Abstract
This study examined (1) the availability and content of national CPGs for treatment of peripartum depression, including comorbid anxiety, with antidepressants and other psychotropics across Europe and (2) antidepressant and other psychotropic utilization data as an indicator of prescribers’ compliance to the guidelines. We conducted a search using Medline and the Guidelines International Network database, combined with direct e-mail contact with national Riseup-PPD COST ACTION members and researchers within psychiatry. Of the 48 European countries examined, we screened 41 records and included 14 of them for full-text evaluation. After exclusion of ineligible and duplicate records, we included 12 CPGs. Multiple CPGs recommend antidepressant initiation or continuation based on maternal disease severity, non-response to first-line non-pharmacological interventions, and after risk-benefit assessment. Advice on treatment of comorbid anxiety is largely missing or unspecific. Antidepressant dispensing data suggest general prescribers’ compliance with the preferred substances of the CPG, although country-specific differences were noted. To conclude, there is an urgent need for harmonized, up-to-date CPGs for pharmacological management of peripartum depression and comorbid anxiety in Europe. The recommendations need to be informed by the latest available evidence so that healthcare providers and women can make informed, evidence-based decisions about treatment choices.
1. Introduction
Peripartum or perinatal depression, which is depression arising in the period between the start of a pregnancy and the end of the first postpartum year, to use a broad definition, affects approximately one in eight women [1]. Peripartum and perinatal depression are used interchangeably, although the former term relates more specifically to the woman. The disorder often persists throughout the peripartum period, with as many as 47% of women with postnatal depression having experienced an antenatal episode [2]. In many cases, depression concurs with anxiety, and this adds a substantial mental health burden to the woman [3]. One recent study has proposed multiple subtypes of perinatal depression, which differ in terms of symptom dimension and time of onset [4]. Women may, therefore, need tailored treatment strategies, including pharmacotherapy, depending on their individual depression course, timing of onset, and prominent symptom typology.
Perinatal depression is associated with a spectrum of obstetric and long-term negative outcomes in the offspring [5,6], including possible adverse impacts on the mother-infant relationship [7,8]. It also substantially affects women’s well-being and functioning, and it can even lead to suicide [9]. In moderate to severe cases or after non-response to first-line psychotherapy, pharmacotherapy with antidepressants is often needed [10]. Pooled results from 40 cohort studies [11] indicate that selective serotonin reuptake inhibitors (SSRIs) are the most commonly used antidepressants, with a population prevalence of filled prescriptions ranging from 3.5% before pregnancy to 3.0% during gestation and 4.7% in the first year postpartum. Augmentation with antipsychotics or adjuvant pharmacotherapy with benzodiazepines or sedative antihistamines may be needed in some cases [10]. Nevertheless, pregnancy remains a major driver for discontinuation of antidepressants, and 49% of those individuals who chose to continue have low antidepressant adherence [12,13].
The decision-making process about antidepressant treatment during pregnancy or lactation is complex, as it involves weighing the possible risk of exposure in utero or in breast milk against the potential adverse effects of sub-optimally treated maternal peripartum depression to both the mother and child. Clinical practice guidelines (CPGs) for peripartum depression management may facilitate this decision-making process. However, many countries have not established CPGs for peripartum depression, and for those available, the recommendations are not always uniform [14]. In 2018, one systematic review evaluated the content of the available CPGs, and it was found that only four countries recommend continuation into pregnancy of a pre-existing antidepressant treatment [14]. This prior work extracted only recommendations from CPGs adhering to the quality criteria of the Appraisal of Guidelines for Research and Evaluation (AGREE) instrument. Thus, there are still knowledge gaps on current clinical practices from CPGs not meeting such quality criteria. Furthermore, the extent to which national CPGs are followed in relation to antidepressant and other psychotropic prescribing remains unknown.
Therefore, the aim of this review was to examine the availability of national CPGs for treatment of peripartum depression with antidepressants across Europe and review their content and recommendations for the pregnancy and postpartum periods. We further evaluate antidepressant utilization data in women during the perinatal period as an indicator of compliance to the guidelines. To shed additional light on mental disorder co-morbidity, we evaluated whether CPGs for peripartum depression provide guidance on psychopharmacological treatment for co-morbid anxiety, along with prescription fill data for other psychotropics (i.e., antipsychotics and benzodiazepines) and sedative antihistamines during pregnancy and postpartum.
2. Materials and Methods
2.1. Search and Selection Criteria for Clinical Practice Guidelines
We conducted an extensive search of CPGs for treatment of peripartum depression in 48 countries in Europe, including member countries of the European Union, Schengen states, and other European countries. San Marino and the Holy See, both located geographically in Italy, were not included, as the former follows guidelines in Italy and the latter was not relevant. We combined multiple search strategies. First, we searched the literature in the Medline database (via PubMed) from inception to 31 August 2021 using the free text terms “antidepressant, peripartum, perinatal, pregnancy, postpartum, antenatal period, prenatal period, postnatal period, depression, mental health, psychiatric” and applied the filter for guidelines only. Second, we searched the Guidelines International Network (GIN) database using the terms “depression, peripartum, perinatal, pregnancy” on 31 August 2021. Third, we contacted directly via email the national members of Riseup-PPD COST ACTION (CA18138–Research Innovation and Sustainable Pan-European Network in Peripartum Depression Disorder) with an inquiry about the existence of a CPG for peripartum depression in the country. Last, we contacted researchers within peripartum psychiatry in various countries. No exclusion criteria were employed based on language. In the searches in Medline and GIN, we did not include published CPGs from countries outside Europe. We did not restrict the search to CPGs meeting the quality criteria of the AGREE instrument, as we aimed to gather as much information as possible about current clinical practices. Case reports and animal studies were excluded. We excluded CPGs on depression or mental health in adults which did not cover or mention peripartum depression within them and CPGs on peripartum depression that did not mention pharmacotherapy interventions. Clinical recommendations without clear references or without a description of the process that led to the recommendation were also excluded. The literature searches and abstract screenings were performed by a single author. The selection of the CPGs eligible for inclusion were agreed upon by all authors.
