin. Pract. 2019, 13, 150–155. [Google Scholar] [CrossRef]
- Laine, M.K.; Masalin, S.; Rönö, K.; Kautiainen, H.; Gissler, M.; Pennanen, P.; Eriksson, J.G. Risk of preterm birth in primiparous women with exposure to antidepressant medication before pregnancy and/or during pregnancy-Impact of body mass index. Ann. Med. 2019, 51, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Boudet-Berquier, J.; Salanave, B.; Desenclos, J.C.; Castetbon, K. Sociodemographic factors and pregnancy outcomes associated with prepregnancy obesity: Effect modification of parity in the nationwide Epifane birth-cohort. BMC Pregnancy Childbirth 2017, 17, 273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Janevic, T.; Zeitlin, J.; Egorova, N.; Balbierz, A.; Howell, E.A. The role of obesity in the risk of gestational diabetes among immigrant and U.S.-born women in New York City. Ann. Epidemiol. 2018, 28, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.G.; Spicer, P.; Peercy, M.T. Obesity, Diabetes, and Birth Outcomes Among American Indians and Alaska Natives. Matern. Child Health J. 2016, 20, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Kapoor, A.; Nelson, L.A.; Buchwald, D.S.; Walker, L.R.; Mueller, B.A. Pre-eclampsia in American Indians/Alaska Natives and Whites: The Significance of Body Mass Index. Matern. Child Health J. 2016, 20, 2233–2238. [Google Scholar] [CrossRef] [PubMed]
- Gernand, A.D.; Simhan, H.N.; Caritis, S.; Bodnar, L.M. Maternal vitamin D status and small-for-gestational-age offspring in women at high risk for preeclampsia. Obstet. Gynecol. 2014, 123, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, A.; Jauk, V.C.; Tita, A.; Harper, L.M. Interaction between maternal obesity and 1-hour glucose challenge test results on maternal and perinatal outcomes. Am. J. Perinatol. 2015, 32, 771–778. [Google Scholar] [CrossRef] [PubMed]
- MBRRACE-UK. Saving Lives, Improving Mothers’ Care-Lessons Learned to Inform Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2015–17; Knight, M., Tuffnell, D., Shakespeare, J., Kotnis, R., Kenyon, S., Kurinczuk, J.J., Eds.; National Perinatal Epidemiology Unit University of Oxford: Oxford, UK, 2019. [Google Scholar]
- Vieira, M.C.; White, S.L.; Patel, N.; Seed, P.T.; Briley, A.L.; Sandall, J.; Welsh, P.; Sattar, N.; Nelson, S.M.; Lawlor, D.A.; et al. Prediction of uncomplicated pregnancies in obese women: A prospective multicentre study. BMC Med. 2017, 15, 194. [Google Scholar] [CrossRef] [PubMed]
- Relph, S.; Guo, Y.; Harvey, A.L.J.; Vieira, M.C.; Corsi, D.J.; Gaudet, L.M.; Pasupathy, D. Characteristics associated with uncomplicated pregnancies in women with obesity: A population-based cohort study. BMC Pregnancy and Childbirth 2021, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Guidelines, Q.C. Obesity in Pregnancy. 2015. Available online: https://www.health.qld.gov.au/__data/assets/pdf_file/0019/142309/g-obesity.pdf (accessed on 8 February 2022).
