Reduced n-3 and n-6 PUFA (DHA and AA) Concentrations in Breast Milk and Erythrocytes Phospholipids during Pregnancy and Lactation in Women with Obesity
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design and Participants
2.2. Clinical and Nutritional Evaluation
2.3. Dietary Intake
2.4. Blood and Breast Milk Samples
2.5. Lipids Extraction from Erythrocytes and Breast Milk
2.6. Gas Chromatography Analysis of Fatty Acid Methyl Esters
2.7. Statistical Analysis
3. Results
3.1. Background Characteristics
3.2. Food Groups Consumption
3.3. Energy, Nutrients, and FA Dietary Intake
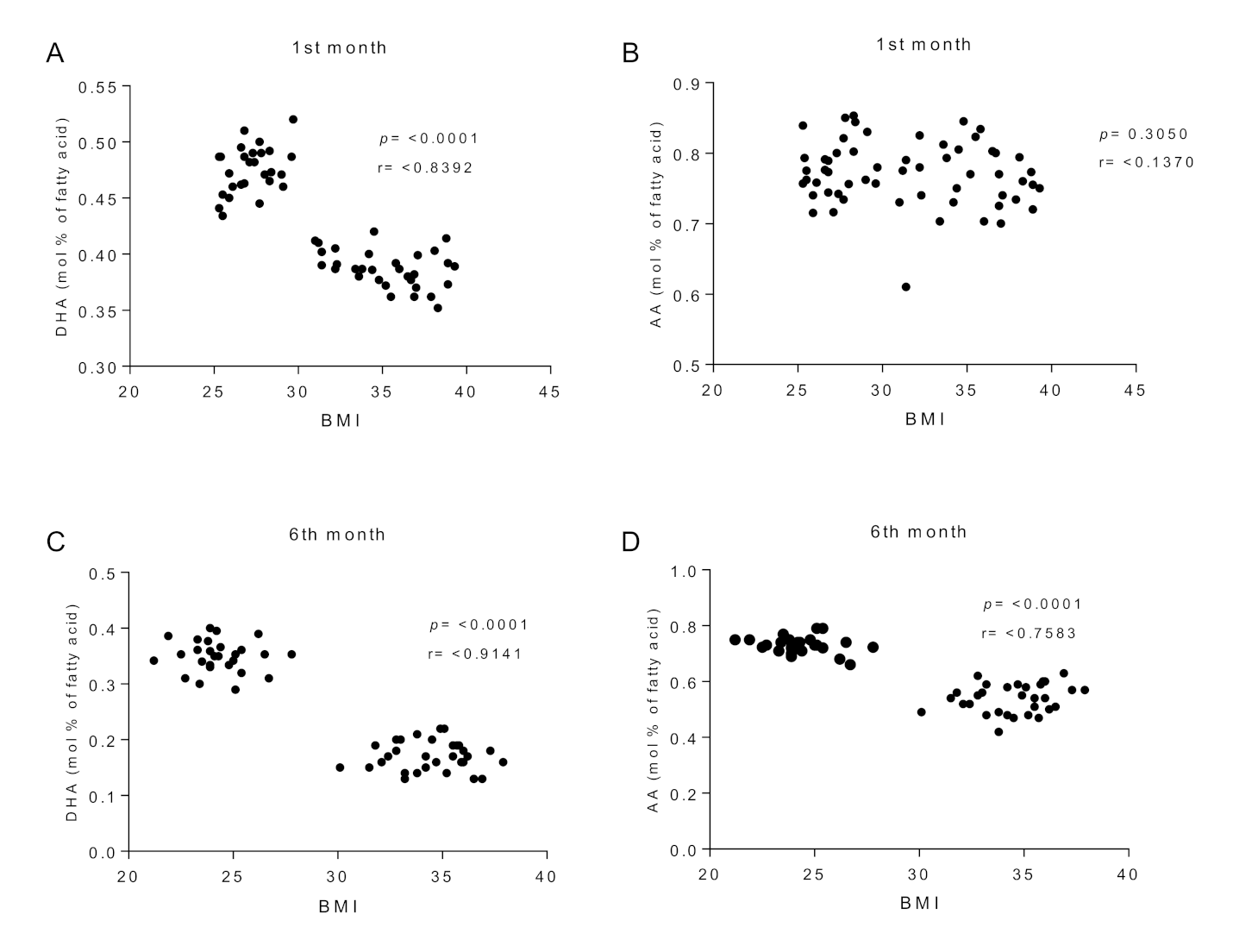
3.4. Fatty Acid Composition of Erythrocyte Phospholipids
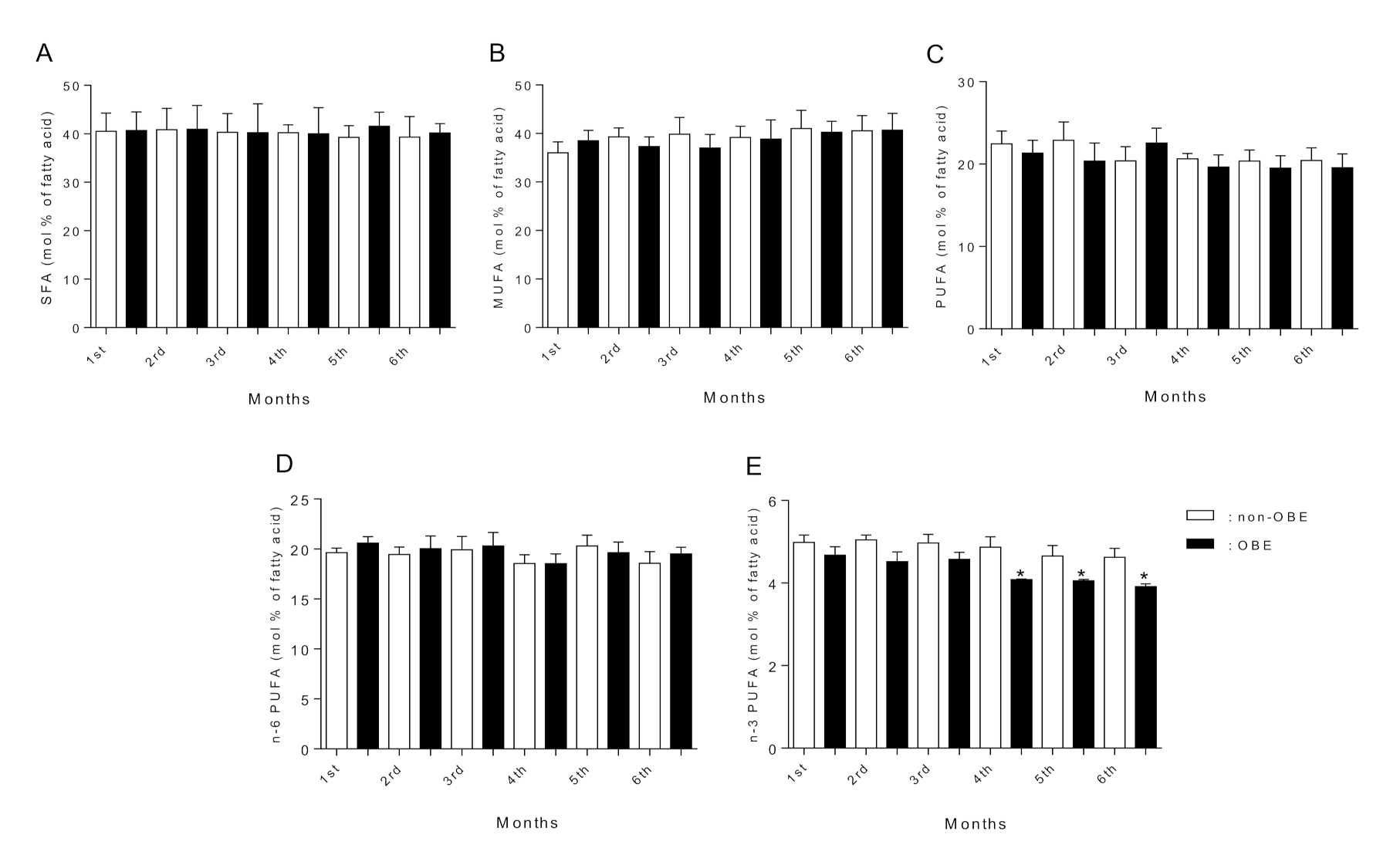
3.5. Fatty Acid Composition of Breast Milk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harding, J.E. The nutritional basis of the fetal origins of adult disease. Int. J. Epidemiol. 2001, 30, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodd, J.M.; Deussen, A.R.; Louise, J. Optimising gestational weight gain and improving maternal and infant health outcomes through antenatal dietary, lifestyle and physical activity advice: The OPTIMISE randomised controlled trial protocol. BMJ Open 2018, 8, e019583. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Modi, N.; Uthaya, S. Impact of breast milk intake on body composition at term in very preterm babies: Secondary analysis of the Nutritional Evaluation and Optimisation in Neonates randomised controlled trial. Arch. Dis. Child Fetal Neonatal Ed. 2019, 104, F306–F312. [Google Scholar] [CrossRef] [PubMed]
- Rees, W.D. Interactions between nutrients in the maternal diet and the implications for the long-term health of the offspring. Proc. Nutr. Soc. 2019, 78, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Ehrenberg, H.M. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG 2006, 113, 1126–1133. [Google Scholar] [CrossRef]
- Catalano, P.M.; Shankar, K. Obesity and pregnancy: Mechanisms of short term and long- term adverse consequences for mother and child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 15 September 2021).