Data abstraction was performed by one author depending on the relevant language and, thereafter, quality-checked by another author. We extracted recommendations regarding (1) initiation, continuation or discontinuation, and switching of the antidepressant for both new and preexisting depression in pregnancy or postpartum, (2) preferred and non-preferred antidepressants in pregnancy and while breastfeeding, (3) compatibility of antidepressants with breastfeeding, (4) antidepressant level monitoring or dose adjustment, and (5) recommendations for pharmacological treatment of comorbid anxiety in pregnancy and postpartum.
2.2. Search and Selection Criteria for Antidepressant and Psychotropic Utilization Studies
We searched the literature in the Medline database (via PubMed) from inception to 31 August 2021 using the free text terms “antidepressant, psychotropic, antipsychotic, anxiolytic, “medication use”, “drug use”, peripartum, perinatal, pregnancy, postpartum, antenatal period, prenatal period, postnatal period, depression, mental health, psychiatric”. We extracted the most complete or recent antidepressant drug utilization studies among those published in the last 10–15 years originating from countries in Europe. We applied no restriction as to the way antidepressant use in pregnancy and postpartum was measured in the studies (e.g., based on self-reporting, prescription fills, or medical records). The outcome criteria were prevalence estimates for antidepressant use before, during, and after pregnancy. The same criteria applied to the search and data extraction for other psychotropic medications. If available, we extracted prevalence estimates from more than one study.
2.3. Ethics Statement
No ethics approval was sought, as this review evaluated existing clinical practice guidelines. No informed consent was collected, as the study did not involve patients. The synthesis was not registered in PROSPERO.
3. Results
3.1. Identified Clinical Practice Guidelines
Figure 1 describes the flow diagram of the various search strategies to achieve the final sample of CPGs included in the study. Across the 48 countries examined, we were unable to identify a contact person or did not receive a response in 22 (45.8%) of the countries. We received a response or identified a CPG in the literature search for 26 countries in Europe, of which 10 (38.5%) (i.e., Austria, Bulgaria, Croatia, Cyprus, France, Greece, Iceland, Portugal, Turkey, and Bosnia and Herzegovina) did not have a national CPG for intervention strategies of peripartum depression or mental health, either specific or broader for the adult population, with mention of the peripartum population. In Ireland, we could only retrieve an information leaflet on peripartum depression for women, which is not classified as a CPG. In Ukraine (personal communication), the criteria for treatment of peripartum depression were reported to be in place, which included pharmacotherapy interventions with amitriptyline, phenazepam, relanium, frenolone, and with vitamins (e.g., ascorbic acid). However, no further information was obtained. Belgium and Sweden used protocols or guidelines for screening and treatment of peripartum depression based on international guidelines (NICE). However, pharmacotherapy interventions are not mentioned [15,16]. Of the searches in PubMed and the GIN database, we screened three CPGs from Spain, Poland, and the UK, which were duplicates of the ones obtained via the contact persons in these countries. We included and fully evaluated 12 CPGs. In the CPG from Latvia, recommendations on pharmacological interventions were only provided for the postpartum period.
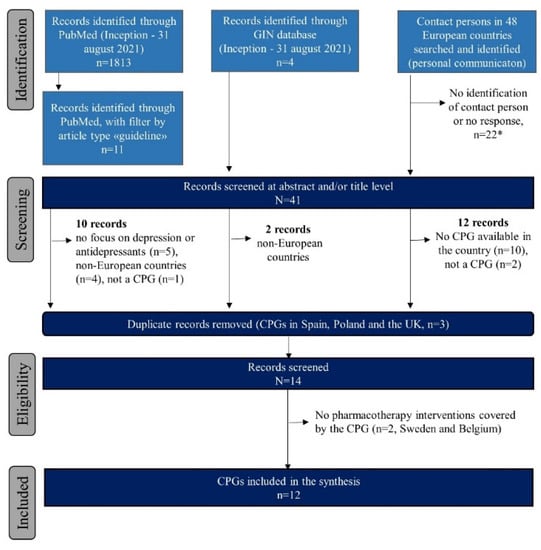
Figure 1.
Flow chart of the review process for the clinical practice guideline synthesis. Abbreviations: CPG = clinical practice guideline; GIN = Guidelines International Network. * No response or identification in Albania, Andorra, Armenia, Azerbaijan, Belarus, Croatia, Czech Republic, Estonia, Georgia, Hungary, Kazakhstan, Kosovo, Liechtenstein, Luxembourg, Moldova, Montenegro, North Macedonia, Romania, Russia, Slovakia, Slovenia, and Switzerland.
3.2. Pharmacological Interventions for Treatment of Antenatal Depression
Table 1 shows that most CPGs advise initiation of antidepressants in women with new onset or moderate-to-severe antenatal depression. This treatment should be undertaken after an individualized risk–benefit evaluation and following non-response to psychotherapy. In contrast, the CPG in Poland discourages the use of antidepressants in the first trimester and states that this medication should be discontinued before delivery. All the CPGs seem unanimous in recommending or mentioning the possibility to continue antidepressants in pregnancy for preexisting moderate-to-severe depression (Table 1). In the UK CPG, monotherapy (if possible) and the lowest effective dose are advised in the context of both initiation and continuation of the antidepressant. On the basis of filled prescription and drug utilization data, there was a decrease in the prevalence of antidepressant use from preconception (range: 1.6–9.6%) into pregnancy (range: 0.3–4.1%), with SSRI being the most commonly prescribed group in most countries (Table 1). For many countries in Eastern Europe, no such utilization data were available.

Table 1.
Overview of recommendations in the CPGs about antidepressant treatment in women with antenatal depression, with prevalence estimates of antidepressant and other psychotropic medication use in the country.
There is general agreement between the CPGs in evaluating individual drug response in the period prior to pregnancy in the decision making about antidepressant continuation during pregnancy. The CPGs provide less uniform guidance regarding switching antidepressants during pregnancy (Table 1). In the CPGs of Malta and Norway, switching is discouraged unless the drug is ineffective. The CPG in the Netherlands considers switching from paroxetine to a preferred antidepressant but before pregnancy. Multiple CPGs (Finland, Germany, Italy, and Serbia) do not provide guidance on switching. Likewise, information on antidepressant level monitoring in serum or plasma and on dosage adjustment is missing for the CPGs in Italy and Denmark.