- Goodnight, W.; Newman, R.; Society of Maternal-Fetal, M. Optimal nutrition for improved twin pregnancy outcome. Obstet. Gynecol. 2009, 114, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Bolognani, C.V.; de Sousa Moreira Reis, L.B.; de Souza, S.S.; Dias, A.; Rudge, M.V.; de Mattos Paranhos Calderon, I. Waist circumference in predicting gestational diabetes mellitus. J. Matern. Fetal Neonatal. Med. 2014, 27, 943–948. [Google Scholar] [CrossRef] [PubMed]
- McDonnold, M.; Mele, L.M.; Myatt, L.; Hauth, J.C.; Leveno, K.J.; Reddy, U.M.; Mercer, B.M.; Eunice Kennedy Shriver National Institute of Child Health Human Development Maternal-Fetal Medicine Units (MFMU) Network. Waist-to-Hip Ratio versus Body Mass Index as Predictor of Obesity-Related Pregnancy Outcomes. Am. J. Perinatol. 2016, 33, 618–624. [Google Scholar]
- Sween, L.K.; Althouse, A.D.; Roberts, J.M. Early-pregnancy percent body fat in relation to preeclampsia risk in obese women. Am. J. Obstet. Gynecol. 2015, 212, e81–e87. [Google Scholar] [CrossRef] [PubMed]
- Toro-Ramos, T.; Sichieri, R.; Hoffman, D.J. Maternal fat mass at mid-pregnancy and birth weight in Brazilian women. Ann. Hum. Biol. 2016, 43, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, N.J.; Peek, M.J.; Quinton, A.E.; Lanzarone, V.; Martin, A.; Benzie, R.; Nanan, R. Maternal abdominal subcutaneous fat thickness as a predictor for adverse pregnancy outcome: A longitudinal cohort study. BJOG 2016, 123, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Gur, E.B.; Ince, O.; Turan, G.A.; Karadeniz, M.; Tatar, S.; Celik, E.; Yalcin, M.; Guclu, S. Ultrasonographic visceral fat thickness in the first trimester can predict metabolic syndrome and gestational diabetes mellitus. Endocrine 2014, 47, 478–484. [Google Scholar] [CrossRef]



| Author (Year) | Countries of Conduct | Setting | Total Study Size | Focal Risk Factor Evaluated | Outcomes Reported | ||||
|---|---|---|---|---|---|---|---|---|---|
| Preeclampsia | Low Birth Weight (and SGA) | Gestational Diabetes | Preterm Birth | Stillbirth | |||||
| Sen [13] (2016) | USA | PC | 261 * | DII (change per unit increase) | X | X | X | X | |
| Davies-Tuck [14] (2016) | Australia | RC | 6038 * | Race (AUS/NZ versus South Asian) | X | X | X | X | |
| Marshall [15] (2014) | USA | RC | 61,191 * | Race (Caucasian versus African American) | X | X | |||
| Machtinger [16] (2015) | USA | RC | 1015 * | Mode of conception (spontaneous versus IVF) | X | X | X | X | |
| Snowden [17] (2016) | USA | RC | 76,174 * | Race (Caucasian, Hispanic, African American, Asian American) | X | X | X | X | |
| Elkholi [18] (2014) | Egypt | PC | 400 | PCOS (yes versus no), Obesity type (android versus gynoid) | X | X | X | ||
| Parker [19] (2015) | USA | RC | 186,705 | History of gastric bypass surgery (yes versus no) | X | X | |||
| Persson [20] (2012) | Sweden | PC | 82,949 * | History of type 1 diabetes (yes versus no) | X | X | |||
| Lamminpaa [21] (2016) | Finland | PC | 29,995 * | Advanced maternal age (≥35 y versus >35 y) | X | X | X | X | |
| Metsälä [22] (2015) | Finland | PC | 11,404 * | Histories of diabetes, hypertension (yes versus no) | X | ||||
| Houde [23] (2015) | USA | RC | 790,721 * | Maternal adolescent age (12–19 versus ≥20); adequacy of GWG | X | X | |||
| Masho [24] (2012) | USA | RC | 2960 * | Maternal weight gain during pregnancy(quartiles) | X | ||||
| Kim [25] (2013) | USA | RC | 251,237 * | Ethnicity (white, black, Asian, Hispanic, American Indian) | X | ||||
| Ducarme [26] (2013) | France | RC | 79 women (94 pregnancies) | Type of bariatric surgery (LAGB versus RYGB) | X | X | |||