- Chen, C.; Xu, X.; Yan, Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PLoS ONE 2018, 13, e0202183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kominiarek, M.A.; Peaceman, A.M. Gestational weight gain. Am. J. Obstet. Gynecol. 2017, 217, 642–651. [Google Scholar] [CrossRef]
- Heslehurst, N.; Rankin, J.; Wilkinson, J.R.; Summerbell, C.D. A nationally representative study of maternal obesity in England, UK: Trends in incidence and demographic inequalities in 619,323 births, 1989–2007. Int. J. Obes. 2010, 34, 420–428. [Google Scholar] [CrossRef] [Green Version]
- Catalano, P.M.; Farrell, K.; Thomas, A.; Huston-Presley, L.; Mencin, P.; de Mouzon, S.H.; Amini, S.B. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am. J. Clin. Nutr. 2009, 90, 1303–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahti-Pulkkinen, M.; Bhattacharya, S.; Wild, S.H.; Lindsay, R.S.; Räikkönen, K.; Norman, J.E.; Bhattacharya, S.; Reynolds, R.M. Consequences of being overweight or obese during pregnancy on diabetes in the offspring: A record linkage study in Aberdeen, Scotland. Diabetologia 2019, 62, 1412–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of gestational weight gain with maternal and Infant outcomes: A systematic review and meta-analysis. JAMA 2017, 317, 2207–2225. [Google Scholar] [CrossRef]
- Dude, A.M.; Grobman, W.; Haas, D.; Mercer, B.M.; Parry, S.; Silver, R.M.; Wapner, R.; Wing, D.; Saade, G.; Reddy, U.; et al. Gestational weight gain and pregnancy outcomes among nulliparous women. Am. J. Perinatol. 2021, 38, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Carroll, M.D.; Kit, B.K.; Ogden, C.L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012, 307, 491–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, L.; Wertz, M.; McDowell, I. Obesity in Pregnancy: Risks and Management. Am. Fam. Physician 2018, 97, 559–561. [Google Scholar]
- Catalano, P.M.; Presley, L.; Minium, J.; Hauguel-de Mouzon, S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 2009, 32, 1076–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paredes, C.; Hsu, R.C.; Tong, A.; Johnson, J.R. Obesity and Pregnancy. Neoreviews 2021, 22, e78–e87. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Neal-Perry, G. Long-term consequences of obesity on female fertility and the health of the offspring. Curr. Opin. Obstet. Gynecol. 2017, 29, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M. Obesity, insulin resistance, and pregnancy outcome. Reproduction 2010, 140, 365–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Y.; Liu, X.; Zhou, B.; Jiang, A.C.; Chai, L. An updated review of worldwide levels of docosahexaenoic and arachidonic acid in human breast milk by region. Public Health Nutr. 2016, 19, 2675–2687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, U.N. Influence of polyunsaturated fatty acids and their metabolites on stem cell biology. Nutrition 2011, 27, 21–22. [Google Scholar] [CrossRef] [PubMed]
- Hadley, K.B.; Ryan, A.S.; Forsyth, S.; Gautier, S.; Salem, N., Jr. The Essentiality of Arachidonic Acid in Infant Development. Nutrients 2016, 8, 216. [Google Scholar] [CrossRef] [Green Version]
- Echeverría, F.; Valenzuela, R.; Hernandez-Rodas, M.C.; Valenzuela, A. Docosahexaenoic acid (DHA), a fundamental fatty acid for the brain: New dietary sources. Prostaglandins Leukot. Essent. Fatty Acids. 2017, 124, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sambra, V.; Echeverria, F.; Valenzuela, A.; Chouinard-Watkins, R.; Valenzuela, R. Docosahexaenoic and Arachidonic Acids as Neuroprotective Nutrients throughout the Life Cycle. Nutrients 2021, 13, 986. [Google Scholar] [CrossRef]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of energy metabolism by long-chain fatty acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef] [PubMed]