Multiple CPGs mention sertraline and citalopram as preferred antidepressants in pregnancy, whereas others (i.e., Finland, Serbia, and Spain) list the class of SSRIs. Paroxetine was mentioned as not a preferred antidepressant in most CPGs, except for Serbia, the UK, and Norway. In the two latter countries, the CPGs advise basing the choice of the antidepressant on maternal prior response and its safety profile. Generally, the antidepressants recommended in the CPGs were also the ones most often used in gestation, except in Denmark (for fluoxetine), the Netherlands and Spain (for paroxetine), and Germany (for amitriptyline). Paroxetine ranked among the most commonly used antidepressants in pregnancy in specific countries (i.e., Italy, the Netherlands, or Spain).
3.3. Pharmacological Interventions for Treatment of Postpartum Depression
Table 2 summarizes the content and recommendations of the CPGs for the postpartum period. Most CPGs (n = 11) recommend initiation or continuation of antidepressant medications in women suffering from depression in the postpartum period. Nearly all CPGs suggest an individual risk–benefit evaluation of the antidepressant treatment in the case of breastfeeding. Recommendations about breastfeeding compatibility with maternal antidepressant use were not specified in three GPGs (Spain, Serbia, and Norway). The CPGs in the Netherlands, Italy, and Finland state that antidepressant use does not prevent breastfeeding, whereas the UK and Denmark advise closely monitoring the exposed breastfed infant for potential adverse effects, such as weight gain. The Maltese CPG advises that only healthy and full-term infants should be breastfed when mothers are taking antidepressants. The Polish CPG gives a detailed recommendation about the timing of antidepressant intake and breastfeeding (i.e., to take one daily dose before the longest sleep of the child and breastfeed directly before that). Recommendations about switching antidepressants are either unspecified (n = 5) in the CPG or discouraged, especially if it affects the woman. The prevalence estimates of antidepressant use postpartum were greater than in the antenatal period and generally returned to the magnitude seen pre-pregnancy. For most of the countries included in this work, no antidepressant utilization data postpartum are available.

Table 2.
Overview of recommendations in the CPGs about antidepressant treatment in women with postnatal depression, with prevalence estimates of antidepressants and other psychotropic medication use in the country.
The specific substances recommended and not recommended vary considerably between the CPGs, but taken together, sertraline (8/12 CPGs) and paroxetine (5/12 CPGs) are the ones most commonly preferred, while fluoxetine is not preferred in most CPGs (8/12 CPGs) due to its very long half-life with the risk of accumulation in the infant. Paroxetine, citalopram, sertraline, or SSRI in general are also the antidepressants most commonly taken by women postpartum. Fluoxetine does not rank high in drug utilization studies in the postpartum period.
3.4. Pharmacological Interventions for Antenatal or Postpartum Comorbid Anxiety and Use of Other Psychotropics
Treatment recommendations for comorbid anxiety are largely missing for both the antenatal and the postpartum period (Table 1 and Table 2). Only seven GPGs state that benzodiazepines can be offered in the case of severe anxiety during pregnancy but only for short-term treatment. In Malta, benzodiazepines are recommended only as needed, and the treatment of choice is augmentation with quetiapine, both during pregnancy and postpartum. During the latter period, sedative antihistamines represent a treatment option. In the UK, it is advised to treat comorbid anxiety with antidepressants during pregnancy or short-term benzodiazepines, and the latter medication is discouraged at postpartum in case of breastfeeding. The CPG in Latvia recommends treatment of comorbid anxiety postpartum with mirtazapine or atypical antipsychotics, including olanzapine at a low dose, while benzodiazepines should be avoided.
Prenatal use data for benzodiazepines, antipsychotics, and quetiapine specifically are lacking for some countries, and for sedative antihistamines, data are very sparse. During pregnancy, benzodiazepines are often used to a larger extent than antidepressants in specific countries (i.e., Germany, Poland, Serbia, and Spain), while in Norway, the use of benzodiazepine and sedative antihistamines is comparable (about 1%). With regard to the use of other psychotropic medication (as an add-on) in the postpartum period, utilization data are largely unavailable, as only the Nordic countries and the UK report postpartum use of benzodiazepines and antipsychotics in the ranges of 0.8–3.2% and 0.2–0.4%, respectively.
4. Discussion
This review across European countries reports important gaps in the availability, agreement, and up-to-date evidence-based content of CPGs for the pharmacological treatment of peripartum depression. This may have implications in the decision making and uptake of effective treatment among perinatal women and consequently in reducing the pervasive costs of peripartum depression. Several of our findings are important for clinical practice and perinatal drug research at large. First, we identified a national CPG only in 12 out of the 48 countries in Europe, adding 6 guidelines to the latest synthesis by Molenaar et al. in 2018 [14]. Nevertheless, the absence of a CPG in most countries raises clear concerns about the pharmacological management of depression in pregnant women and new mothers [10], especially in countries where higher rates of peripartum depression [50,51] are paralleled by low use of antidepressants and greater use of benzodiazepines [24]. Second, we found general agreement within the CPGs in recommending psychotherapy as first-line intervention, as well as antidepressant initiation or continuation based on psychotherapy non-response or depression severity. However, the recommendations are sometimes unspecific and not uniform across guidelines. Third, emerging issues and questions that are met in the real-world practice are not covered with the latest available evidence (e.g., drug monitoring or dose adjustments, antidepressant switching and treatment augmentation, adjuvant strategies for comorbid anxiety, and compatibility of breastfeeding with antidepressant treatment). Finally, the unavailability of antidepressant and other psychotropic utilization data from pre-pregnancy through the end of the first postpartum year, especially in some countries, impedes the evaluation of prescribers’ compliance to a CPG and calls for ad hoc perinatal drug utilization research.
The available evidence about antidepressant safety and antidepressant effectiveness in the context of continuation, discontinuation, or initiation is limited, especially for the pregnancy period [50,52,53,54,55,56,57]. It is now widely acknowledged that intrauterine exposure to SSRIs does not substantially increase the risk of congenital anomalies in offspring, while the risk for negative longer-term developmental outcomes is less clear [58,59,60]. However, only more recent studies have compared outcomes in offspring born to continuers versus discontinuers [61]. Antidepressant continuation in pregnancy was found to increase the risk of low birth weight, premature birth, or affective disorder diagnosis later in childhood [62,63,64,65]. Yet, the role of confounding by maternal disease severity remains an important concern in this research. Regarding antidepressant effectiveness, a recent meta-analysis [53] found a 74% increased risk of depression relapse during pregnancy with antidepressant discontinuation relative to continuation in pregnancy. The four included studies were, however, very heterogeneous and adopted an oversimplified definition of antidepressant continuation or discontinuation that did not reflect the treatment intensity, dose changes, or timing of exposure as in real-world settings [66,67,68].