| Halloran [27] (2012) | USA | RC | 2815 * | Ethnicity (Caucasian versus African American) | X | X | |||
| Louis [28] (2012) | USA | PC | 161 | Obstructive sleep apnea (yes versus no) | X | X | |||
| Hedderson [29] (2012) | USA | RC | 40,279 * | Race (White, Hispanic, African American, Asian, Filipina) | X | ||||
| Olivarez [30] (2011) | USA | PC | 50 * | Obstructive sleep apnea (yes versus no) | X | X | X | ||
| Salihu [31] (2010) | USA | RC | 132,894 * | Nulliparity (nulliparious versus multiparous), race (white, black, hispanic) | X | ||||
| Aliyu [32] (2010) | USA | RC | 3278 * | Maternal age (20–24 versus ≥35) | X | ||||
| Aliyu [33] (2010) | USA | RC | 51,427 * | Adolescent maternal age (<20 years versus 20–24 years) | X | ||||
| Shachar [34] (2015) | USA | RC | 19,664 * | Maternal height (five size categories considered) | X | ||||
| Salihu [35] (2009) | USA | RC | 149,532 * | Ethnicity (Hispanic, Caucasian, African American) | X | ||||
| Thrift [36] (2014) | Australia | RC | 55,275 * | Indigenous status (indigenous versus non-indigenous) | X | X | X | ||
| Barton [37] (2014) | USA | RC | 9452 * | Maternal age (20–29 versus >40) | X | X | X | X | X |
| Kim [38] (2014) | USA | RC | 462,296 * | Ethnicity (Caucasian, African American), Age (20–29, >40) | X | ||||
| Reeske [39] (2012) | Germany | RC | 3338 | Ethnicity (Turkish versus German) | X | ||||
| Lynch [40] (2012) | USA | PC | 1013 * | Complement activation fragments (Bb, C3a; quartiles) | X | ||||
| Belogolovkin [41] (2012) | USA | RC | 131,166 * | Prior bariatric surgery (yes versus no) | X | X | X | X | |
| Hogh [42] (2020) | Denmark | PC | 15,154 * | Multivitamin use (non-users versus periconceptional use versus early pregnancy use) | X | ||||
| Njagu [43] (2020) | USA | RC | 374 * | GWG (≤20 lbs versus >20 lbs weight gain) | X | X | |||
| Malik [44] (2020) | Singapore | PC | 55 * | Postbariatric surgery (yes versus no) | X | X | |||
| Porteous [45] (2020) | Australia | RC | 5426 | Referral to an Ante-natal dietitian (yes versus no); number of appointments attended | X | ||||
| Pratt [46] (2019) | Australia | RC | 18,402 * | Hypertensive disorder | X | X | X | X | |
| Dolin [47] (2019) | USA | RC | 76 * | Bariatric surgery (<12 months before versus ≥12 months before) | X | X | X | ||
| Browne [48] (2019) | USA | RC | 3,097,123 * | Diabetes (nondiabetic versus pregestational diabetic) | X | ||||
| Ijas [49] (2019) | Finland | RC | 24,577 * | Age (<19, 20–29, 30–39, ≥40), parity (primiparous versus multiparous), SES (upper, lower, manual, other) | X | ||||
| Karadag [50] (2020) | Turkey | RC | 144 * | LSG (≤1 year versus >1 year before pregnancy) | X | X | X | X | |
| Ram [51] (2019) | Canada | RC | 487,870 * | Singleton versus twin pregnancies | X | X | X | X | X |
| Meghelli [52] (2020) | France | RC | 472 * | Age, GWG, hospitalization | X | X | X | X | |
| Fallatah [53] (2019) | Saudi Arabia | RC | 132 * | Vitamin D levels (deficient versus optimal versus therapeutic versus excess) | X | X | X | X | |
| Bar-Zeev [54] (2020) | USA | PC | 222,408 * | Prenatal smoking (non-smoker, quit smoking, reduced the amount smoked, smoked the same or more) | X | ||||
| Kong [55] (2019) | Finland | RC | 649,043 * | Prematurity, diabetes (no versus insulin treated versus type II) | X | ||||
| Ukah [56] (2019) | USA | RC | 165,908 * | GWG; race (Black, Native American, Hispanic, White) | X | X | |||
| Feghali [57] (2019) | USA | RC | 5814 * | GWG (adequate, inadequate, excess) | X | X | X | ||
| Roussel [58] (2019) | France | RC | 996 * | GWG (recommended weight gain, low weight gain, weight loss) | X | X | X | ||