- Demmelmair, H.; Koletzko, B. Importance of fatty acids in the perinatal period. World Rev. Nutr. Diet. 2015, 112, 31–47. [Google Scholar] [PubMed]
- Araya, J.; Rodrigo, R.; Pettinelli, P.; Araya, A.V.; Poniachik, J.; Videla, L.A. Decreased liver fatty acid delta-6 and delta-5 desaturase activity in obese patients. Obesity 2010, 18, 1460–1463. [Google Scholar] [CrossRef] [PubMed]
- Elizondo, A.; Araya, J.; Rodrigo, R.; Signorini, C.; Sgherri, C.; Comporti, M.; Poniachik, J.; Videla, L.A. Effects of weight loss on liver and erythrocyte polyunsaturated fatty acid pattern and oxidative stress status in obese patients with non-alcoholic fatty liver disease. Biol. Res. 2008, 41, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Gázquez, A.; Prieto-Sánchez, M.T.; Blanco-Carnero, J.E.; Ruíz-Palacios, M.; Nieto, A.; van Harskamp, D.; Oosterink, J.E.; Schierbeek, H.; van Goudoever, J.B.; Demmelmair, H.; et al. Altered materno-fetal transfer of 13C-polyunsaturated fatty acids in obese pregnant women. Clin. Nutr. 2019, 39, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Barrera, C.; Valenzuela, R.; Chamorro, R.; Bascuñán, K.; Sandoval, J.; Sabag, N.; Valenzuela, F.; Valencia, M.P.; Puigrredon, C.; Valenzuela, A. The Impact of Maternal Diet during Pregnancy and Lactation on the Fatty Acid Composition of Erythrocytes and Breast Milk of Chilean Women. Nutrients 2018, 10, 839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atalah, E.; Castillo, C.; Castro, R.; Aldea, A. Proposal of a new standard for the nutritional assessment of pregnant women. Rev. Med. Chil. 1997, 125, 1429–1436. [Google Scholar] [PubMed]
- WHO/FAO/UNU. Human Energy Requirements, Report of a Joint FAO/WHO/UNU Expert Consultation; WHO: Rome, Italy, 2014. [Google Scholar]
- Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes: Guiding Principles for Nutrition Labeling and Fortification; Institute of Medicine of the National Academies: Washington, DC, USA, 2001; 224p. [Google Scholar]
- Ministery of Health (MINSAL). Perinatal Guide, 1st ed.; MINSAL: Santiago, Chile, 2015; ISBN 978-956-348-076-4. [Google Scholar]
- Cerda, R.; Barrera, C.; Arena, M.; Bascuñán, K.A.; Jimenez, G. Photographic Atlas of Chilean Food and Typical Preparations. In National Survey of Food Consumption, 1st ed.; Government of Chile, Ministry of Health: Santiago, Chile, 2010. [Google Scholar]
- Bascuñán, K.A.; Valenzuela, R.; Chamorro, R.; Valencia, A.; Barrera, C.; Puigrredon, C.; Sandoval, J.; Valenzuela, A. Polyunsaturated fatty acid composition of maternal diet and erythrocyte phospholipid status in Chilean pregnant women. Nutrients 2014, 6, 4918–4934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gutierrez, V.; Cert, A.; Rios, J.J. Determination of phospholipid fatty acid and triacylglycerol composition of rat caecal mucosa. J. Chromatogr. 1992, 575, 1–6. [Google Scholar] [CrossRef]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride—Methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Valenzuela, R.; Bascuñán, K.; Chamorro, R.; Barrera, C.; Sandoval, J.; Puigrredon, C.; Parraguez, G.; Orellana, P.; Gonzalez, V.; Valenzuela, A. Modification of docosahexaenoic acid composition of milk from nursing women who received alpha linolenic acid from chia oil during gestation and nursing. Nutrients 2015, 7, 6405–6424. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.W.; Brown, R.; Nitzke, S. Fast Food Intake in Relation to Employment Status, Stress, Depression, and Dietary Behaviors in Low-Income Overweight and Obese Pregnant Women. Matern. Child Health J. 2016, 20, 1506–1517. [Google Scholar] [CrossRef]