No observational or randomized study to date has investigated the benefit of antidepressant initiation in pregnancy on relapse or remission of peripartum depression. The need for clinical drug trials in pregnant and postpartum women has never been greater [69]. The findings from the “stop or go” randomized trial indicated no significant difference in the risk of relapse of depression in women who tapered SSRIs with additional preventive cognitive therapy, relative to those who continued SSRIs [70]. However, the study included only 44 women, demonstrating the need for larger trials which also address the efficacy of antidepressant initiation in pregnancy. In 2019, the Food and Drug Administration in the US approved the first drug specifically for the treatment of postpartum depression: the GABA-A receptor modulator brexanolone. Brexanolone is not yet approved in the EU, but regulatory pathways have been initiated for future marketing authorization. This new drug constitutes an important therapeutic option for women with severe postpartum depression, but its difficult administration in terms of duration and form (i.e., intravenously) may limit its usage. Determining the comparative effectiveness and safety of brexanolone versus any other treatment for postnatal depression [54] will be crucial to inform clinical decisions involving CPGs at an international level. Similarly, more research is needed about the comparative effectiveness of different pharmacological interventions versus other therapeutic options, such as electroconvulsive therapy for treatment of severe perinatal depression.
There remains a need for more unified guidelines on the use of antidepressants to treat peripartum depression to guide clinical decision making. However, the decision to treat peripartum depression with antidepressants must always consider the individualized risk–benefit profile of the medication for each woman [54]. Most CPGs recommend an individualized risk–benefit assessment, which should consider the psychiatric history of the woman, her response to prior or ongoing antidepressants, mental health outcomes following prior attempts to discontinue the medication, the woman’s treatment preference, and her desire to breastfeed. The antidepressant with the lowest known risk for breastfed children in the lowest effective dose and in the lowest effective drug serum concentration should be prescribed [60]. To the best of our knowledge, there is no evidence base to discourage breastfeeding of preterm or low birth weight infants. However, caution is needed due to the immature liver metabolic capacity in preterm infants, especially in combination with maternal fluoxetine use, which has a long half-life and increased risk of accumulation in the breastfed infant [60]. The decision making in pregnancy and while breastfeeding could be aided by further development of patient decision aid (PDA) tools. Early data suggest that they are acceptable to users and reduce decisional conflict [71,72].
Generally, there was satisfactory compliance in prescribing preferred antidepressants during pregnancy (e.g., sertraline and citalopram), although exceptions were noted. Paroxetine ranked among the most commonly used antidepressants in pregnancy in specific countries, despite being a non-preferred antidepressant. However, we could not corroborate whether this drug choice was derived from an individualized assessment based on maternal prior response to the drug or whether it reflects poor prescriber compliance to the CPG. One Dutch study [73] found that gynecologists and midwives were aware of the national CPG on antidepressants in pregnancy, yet only 13.9% of them adhered to its recommendations. Efforts are, therefore, necessary to facilitate the uptake of the CPG recommendations in routine clinical practice by all healthcare professionals involved in the care of women with peripartum depression.
One key finding is that guidance on intervention strategies for comorbid anxiety and advice on augmentation with antipsychotics are largely missing across the examined CPGs, and when present, it is too unspecific with regard to drug selection and maximum permissible doses. Indeed, we observed important country-specific fluctuations in the utilization of benzodiazepines that need to be addressed. Uniform, specific recommendations for this problem are needed for multiple reasons: (1) some women manifest active depressive symptoms despite antidepressant treatment, and clinicians need evidence-based guidance to treat them; (2) anxiety is a prominent symptom of severe peripartum depression [4]; and (3) benzodiazepines should be used only sporadically during pregnancy or postpartum, and alternative interventions are necessary for protracted treatments [60]. Yet, to date, evidence does not exist to help make recommendations for the perinatal population, which calls for urgent population-based perinatal drug research.
Strengths and Limitations
Several strengths and limitations need mentioning. One of the main strengths of this synthesis is that we provided a global view of the existing CPGs across Europe. We applied multiple search strategies, our search was not restricted to CPGs meeting the AGREE instrument, and we applied no language restrictions, which enabled us to gather as many CPGs as possible, including current clinical practices. Direct contact with representatives of the COST network and experts in psychiatry and psychology allowed us to examine CPG availability in low- and middle-income countries in Europe, which are unlikely to publish national CPGs. Our review did not include consensus statements or expert opinion articles, as these items only reflect individuals’ perspectives or practices. In addition, we extracted psychotropic utilization data from the literature as a proxy of prescribers’ compliance to their national CPGs. However, such a proxy is not ideal, and specific field studies are necessary to accurately measure prescribers’ adherence to the CPGs [73]. Identification of CPGs eligible for inclusion in the review was performed by a single author, but the final decision for inclusion or exclusion was agreed upon by all authors. We did not assess the quality of the included CPGs based on the AGREE instrument, and therefore, we could not assess the degree of the evidence upon which the different CPG recommendations were based. Lastly, our review was restricted to European countries, and so our results are not generalizable to countries outside Europe.
5. Conclusions
Many countries in Europe do not have a CPG for pharmacological treatment of peripartum depression, and where present, recommendations are not fully uniform and not up to date with the latest available evidence. This review expresses the urgent need for a harmonized, up-to-date CPG for pharmacological management of peripartum depression and comorbid anxiety in Europe. Treatment recommendations need to be informed by the latest available evidence and cover emerging issues that are met in the current clinical practice. Our work is only the first step in facilitating the complex decision making in pharmacological treatment of women with peripartum depression. Women across Europe should be empowered to make informed, evidence-based decisions about their treatments during pregnancy and while breastfeeding.