| Nowak [59] (2019) | Poland | 63RC | 474 * | GWG (inadequate versus excess) | X | ||||
| Thompson [60] (2019) | USA | RC | 10,811,496 * | GWG (<5 kg, 6–9 kg, >9 kg), gestational hypertension | X | X | X | ||
| Benjamin [61] (2019) | USA | RC | 694 * | LGA, GWG (inadequate versus adequate versus excess), height (<1.60 m, 1.6 m to <1.65 m, 1.65 m to <1.7 m, ≥1.7 m) | X | X | |||
| Thagaard [62] (2019) | Denmark | RC | 2503 * | Adiponectin and leptin concentrations | X | ||||
| Grove [63] (2019) | England | RC | 20,069 * | GWG (decrease in BMI versus increase in BMI) | X | ||||
| Shaukat [64] (2019) | Qatar | RC | 1134 * | Ethnicity (Arab versus non-Arab), hypertension | X | X | X | X | |
| Moore Simas [65] (2019) | USA | RC | 2039 * | GWG (low, appropriate, excess), AGT (yes versus no) | X | ||||
| Frankenthal [66] (2019) | Israel | PC | 1058 * | GWG (low, appropriate, excess), assisted reproduction treatment (yes versus no) | X | X | |||
| Laine [67] (2019) | Finland | RC | 6920 * | Antidepressant use (yes versus no) | X | ||||
| Boudet-Berquier [68] (2017) | France | RC | 3208 * | Parity (primiparous versus multiparous), GWG (low, appropriate, excess), hypertensive complications (yes versus no), vaginal birth (yes versus no), smoking (non-smoker, quit smoking, smoke the same); maternal age (18–24, 25–29, 30–34, ≥35) | X | X | |||
| Janevic [69] (2018) | USA | RC | 668,035 * | Ethnicity (Black, White, all Hispanic, all Asian, Mexican, Chinese, Indian); place of birth (foreign born versus USA born) | X | ||||
| Anderson [70] (2016) | USA | RC | 5,193,386 * | Ethnicity (American Indian/Alaska Native, Black, White, Hispanic) | X | X | |||
| Zamora-Kapoor [71] (2016) | USA | RC | 71,080 * | Ethnicity (American Indian/Alaska Native versus White) | X | ||||
| Gernand [72] (2014) | USA | PC | 792 * | Vitamin D status | X | ||||
| Subramaniam [73] (2015) | USA | RC | 14,525 * | LGA, macrosomia, shoulder dystocia, hypertension | X | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakhraei, R.; Denize, K.; Simon, A.; Sharif, A.; Zhu-Pawlowsky, J.; Dingwall-Harvey, A.L.J.; Hutton, B.; Pratt, M.; Skidmore, B.; Ahmadzai, N.; et al. Predictors of Adverse Pregnancy Outcomes in Pregnant Women Living with Obesity: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 2063. https://doi.org/10.3390/ijerph19042063
Fakhraei R, Denize K, Simon A, Sharif A, Zhu-Pawlowsky J, Dingwall-Harvey ALJ, Hutton B, Pratt M, Skidmore B, Ahmadzai N, et al. Predictors of Adverse Pregnancy Outcomes in Pregnant Women Living with Obesity: A Systematic Review. International Journal of Environmental Research and Public Health. 2022; 19(4):2063. https://doi.org/10.3390/ijerph19042063
Chicago/Turabian StyleFakhraei, Romina, Kathryn Denize, Alexandre Simon, Ayni Sharif, Julia Zhu-Pawlowsky, Alysha L. J. Dingwall-Harvey, Brian Hutton, Misty Pratt, Becky Skidmore, Nadera Ahmadzai, and et al. 2022. "Predictors of Adverse Pregnancy Outcomes in Pregnant Women Living with Obesity: A Systematic Review" International Journal of Environmental Research and Public Health 19, no. 4: 2063. https://doi.org/10.3390/ijerph19042063
APA StyleFakhraei, R., Denize, K., Simon, A., Sharif, A., Zhu-Pawlowsky, J., Dingwall-Harvey, A. L. J., Hutton, B., Pratt, M., Skidmore, B., Ahmadzai, N., Heslehurst, N., Hayes, L., Flynn, A. C., Velez, M. P., Smith, G., Lanes, A., Rybak, N., Walker, M., & Gaudet, L. (2022). Predictors of Adverse Pregnancy Outcomes in Pregnant Women Living with Obesity: A Systematic Review. International Journal of Environmental Research and Public Health, 19(4), 2063. https://doi.org/10.3390/ijerph19042063