- Garmendia, M.L.; Alonso, F.T.; Kain, J.; Uauy, R.; Corvalan, C. Alarming weight gain in women of a post-transitional country. Public Health Nutr. 2014, 17, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Araya, M.B.; Padilla, O.; Garmendia, M.L.; Atalah, E.; Uauy, R. Prevalence of obesity among chilean women in childbearing ages. Rev. Med. Chil. 2014, 142, 1440–1448. [Google Scholar]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.; Eriksson, J.G.; Broekman, B.F. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Andres, A.; Hull, H.R.; Shankar, K.; Casey, P.H.; Cleves, M.A.; Badger, T.M. Longitudinal body composition of children born to mothers with normal weight, overweight, and obesity. Obesity 2015, 23, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Puentes, A.G.; Alemany, A.M.; Chisaguano, A.M.; Montes Goyanes, R.; Castellote, A.I.; Torres-Espínola, F.J.; García-Valdés, L.; Escudero-Marín, M.; Segura, M.T.; Campoy, C.; et al. The effect of maternal obesity on breast milk fatty acids and its association with infant growth and cognition-The PREOBE Follow-Up. Nutrients 2019, 11, 2154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez, D.; Muñoz, Y.; Ortiz, M.; Maliqueo, M.; Chouinard-Watkins, R.; Valenzuela, R. Impact of maternal obesity on the metabolism and bioavailability of polyunsaturated fatty acids during pregnancy and breastfeeding. Nutrients 2020, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Videla, L.A. The importance of the long-chain polyunsaturated fatty acid n-6/n-3 ratio in development of non-alcoholic fatty liver associated with obesity. Food Funct. 2011, 2, 644–648. [Google Scholar] [CrossRef]
- Gonzalez-Soto, M.; Mutch, D.M. Diet Regulation of Long-Chain PUFA Synthesis: Role of Macronutrients, Micronutrients, and Polyphenols on delta-5/delta-6 Desaturases and Elongases 2/5. Adv. Nutr. 2021, 12, 980–994. [Google Scholar] [CrossRef]
- Gura, T. Nature’s first functional food. Science 2014, 345, 747–749. [Google Scholar] [CrossRef]
- Walker, A. Breast milk as the gold standard for protective nutrients. J. Pediatr. 2010, 156, S3–S7. [Google Scholar] [CrossRef]
- Sheila, M.I. Human milk: Maternal dietary lipids and infant development. Proc. Nutr. Soc. 2007, 66, 397–404. [Google Scholar]
- Koletzko, B.; Thiel, I.; Abiodun, P.O. The fatty acid composition of human milk in Europe and Africa. J. Pediatr. 1992, 120, S62–S70. [Google Scholar] [CrossRef]
- Antonakou, A.; Skenderi, K.P.; Chiou, A.; Anastasiou, C.A.; Bakoula, C.; Matalas, A.L. Breast milk fat concentration and fatty acid pattern during the first six months in exclusively breastfeeding Greek women. Eur. J. Nutr. 2013, 52, 963–973. [Google Scholar] [CrossRef]
- Campoy, C.; Chisaguano Tonato, A.M.; de la Garza Puentes, A.; Sáenz de Pipaón, M.; Verduci, E.; Koletzko, B.; González Casanova, I.; Larqué, E.; Valenzuela, R.; Moreno Villares, J.M.; et al. Controversy about the critical role of long-chain polyunsaturated fatty acids, arachidonic acid (ARA) and docosahexaenoic acid (DHA), during infancy. Nutr. Hosp. 2021, 38, 1101–1112. [Google Scholar] [PubMed]
- Spector, A.A.; Kim, H.Y. Discovery of essential fatty acids. J. Lipid Res. 2015, 56, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rincón-Cervera, M.Á.; Valenzuela, R.; Hernandez-Rodas, M.C.; Barrera, C.; Espinosa, A.; Marambio, M.; Valenzuela, A. Vegetable oils rich in alpha linolenic acid increment hepatic n-3 LCPUFA, modulating the fatty acid metabolism and antioxidant response in rats. Prostaglandins Leukot. Essent. Fatty Acids 2016, 111, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Essential fatty acids: Biochemistry, physiology and pathology. Biotechnol. J. 2006, 1, 420–439. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.; Rincón-Cervera, M.Á.; Echeverría, F.; Barrera, C.; Espinosa, A.; Hernández-Rodas, M.C.; Ortiz, M.; Valenzuela, A.; Videla, L.A. Iron-induced pro-oxidant and pro-lipogenic responses in relation to impaired synthesis and accretion of long-chain polyunsaturated fatty acids in rat hepatic and extrahepatic tissues. Nutrition 2018, 45, 49–58. [Google Scholar] [CrossRef]