Author Contributions
All authors participated in the conceptualization, methodology, review, and editing; formal analysis, A.L.; writing—original draft preparation, A.L., S.K.-S., E.F., R.B., M.L.-v.d.B., C.A.W., V.B.B., K.S.V., B.M., A.F. and A.O.; data curation, A.L.; project administration, A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was supported by COST under COST Action Riseup-PPD CA18138 www.cost.eu (access date 30 September 2021). A.L. was supported by the Norwegian Research Council (grant number 288696). C.A.W. was funded by the UK’s National Institute for Health Research (NIHR). The funders had no role in the analyses, interpretation of results, or the writing of this manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available online in Medline (via PubMed).
Acknowledgments
We are grateful to all national members of Riseup-PPD COST ACTION and the researchers and clinicians that supported our search for clinical practice guidelines. This paper is based upon work from the COST Action Riseup-PPD CA 18138 and was supported by COST under COST Action Riseup-PPD CA18138 www.cost.eu (access date 30 September 2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Woody, C.A.; Ferrari, A.J.; Siskind, D.J.; Whiteford, H.A.; Harris, M.G. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J. Affect. Disord. 2017, 219, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Underwood, L.; Waldie, K.; D’Souza, S.; Peterson, E.R.; Morton, S. A review of longitudinal studies on antenatal and postnatal depression. Arch. Womens Ment. Health 2016, 19, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Falah-Hassani, K.; Shiri, R.; Dennis, C.L. The prevalence of antenatal and postnatal co-morbid anxiety and depression: A meta-analysis. Psychol. Med. 2017, 47, 2041–2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putnam, K.T.; Wilcox, M.; Robertson-Blackmore, E.; Sharkey, K.; Bergink, V.; Munk-Olsen, T.; Deligiannidis, K.M.; Payne, J.; Altemus, M.; Newport, J.; et al. Clinical phenotypes of perinatal depression and time of symptom onset: Analysis of data from an international consortium. Lancet Psychiatry 2017, 4, 477–485. [Google Scholar] [CrossRef] [Green Version]
- Stein, A.; Pearson, R.M.; Goodman, S.H.; Rapa, E.; Rahman, A.; McCallum, M.; Howard, L.M.; Pariante, C.M. Effects of perinatal mental disorders on the fetus and child. Lancet 2014, 384, 1800–1819. [Google Scholar] [CrossRef]
- Slomian, J.; Honvo, G.; Emonts, P.; Reginster, J.-Y.; Bruyère, O. Consequences of maternal postpartum depression: A systematic review of maternal and infant outcomes. Womens Health 2019, 15, 1745506519844044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigoriadis, S.; VonderPorten, E.H.; Mamisashvili, L.; Tomlinson, G.; Dennis, C.L.; Koren, G.; Steiner, M.; Mousmanis, P.; Cheung, A.; Radford, K.; et al. The impact of maternal depression during pregnancy on perinatal outcomes: A systematic review and meta-analysis. J. Clin. Psychiatry 2013, 74, e321–e341. [Google Scholar] [CrossRef]
- Gordon, H.; Nath, S.; Trevillion, K.; Moran, P.; Pawlby, S.; Newman, L.; Howard, L.M.; Molyneaux, E. Self-Harm, Self-Harm Ideation, and Mother-Infant Interactions: A Prospective Cohort Study. J. Clin. Psychiatry 2019, 80, 18m12708. [Google Scholar] [CrossRef]
- Cantwell, R.; Clutton-Brock, T.; Cooper, G.; Dawson, A.; Drife, J.; Garrod, D.; Harper, A.; Hulbert, D.; Lucas, S.; McClure, J.; et al. Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer: 2006–2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG 2011, 118 (Suppl. 1), 1–203. [Google Scholar] [CrossRef]
- Hendrick, V. Psychiatric Disorders in Pregnancy and the Postpartum: Principles and Treatment; Humana Press: Totowa, NJ, USA, 2006. [Google Scholar]
- Molenaar, N.M.; Bais, B.; Lambregtse-van den Berg, M.P.; Mulder, C.L.; Howell, E.A.; Fox, N.S.; Rommel, A.S.; Bergink, V.; Kamperman, A.M. The international prevalence of antidepressant use before, during, and after pregnancy: A systematic review and meta-analysis of timing, type of prescriptions and geographical variability. J. Affect. Disord. 2020, 264, 82–89. [Google Scholar] [CrossRef]
- Lupattelli, A.; Spigset, O.; Bjornsdottir, I.; Hameen-Anttila, K.; Mardby, A.C.; Panchaud, A.; Juraski, R.G.; Rudolf, G.; Odalovic, M.; Drozd, M.; et al. Patterns and factors associated with low adherence to psychotropic medications during pregnancy—A cross-sectional, multinational web-based study. Depress. Anxiety 2015, 32, 426–436. [Google Scholar] [CrossRef]
- Petersen, I.; Gilbert, R.E.; Evans, S.J.; Man, S.L.; Nazareth, I. Pregnancy as a major determinant for discontinuation of antidepressants: An analysis of data from The Health Improvement Network. J. Clin. Psychiatry 2011, 72, 979–985. [Google Scholar] [CrossRef]
- Molenaar, N.M.; Kamperman, A.M.; Boyce, P.; Bergink, V. Guidelines on treatment of perinatal depression with antidepressants: An international review. Aust. N. Z. J. Psychiatry 2018, 52, 320–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Damme, R.; Van Parys, A.S.; Vogels, C.; Roelens, K.; Lemmens, G.M.D. A mental health care protocol for the screening, detection and treatment of perinatal anxiety and depressive disorders in Flanders. J. Psychosom. Res. 2020, 128, 109865. [Google Scholar] [CrossRef]
- Swedish National Board of Health and Welfare. National Guidelines for Care for Depression and Anxiety Syndrome—Support for Control and Management. Available online: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/nationella-riktlinjer/2017-12-1.pdf (accessed on 30 March 2021).
- Middelboe, T.; Wøjdemann, K.; Bjergager, M.; Klindt Poulsen, B. Anvendelse af Psykofarmaka Ved Graviditet Og Amning—Kliniske Retningslinjer; Dansk Psykiatrisk Selskab; Dansk Selskab for Obstetrik og Gynækologi; Dansk Pædiatrisk Selskab og Dansk Selskab for Klinisk Farmakologi: Denmark, 27 October 2014; Available online: https://www.dpsnet.dk/wp-content/uploads/2021/02/anvendelse_af_psykofarmaka_okt_2014.pdf (accessed on 3 February 2022).