- Brei, C.; Stecher, L.; Much, D.; Karla, M.T.; Amann-Gassner, U.; Shen, J.; Ganter, C.; Karampinos, D.C.; Brunner, S.; Hauner, H. Reduction of the n-6:n-3 long-chain PUFA ratio during pregnancy and lactation on offspring body composition: Follow-up results from a randomized controlled trial up to 5 y of age. Am. J. Clin. Nutr. 2016, 103, 1472–1481. [Google Scholar] [CrossRef]
- Hauner, H.; Much, D.; Vollhardt, C.; Brunner, S.; Schmid, D.; Sedlmeier, E.M.; Heimberg, E.; Schuster, T.; Zimmermann, A.; Schneider, K.T.M.; et al. Effect of reducing the n-6:n-3 long-chain PUFA ratio during pregnancy and lactation on infant adipose tissue growth within the first year of life: An open-label randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Rahmawaty, S.; Lyons-Wall, P.; Charlton, K.; Batterham, M.; Meyer, B.J. Effect of replacing bread, egg, milk, and yogurt with equivalent ω-3 enriched foods on ω-3 LCPUFA intake of Australian children. Nutrition 2014, 30, 1337–1343. [Google Scholar] [CrossRef] [Green Version]
- Miles, E.A.; Noakes, P.S.; Kremmyda, L.S.; Vlachava, M.; Diaper, N.D.; Rosenlund, G.; Urwin, H.; Yaqoob, P.; Rossary, A.; Farges, M.C.; et al. The salmon in pregnancy study: Study design, subject characteristics, maternal fish and marine n-3 fatty acid intake, and marine n-3 fatty acid status in maternal and umbilical cord blood. Am. J. Clin. Nutr. 2011, 94, 1986S–1992S. [Google Scholar] [CrossRef] [Green Version]
- Sherry, C.L.; Oliver, J.S.; Marriage, B.J. Docosahexaenoic acid supplementation in lactating women increases breast milk and plasma docosahexaenoic acid concentrations and alters infant omega 6:3 fatty acid ratio. Prostaglandins Leukot. Essent. Fatty Acids 2015, 95, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smit, E.N.; Koopmann, M.; Boersma, E.R.; Muskiet, F.A. Effect of supplementation of arachidonic acid (AA) or a combination of AA plus docosahexaenoic acid on breastmilk fatty acid composition. Prostaglandins Leukot. Essent. Fatty Acids 2000, 62, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Monthé-Drèze, C.; Penfield-Cyr, A.; Smid, M.C.; Sen, S. Maternal pre-pregnancy obesity attenuates response to omega-3 fatty acids supplementation during pregnancy. Nutrients 2018, 10, 1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archer, E.; Marlow, M.L.; Lavie, C.J. Controversy and debate: Memory-Based Methods Paper 1: The fatal flaws of food frequency questionnaires and other memory-based dietary assessment methods. J. Clin. Epidemiol. 2018, 104, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Primorac, L.; Mandić, M.L.; Klapec, T.; Folivarski, K.; Perl, A.; Sudar, A. Adequacy of a food composition database to estimate fat and fatty acid intake. Int. J. Food Sci. Nutr. 2000, 51, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Pennington, J.A.T. Applications of food composition data: Data sources and considerations for use. J. Food Comp. Anal. 2008, 21, S3–S12. [Google Scholar] [CrossRef]

| Non-OBE (n = 31) | OBE (n = 30) | p-Value | |
|---|---|---|---|
| Age (years) | 28.5 ± 4.5 | 27.1 ± 5.2 | 0.784 |
| Preconception weight (kg) | 54.7 ± 4.7 | 76.3 ± 6.6 | 0.031 * |
| Height (m) | 1.56 ± 0.15 | 1.62 ± 0.17 | 0.684 |
| Preconception BMI (kg/m2) | 22.6 ± 2.2 | 29.1 ± 2.9 | 0.039 * |
| At enrollment | |||