- Jimenez-Solem, E.; Andersen, J.T.; Petersen, M.; Broedbaek, K.; Andersen, N.L.; Torp-Pedersen, C.; Poulsen, H.E. Prevalence of antidepressant use during pregnancy in Denmark, a nation-wide cohort study. PLoS ONE 2013, 8, e63034. [Google Scholar] [CrossRef] [Green Version]
- Zoega, H.; Kieler, H.; Norgaard, M.; Furu, K.; Valdimarsdottir, U.; Brandt, L.; Haglund, B. Use of SSRI and SNRI Antidepressants during Pregnancy: A Population-Based Study from Denmark, Iceland, Norway and Sweden. PLoS ONE 2015, 10, e0144474. [Google Scholar] [CrossRef] [PubMed]
- Bais, B.; Munk-Olsen, T.; Bergink, V.; Liu, X. Prescription patterns of benzodiazepine and benzodiazepine-related drugs in the peripartum period: A population-based study. Psychiatry Res. 2020, 288, 112993. [Google Scholar] [CrossRef] [PubMed]
- Reutfors, J.; Cesta, C.E.; Cohen, J.M.; Bateman, B.T.; Brauer, R.; Einarsdottir, K.; Engeland, A.; Furu, K.; Gissler, M.; Havard, A.; et al. Antipsychotic drug use in pregnancy: A multinational study from ten countries. Schizophr. Res. 2020, 220, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Current Clinical Care—Depression. Finnish Medical Association’s Duodecim and the Finnish Psychiatric Association. Available online: https://www.kaypahoito.fi/hoi50023#s13 (accessed on 30 June 2021).
- Gissler, M.; Artama, M.; Ritvanen, A.; Wahlbeck, K. Use of psychotropic drugs before pregnancy and the risk for induced abortion: Population-based register-data from Finland 1996–2006. BMC Public Health 2010, 10, 383. [Google Scholar] [CrossRef]
- Lupattelli, A.; Spigset, O.; Twigg, M.J.; Zagorodnikova, K.; Mardby, A.C.; Moretti, M.E.; Drozd, M.; Panchaud, A.; Hameen-Anttila, K.; Rieutord, A.; et al. Medication use in pregnancy: A cross-sectional, multinational web-based study. BMJ Open 2014, 4, e004365. [Google Scholar] [CrossRef] [Green Version]
- Bais, B.; Molenaar, N.M.; Bijma, H.H.; Hoogendijk, W.J.G.; Mulder, C.L.; Luik, A.I.; Lambregtse-van den Berg, M.P.; Kamperman, A.M. Prevalence of benzodiazepines and benzodiazepine-related drugs exposure before, during and after pregnancy: A systematic review and meta-analysis. J. Affect. Disord. 2020, 269, 18–27. [Google Scholar] [CrossRef] [PubMed]
- German S3 Guideline/National Health Care Guideline. Unipolar Depression; ÄZQ—Redaktion Nationale VersorgungsLeitlinien: Berlin, Germany, Peripartum Depression 2017; Chapter 3.9.1; pp. 151–159.
- Lewer, D.; O’Reilly, C.; Mojtabai, R.; Evans-Lacko, S. Antidepressant use in 27 European countries: Associations with sociodemographic, cultural and economic factors. Br. J. Psychiatry 2015, 207, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Anniverno, R.; Bramante, A.; Petrilli, G.; Mencacci, C. Prevenzione, Diagnosi E Trattamento Della Psicopatologia Perinatale: Linee Guida Per Professionisti Della Salute; Osservatorio Nazionale sulla Salute della Donna: Milano, Italy, 2010. [Google Scholar]
- Charlton, R.A.; Jordan, S.; Pierini, A.; Garne, E.; Neville, A.J.; Hansen, A.V.; Gini, R.; Thayer, D.; Tingay, K.; Puccini, A.; et al. Selective serotonin reuptake inhibitor prescribing before, during and after pregnancy: A population-based study in six European regions. BJOG 2015, 122, 1010–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupattelli, A.; Picinardi, M.; Cantarutti, A.; Nordeng, H. Use and Intentional Avoidance of Prescribed Medications in Pregnancy: A Cross-Sectional, Web-Based Study among 926 Women in Italy. Int. J. Environ. Res. Public Health 2020, 17, 3830. [Google Scholar] [CrossRef] [PubMed]
- Barbui, C.; Conti, V.; Purgato, M.; Cipriani, A.; Fortino, I.; Rivolta, A.L.; Lora, A. Use of antipsychotic drugs and mood stabilizers in women of childbearing age with schizophrenia and bipolar disorder: Epidemiological survey. Epidemiol. Psychiatr. Sci. 2013, 22, 355–361. [Google Scholar] [CrossRef]
- Agius, R.; Felice, E.; Buhagia, R. Women with Mental Health Problems—During Pregnancy, Birth and the Postnatal Period. Malta. unpublished.
- Federatie Medisch Specialisten. SSRI-Gebruik en Zwangerschap. Available online: https://richtlijnendatabase.nl/richtlijn/ssri_en_zwangerschap/ssri-gebruik_en_zwangerschap_-_startpagina.html (accessed on 23 June 2021).
- Molenaar, N.M.; Lambregtse-van den Berg, M.P.; Bonsel, G.J. Dispensing patterns of selective serotonin reuptake inhibitors before, during and after pregnancy: A 16-year population-based cohort study from the Netherlands. Arch. Womens Ment. Health 2020, 23, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Radojcic, M.R.; El Marroun, H.; Miljkovic, B.; Stricker, B.H.C.; Jaddoe, V.W.V.; Verhulst, F.C.; White, T.; Tiemeier, H. Prenatal exposure to anxiolytic and hypnotic medication in relation to behavioral problems in childhood: A population-based cohort study. Neurotoxicol. Teratol. 2017, 61, 58–65. [Google Scholar] [CrossRef]
- Thorbjørn, B.S.; Eberhard-Gran, M.; Nordeng, H.; Nerum, H.; Lyng, S. Mental Helse i Svangerskapet. Veileder i Fødselshjelp 2020. Available online: https://www.legeforeningen.no/foreningsledd/fagmed/norsk-gynekologisk-forening/veiledere/veileder-i-fodselshjelp/ (accessed on 26 October 2020).
- Engeland, A.; Bjorge, T.; Klungsoyr, K.; Hjellvik, V.; Skurtveit, S.; Furu, K. Trends in prescription drug use during pregnancy and postpartum in Norway, 2005 to 2015. Pharmacoepidemiol. Drug Saf. 2018, 27, 995–1004. [Google Scholar] [CrossRef]
- Riska, B.S.; Skurtveit, S.; Furu, K.; Engeland, A.; Handal, M. Dispensing of benzodiazepines and benzodiazepine-related drugs to pregnant women: A population-based cohort study. Eur. J. Clin. Pharmacol. 2014, 70, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Samochowiec, J.; Rybakowski, J.; Galecki, P.; Szulc, A.; Rymaszewska, J.; Cubala, W.J.; Dudek, D. Recommendations of the Polish Psychiatric Association for treatment of affective disorders in women of childbearing age. Part I: Treatment of depression. Psychiatr. Pol. 2019, 53, 245–262. [Google Scholar] [CrossRef]
- Milasinovic, G.; Vukicevic, D. Nacionalni Vodic Dobre Klinicke Orakse Za Djagnostikovanje i Lecenje Depresije; Ministarstvo zdravlja Republike Srbije: Belgrade, Serbia, 2011. [Google Scholar]
- García-Herrera, P.B.J.M.; Nogueras Morillas, E.V.; Muñoz Cobos, F.; Morales Asencio, J.M. Guía de Práctica Clínica Para el Tratamiento de la Depresión en Atención Primaria; Distrito Sanitario Málaga-UGC Salud Mental Hospital Regional Universitario “Carlos Haya”: Málaga, Spain, 2011. [Google Scholar]
- Lendoiro, E.; Gonzalez-Colmenero, E.; Concheiro-Guisan, A.; de Castro, A.; Cruz, A.; Lopez-Rivadulla, M.; Concheiro, M. Maternal hair analysis for the detection of illicit drugs, medicines, and alcohol exposure during pregnancy. Ther. Drug Monit. 2013, 35, 296–304. [Google Scholar] [CrossRef] [PubMed]
- De Las Cuevas, C.; de la Rosa, M.A.; Troyano, J.M.; Sanz, E.J. Are psychotropics drugs used in pregnancy? Pharmacoepidemiol. Drug Saf. 2007, 16, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- NICE—National Institute for Health and Care Excellence. Antenatal and Postnatal Mental Health: Clinical Management and Service Guidance. 11 February 2020. Available online: https://www.nice.org.uk/guidance/cg192 (accessed on 31 August 2021).
- McAllister-Williams, R.H.; Baldwin, D.S.; Cantwell, R.; Easter, A.; Gilvarry, E.; Glover, V.; Green, L.; Gregoire, A.; Howard, L.M.; Jones, I.; et al. British Association for Psychopharmacology consensus guidance on the use of psychotropic medication preconception, in pregnancy and postpartum 2017. J. Psychopharmacol. 2017, 31, 519–552. [Google Scholar] [CrossRef] [PubMed]
- Margulis, A.V.; Kang, E.M.; Hammad, T.A. Patterns of prescription of antidepressants and antipsychotics across and within pregnancies in a population-based UK cohort. Matern. Child Health J. 2014, 18, 1742–1752. [Google Scholar] [CrossRef] [PubMed]
- Raitasalo, K.; Holmila, M.; Autti-Ramo, I.; Martikainen, J.E.; Sorvala, V.M.; Makela, P. Benzodiazepine use among mothers of small children: A register-based cohort study. Addiction 2015, 110, 636–643. [Google Scholar] [CrossRef]
- Slimību Profilakses un Kontroles Centrs. Klīniskie Algoritmi un Pacientu Ceļi. Available online: https://www.spkc.gov.lv/lv/kliniskie-algoritmi-un-pacientu-celi (accessed on 23 June 2021).
- Petersen, I.; Peltola, T.; Kaski, S.; Walters, K.R.; Hardoon, S. Depression, depressive symptoms and treatments in women who have recently given birth: UK cohort study. BMJ Open 2018, 8, e022152. [Google Scholar] [CrossRef] [Green Version]
- Lupattelli, A.; Twigg, M.J.; Zagorodnikova, K.; Moretti, M.E.; Drozd, M.; Panchaud, A.; Rieutord, A.; Juraski, R.G.; Odalovic, M.; Kennedy, D.; et al. Self-reported perinatal depressive symptoms and postnatal symptom severity after treatment with antidepressants in pregnancy: A cross-sectional study across 12 European countries using the Edinburgh Postnatal Depression Scale. Clin. Epidemiol. 2018, 10, 655–669. [Google Scholar] [CrossRef] [Green Version]
- Wachs, T.D.; Black, M.M.; Engle, P.L. Maternal Depression: A Global Threat to Children’s Health, Development, and Behavior and to Human Rights. Child Dev. Perspect. 2009, 3, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Molyneaux, E.; Telesia, L.A.; Henshaw, C.; Boath, E.; Bradley, E.; Howard, L.M. Antidepressants for preventing postnatal depression. Cochrane Database Syst. Rev. 2018, 4, CD004363. [Google Scholar] [CrossRef] [Green Version]
- Bayrampour, H.; Kapoor, A.; Bunka, M.; Ryan, D. The Risk of Relapse of Depression During Pregnancy After Discontinuation of Antidepressants: A Systematic Review and Meta-Analysis. J. Clin. Psychiatry 2020, 81, 19r13134. [Google Scholar] [CrossRef]
- Wilson, C.A.; Robertson, L.; Brown, J.V.; Ayre, K.; Khalifeh, H. Brexanolone and related neurosteroid GABA(A) positive allosteric modulators for postnatal depression. Cochrane Database Syst. Rev. 2021, 5, CD014624. [Google Scholar] [CrossRef]