| Weight (kg) | 62.1± 5.5 | 84.9 ± 7.4 | 0.042 * |
| BMI (kg/m2) | 25.5 ± 2.4 | 32.5 ± 2.8 | 0.031 * |
| Gestational age (weeks) | 24.5 ± 1.9 | 24.9 ± 2.2 | 0.814 |
| After delivery | |||
| Weight (kg) | 66.8 ± 6.9 | 89.8 ± 7.2 | 0.035 * |
| BMI (kg/m2) | 27.8 ± 1.9 | 34.2 ± 2.8 | 0.043 * |
| Gestational age (weeks) | 39.1 ± 2.2 | 37.9 ± 2.4 | 0.793 |
| Body weight gain (kg) † | 12.2 ± 4.7 | 13.6 ± 5.9 | 0.629 |
| At 6th postpartum month | |||
| Weight (kg) | 57.6 ± 4.9 | 83.7 ± 6.8 | 0.029 * |
| BMI (kg/m2) | 23.9 ± 1.8 | 32.3 ± 3.0 | 0.037 * |
| 6th Pregnancy Month | 1st Lactation Month | 6th Lactation Month | ||||
|---|---|---|---|---|---|---|
| Food Groups | Non-OBE a | OBE b | Non-OBE c | OBE d | Non-OBE e | OBE f |
| Cereals | 298.2 ± 32.4 | 382.3 ± 51.0 | 284.7 ± 35.8 | 372.5 ± 45.2 | 271.7 ± 39.5 | 366.3 ± 50.4 |
| Fruits and Vegetables | 501.5 ± 47.6 b,d,f | 375.2 ± 32.9 a,c,e | 472.6 ± 58.9 b,d,f | 302.9 ± 31.4 a,c,e | 457.8 ± 38.9 b,d,f | 314.5 ± 25.9 a,c,e |
| Dairy | 599.7 ± 40.9 b,d,f | 325.6 ± 31.9 a,c,e | 492.7 ± 42.6 b,d,f | 294.5 ± 29.9 a,c,e | 479.2 ± 45.2 b,d,f | 239.4 ± 50.6 a,c,e |
| Meats and Eggs | 101.1 ± 12.5 | 119.4 ± 29.9 | 89.5 ± 19.6 | 107.6 ± 19.4 | 85.9 ± 10.8 | 102.8 ± 26.3 |
| Fish and Seafood | 25.3 ± 6.7 | 22.1 ± 6.0 | 28.9 ± 7.4 | 24.5 ± 10.5 | 23.5 ± 5.5 | 20.4 ± 6.9 |
| Legumes | 30.1 ± 8.5 | 18.2 ± 9.6 | 25.9 ± 7.1 | 15.4 ± 7.5 | 35.8 ± 10.2 | 20.1 ± 5.8 |
| High-Fat Foods | 25.2 ± 5.8 b,d,f | 56.9 ± 10.6 a,c,e | 23.6 ± 6.3 b,d,f | 50.1 ± 13.6 a,c,e | 20.9 ± 5.5 b,d,f | 52.8 ± 9.8 a,c,e |
| Oils and Fats | 28.9 ± 6.3 b,d,f | 44.6 ± 9.6 a,c,e | 25.9 ± 5.4 b,d,f | 40.8 ± 10.6 a,c,e | 23.7 ± 8.2 b,d,f | 41.8 ± 12.6 a,c,e |
| Sugar and Processed Foods | 161.4 ± 30.5 b,d,f | 402.6 ± 69.7 a,c,e | 182.6 ± 44.6 b,d,f | 384.5 ± 60.1 a,c,e | 170.5 ± 38.6 b,d,f | 371.5 ± 78.3 a,c,e |
| 6th Month of Pregnancy | 1st Month of Lactation | 6th Month of Lactation | ||||
|---|---|---|---|---|---|---|
| Energy/Nutrients/Fatty Acid | Non-OBE a | OBE b | Non-OBE c | OBE d | Non-OBE e | OBE f |
| Energy (kcal/day) | 2142 ± 274 b | 2895 ± 316 a,c,e | 1852 ± 205 b,d,f | 2659 ± 302 c,e | 1793 ± 199 a,b,d,f | 2438 ± 294 c,e |
| Protein (g/day) | 81.1 ± 26.5 | 98.5 ± 31.5 | 73.1 ± 24.3 | 90.6 ± 29.4 | 66.5 ± 19.2 | 84.5 ± 22.3 |
| Carbohydrate (g/day) | 325.3 ± 62.4 | 449.5 ± 72.3 | 247.2 ± 51.4 | 401.6 ± 82.9 | 232.1 ± 44.6 | 362.4 ± 80.1 |
| Fiber (g/day) | 42.7 ± 14.9 f | 20.1 ± 10.3 | 39.6 ± 12.8 | 18.5 ± 9.3 | 31.7 ± 9.2 | 17.2 ± 6.8 a |
| Sugar (g/day) | 90.5 ± 20.5 b,d,f | 198.5 ± 45.9 a,c,e | 82.6 ± 22.9 b,d,f | 184.3 ± 38.7 a,c,e | 72.4 ± 18.0 b,d,f | 175.2 ± 32.6 a,c,e |
| Fat (g/day) | 74.8 ± 11.4 b,d,f | 124.1 ± 21.3 a,c,e | 69.2 ± 14.1 b,d,f | 121.3 ± 19.1 a,c,e | 69.6 ± 15.8 b,d,f | 115.5 ± 22.0 a,c,e |
| ΣSFA (g/day) | 20.1 ± 2.5 b,d,f | 44.3 ± 3.8 a,c,e | 18.7 ± 2.2 b,d,f | 36.9 ± 3.6 a,c,e | 16.5 ± 2.2 b,d,f | 32.5 ± 3.3 a,c,e |
| ΣMUFA (g/day) | 27.6 ± 3.6 | 35.7 ± 3.0 | 25.9 ± 2.7 | 38.9 ± 2.9 | 25.1 ± 2.4 | 37.7 ± 2.5 |
| ΣPUFA (g/day) | 26.9 ± 2.9 | 42.1 ± 4.1 a,c,e | 24.6 ± 2.5 | 45.4 ± 6.7 a,c,e | 27.9 ± 2.8 | 45.1 ± 6.4 a,c,e |
| Σn-6 PUFA (g/day) | 24.8 ± 2.3 | 38.0 ± 3.5 a,c,e | 21.2 ± 2.1 | 41.9 ± 5.1 a,c,e | 25.9 ± 2.5 | 42.2 ± 4.2 a,c,e |
| 18:2n-6 (LA) (g/day) | 23.2 ± 2.1 | 36.4 ± 3.4 a,c,e | 21.3 ± 2.3 | 39.9 ± 3.7 a,c,e | 24.7 ± 2.2 | 40.8 ± 2.0 a,c,e |
| 20:4n-6 (AA) (g/day) | 0.72 ± 0.2 | 0.86 ± 0.3 | 0.81 ± 0.2 | 0.71 ± 0.1 | 0.82 ± 0.2 | 0.77 ± 0.1 |
| Σn-3 PUFA (g/day) | 2.05 ± 0.6 | 2.82 ± 0.7 | 1.95 ± 0.5 | 2.92 ± 0.8 | 1.92 ± 0.5 | 2.75 ± 0.4 |
| 18:3n-3 (ALA) (g/day) | 1.82 ± 0.6 | 2.65 ± 0.7 | 1.71 ± 0.4 | 2.75 ± 0.7 | 1.71 ± 0.6 | 2.59 ± 0.4 |
| 20:5n-3 (EPA) (g/day) | 0.05 ± 0.02 | 0.04 ± 0.01 | 0.06 ± 0.02 | 0.04 ± 0.01 | 0.05 ± 0.02 | 0.04 ± 0.02 |
| 22:6n-3 (DHA) (g/day) | 0.12± 0.03 | 0.09 ± 0.2 | 0.13 ± 0.04 | 0.10 ± 0.02 | 0.11± 0.03 | 0.08 ± 0.02 |
| n-6/n-3 PUFA ratio | 12.1 ± 1.2 | 13.4 ± 1.4 | 11.5 ± 1.1 | 14.1 ± 1.8 | 13.4 ± 1.4 | 15.3 ± 1.9 |
| 6th Month of Pregnancy | 1st Month of Lactation | 6th Month of Lactation | ||||
|---|---|---|---|---|---|---|
| FA (Expressed as mol % of Fatty Acid) | Non-OBE a | OBE b | Non-OBE c | OBE d | Non-OBE e | OBE f |
| ΣSFA | 49.8 ± 4.7 | 53.7 ± 5.1 | 48.8 ± 5.0 | 52.9 ± 5.6 | 49.3 ± 5.2 | 53.0 ± 5.4 |
| C16:0 | 30.3 ± 3.2 | 33.2 ± 3.7 | 30.9 ± 2.9 | 32.9 ± 3.5 | 31.5 ± 3.3 | 34.5 ± 3.6 |
| C18:0 | 15.8 ± 1.8 | 18.8 ± 1.9 | 16.1 ± 1.4 | 17.9 ± 1.9 | 16.5 ± 1.4 | 18.2 ± 1.9 |
| ΣMUFA | 16.8 ± 1.7 | 15.2 ± 1.4 | 17.3 ± 1.6 | 15.9 ± 1.7 | 18.3 ± 1.8 | 16.9 ± 1.5 |
| C18:1n-9 | 14.5 ± 1.4 | 13.8 ± 1.3 | 15.5 ± 1.7 | 13.6 ± 1.4 | 17.3 ± 1.8 | 15.5 ± 1.2 |
| ΣPUFA | 33.4 ± 3.8 | 31.3 ± 3.2 | 33.9 ± 3.6 | 31.2 ± 3.0 | 32.4 ± 3.7 | 30.1 ± 3.3 |
| Σn-6 PUFA | 24.8 ± 2.5 | 25.9 ± 2.9 | 25.8 ± 2.4 | 26.3 ± 2.7 | 24.5 ± 2.6 | 25.7 ± 2.8 |
| C18:2n-6 (LA) | 11.3 ± 1.3 | 12.6 ± 1.5 | 12.8 ± 1.9 | 13.5 ± 2.2 | 12.7 ± 1.4 | 13.9 ± 1.7 |
| C20:4 n-6 (AA) | 12.5 ± 1.6 | 11.9 ± 1.4 | 11.6 ± 1.3 | 11.2 ± 1.5 | 10.7 ± 1.2 | 9.61 ± 0.5 a |
| C22:5n-6 (DPAn-6) | 0.81 ± 0.1 | 0.79 ± 0.2 | 0.87 ± 0.05 | 0.81 ± 0.1 | 0.95 ± 0.2 | 0.92 ± 0.3 |
| Σ n-3 PUFA | 8.60 ± 1.0 b,d,f | 5.40 ± 0.4 a,c,e | 8.10 ± 0.9 b,d,f | 4.90 ± 0.5 a,c,e | 7.90 ± 1.1 b,d,f | 4.38 ± 0.4 a,c,e |
| C18:3n-3 (ALA) | 1.88 ± 0.3 b,d,f | 1.15 ± 0.2 a,c,e | 2.09 ± 0.5 b,d,f | 1.05 ± 0.3 a,c,e | 2.15 ± 0.6 b,d,f | 1.01 ± 0.4 a,c,e |
| C20:5n-3 (EPA) | 1.57 ± 0.4 b,d,f | 0.93 ± 0.1 a,d,f | 1.24 ± 0.3 f | 0.51 ± 0.2 a,b | 1.19 ± 0.2 d,f | 0.44 ± 0.1 a,b,c,e |
| C22:5n-3 (DPAn-3) | 0.58 ± 0.1 c,d,e,f | 0.40 ± 0.1 d,e,f | 0.38 ± 0.05 a,d,f | 0.21 ± 0.03 a,b,c | 0.29 ± 0.04 a,b,f | 0.17 ± 0.03 a,b,c,e |
| C22:6n-3 (DHA) | 4.53 ± 0.5 b,d,f | 3.04 ± 0.4 a,c,e | 4.39 ± 0.6 b,d,f | 3.01 ± 0.3 a,c,e | 4.22 ± 0.4 b,d,f | 2.55 ± 0.3 a,c,e |
| n-6/n-3 PUFA ratio | 2.87 ± 0.1 b,d,f | 4.77 ± 0.6 a,c,e | 3.16 ± 0.5 b,d,f | 5.34 ± 0.7 a,c,e | 3.09 ± 0.4 b,d,f | 5.84 ± 0.6 a,c,e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamorro, R.; Bascuñán, K.A.; Barrera, C.; Sandoval, J.; Puigrredon, C.; Valenzuela, R. Reduced n-3 and n-6 PUFA (DHA and AA) Concentrations in Breast Milk and Erythrocytes Phospholipids during Pregnancy and Lactation in Women with Obesity. Int. J. Environ. Res. Public Health 2022, 19, 1930. https://doi.org/10.3390/ijerph19041930
Chamorro R, Bascuñán KA, Barrera C, Sandoval J, Puigrredon C, Valenzuela R. Reduced n-3 and n-6 PUFA (DHA and AA) Concentrations in Breast Milk and Erythrocytes Phospholipids during Pregnancy and Lactation in Women with Obesity. International Journal of Environmental Research and Public Health. 2022; 19(4):1930. https://doi.org/10.3390/ijerph19041930
Chicago/Turabian StyleChamorro, Rodrigo, Karla A. Bascuñán, Cynthia Barrera, Jorge Sandoval, Claudia Puigrredon, and Rodrigo Valenzuela. 2022. "Reduced n-3 and n-6 PUFA (DHA and AA) Concentrations in Breast Milk and Erythrocytes Phospholipids during Pregnancy and Lactation in Women with Obesity" International Journal of Environmental Research and Public Health 19, no. 4: 1930. https://doi.org/10.3390/ijerph19041930
APA StyleChamorro, R., Bascuñán, K. A., Barrera, C., Sandoval, J., Puigrredon, C., & Valenzuela, R. (2022). Reduced n-3 and n-6 PUFA (DHA and AA) Concentrations in Breast Milk and Erythrocytes Phospholipids during Pregnancy and Lactation in Women with Obesity. International Journal of Environmental Research and Public Health, 19(4), 1930. https://doi.org/10.3390/ijerph19041930