- Swanson, S.A.; Hernandez-Diaz, S.; Palmsten, K.; Mogun, H.; Olfson, M.; Huybrechts, K.F. Methodological considerations in assessing the effectiveness of antidepressant medication continuation during pregnancy using administrative data. Pharmacoepidemiol. Drug Saf. 2015, 24, 934–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yonkers, K.A.; Gotman, N.; Smith, M.V.; Forray, A.; Belanger, K.; Brunetto, W.L.; Lin, H.; Burkman, R.T.; Zelop, C.M.; Lockwood, C.J. Does antidepressant use attenuate the risk of a major depressive episode in pregnancy? Epidemiology 2011, 22, 848–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, L.S.; Altshuler, L.L.; Harlow, B.L.; Nonacs, R.; Newport, D.J.; Viguera, A.C.; Suri, R.; Burt, V.K.; Hendrick, V.; Reminick, A.M.; et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006, 295, 499–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigoriadis, S.; VonderPorten, E.H.; Mamisashvili, L.; Roerecke, M.; Rehm, J.; Dennis, C.L.; Koren, G.; Steiner, M.; Mousmanis, P.; Cheung, A.; et al. Antidepressant exposure during pregnancy and congenital malformations: Is there an association? A systematic review and meta-analysis of the best evidence. J. Clin. Psychiatry 2013, 74, e293–e308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grigoriadis, S.; VonderPorten, E.H.; Mamisashvili, L.; Eady, A.; Tomlinson, G.; Dennis, C.L.; Koren, G.; Steiner, M.; Mousmanis, P.; Cheung, A.; et al. The effect of prenatal antidepressant exposure on neonatal adaptation: A systematic review and meta-analysis. J. Clin. Psychiatry 2013, 74, e309–e320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spigset, O.; Nordeng, H. Safety of Psychotropic Drugs in Pregnancy and Breastfeeding. In Pharmacovigilance in Psychiatry; Spina, E., Trifirò, G., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 299–319. [Google Scholar] [CrossRef]
- Rommel, A.S.; Bergink, V.; Liu, X.; Munk-Olsen, T.; Molenaar, N.M. Long-Term Effects of Intrauterine Exposure to Antidepressants on Physical, Neurodevelopmental, and Psychiatric Outcomes: A Systematic Review. J. Clin. Psychiatry 2020, 81. [Google Scholar] [CrossRef]
- Park, M.; Hanley, G.E.; Guhn, M.; Oberlander, T.F. Prenatal antidepressant exposure and child development at kindergarten age: A population-based study. Pediatr. Res. 2021, 89, 1515–1522. [Google Scholar] [CrossRef]
- Rommel, A.S.; Momen, N.C.; Molenaar, N.M.; Liu, X.; Munk-Olsen, T.; Bergink, V. Long-term prenatal effects of antidepressant use on the risk of affective disorders in the offspring: A register-based cohort study. Neuropsychopharmacology 2021, 46, 1518–1525. [Google Scholar] [CrossRef]
- Wartko, P.D.; Weiss, N.S.; Enquobahrie, D.A.; Chan, K.C.G.; Stephenson-Famy, A.; Mueller, B.A.; Dublin, S. Association of Antidepressant Continuation in Pregnancy and Infant Birth Weight. J. Clin. Psychopharmacol. 2021, 41, 403–413. [Google Scholar] [CrossRef]
- Wolgast, E.; Lilliecreutz, C.; Sydsjö, G.; Bladh, M.; Josefsson, A. The impact of major depressive disorder and antidepressant medication before and during pregnancy on obstetric and neonatal outcomes: A nationwide population-based study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 257, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.E.; Lupattelli, A.; Palmsten, K.; Bandoli, G.; Hurault-Delarue, C.; Damase-Michel, C.; Chambers, C.D.; Nordeng, H.M.E.; van Gelder, M. Longitudinal Methods for Modeling Exposures in Pharmacoepidemiologic Studies in Pregnancy. Epidemiol. Rev. 2021, 43, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Lupattelli, A.; Mahic, M.; Handal, M.; Ystrom, E.; Reichborn-Kjennerud, T.; Nordeng, H. Attention-deficit/hyperactivity disorder in children following prenatal exposure to antidepressants: Results from the Norwegian mother, father and child cohort study. BJOG 2021, 128, 1917–1927. [Google Scholar] [CrossRef]
- Trinh, N.T.; Nordeng, H.M.; Bandoli, G.; Eberhard-Gran, M.; Lupattelli, A. Antidepressant and mental health care utilization in pregnant women with depression and/or anxiety: An interrupted time-series analysis. medRxiv 2021. [Google Scholar] [CrossRef]
- Scaffidi, J.; Mol, B.W.; Keelan, J.A. The pregnant women as a drug orphan: A global survey of registered clinical trials of pharmacological interventions in pregnancy. BJOG 2017, 124, 132–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molenaar, N.M.; Brouwer, M.E.; Burger, H.; Kamperman, A.M.; Bergink, V.; Hoogendijk, W.J.G.; Williams, A.D.; Bockting, C.L.H.; Lambregtse-van den Berg, M.P. Preventive Cognitive Therapy With Antidepressant Discontinuation During Pregnancy: Results From a Randomized Controlled Trial. J. Clin. Psychiatry 2020, 81, 19l13099. [Google Scholar] [CrossRef]
- Khalifeh, H.; Molyneaux, E.; Brauer, R.; Vigod, S.; Howard, L.M. Patient decision aids for antidepressant use in pregnancy: A pilot randomised controlled trial in the UK. BJGP Open 2019, 3, bjgpopen19X101666. [Google Scholar] [CrossRef] [Green Version]
- Vigod, S.N.; Hussain-Shamsy, N.; Stewart, D.E.; Grigoriadis, S.; Metcalfe, K.; Oberlander, T.F.; Schram, C.; Taylor, V.H.; Dennis, C.L. A patient decision aid for antidepressant use in pregnancy: Pilot randomized controlled trial. J. Affect. Disord. 2019, 251, 91–99. [Google Scholar] [CrossRef]
- Molenaar, N.M.; Brouwer, M.E.; Duvekot, J.J.; Burger, H.; Knijff, E.M.; Hoogendijk, W.J.; Bockting, C.L.H.; de Wolf, G.S.; Lambregtse-van den Berg, M.P. Antidepressants during pregnancy: Guideline adherence and current practice amongst Dutch gynaecologists and midwives. Midwifery 2018, 61, 29–35. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).